Abstract
The HEK 293 cell line (293 cells) was derived from human embryonic kidney (HEK) cells grown in tissue culture. 293 cells are very easy to grow and transfect and have been widely used in cell biological research for many years. 293 cells have many of the properties of immature neurons, suggesting that they represent a transformed neuronal cell present in the original kidney culture, and they are not useful as an in vitro model for kidney cell function. The 293T cell line contains the SV40 large T-antigen, which allows the episomal replication of transfected plasmids containing the SV40 origin of replication, and 293FT cells are a fast-growing variant. A recent report showed that introducing a set of transcription factors associated with pluripotency into human somatic cells can directly reprogr am them to produce induced pluripotent stem (iPS) cells. To date, however, iPS cells have not been generated from immortalized cells. We examined whether iPS cells could be generated from 293 FT cells transfected with four transcription factors (OCT4, SOX2, NANOG, and LIN28). The obtained cells morphologically resembled human ES cells, and showed a similar marker gene expression pattern. These cells had an impaired ability to differentiate, and formed immature ectodermal tumors after they were transplanted into nude mice. Thus, we could not derive fully reprogrammed iPS cells from 293FT cells. We conclude that the 293FT cells transduced with OCT4, SOX2, NANOG, and LIN28 produced aberrant ES-like cells.
Keywords: iPS, 293, reprogramming, stem cells
Introduction
The HEK 293 cell line (293 cells) was derived from human embryonic kidney (HEK) cells grown in tissue culture. 293 cells are very easy to grow and transfect and have been widely used in cell biological research for many years. 293 cells have many of the properties of immature neurons, suggesting that they represent a transformed neuronal cell present in the original kidney culture, and they are not useful as an in vitro model for kidney cell function. The 293T cell line contains the SV40 large T-antigen, which allows the episomal replication of transfected plasmids containing the SV40 origin of replication, and 293FT cells are a fast-growing variant.
Some recent reports showed that introducing a set of transcription factors associated with pluripotency into human somatic cells can directly reprogram them to produce induced pluripotent stem (iPS) cells. (3-6) Specifically, a set of four factors, OCT4, SOX2, NANOG, and LIN28, can reprogram human fetal or postnatal fibroblasts (4). The cellular characteristics of the reprogrammed cells are similar to those of human ES cells. To date, iPS cells have not been generated from immortalized cells.
Here we investigated whether 293FT cells could be reprogrammed by the introduction of OCT4, SOX2, NANOG, and LIN28. We also examined whether completely reprogrammed cells could be derived from 293FT cells.
Materials & Methods
Cell culture
293FT cells (purchased from Invitrogen) were maintained in a standard culture medium. DMEM supplemented with 7% FBS, 2 mM glutamine, and antibiotics (50 U/ml penicillin and 50 mg/ml streptomycin). Mitomycin C-treated MEF feeder cells (purchased from ReproCELL) were plated in 10-cm culture dishes that had been coated with 0.1% gelatin (Sigma), in the same culture medium. iPS cells were generated and maintained in Primate ES medium (ReproCELL) supplemented with 4 ng/ml recombinant human basic fibroblast growth factor (bFGF) (R&D Systems), and the medium was changed daily. For passaging, the iPS cells were rinsed once with Hank’s balanced salt solution (HBSS) (Invitrogen) and incubated with dissociation medium (ReproCELL) at 37°C. All cultures were maintained at 37°C in a humidified 5% CO2/95% air atmosphere.
Plasmid construction
The open reading frames of human OCT4, SOX2, NANOG, and LIN28 were amplified by RT-PCR and subcloned, individually, into pLenti 6.3/V5-TOPO (Invitrogen). We inserted a stop codon after each gene to avoid expressing the V5-tag. The plasmids were constructed by Invitrogen.
Lentivirus production and infection
Lentiviruses were produced using the ViraPowerTM HiPerfomeTM Lentiviral Expression Kit (Invitrogen). 293FT cells were plated at 5-6 X 106 cells per 10-cm dish and incubated overnight. Four dishes of 293FT cells were used to produce each of the lentiviruses (encoding OCT4, SOX2, NANOG, and LIN28). The next day, the 293FT cells were transfected with 3 mg pLenti 6.3/V5- TOPO, 9 mg ViraPower packaging mix (Invitrogen), and the Fugene 6 transfection reagent (Roche), according to the manufacturer’s instructions. Twenty-four hours later, the medium was collected as the first virus-containing supernatant and replaced with new medium. Medium was also collected at the 48- and 72-hour post-transduction time points. The virus-containing supernatants were passed through a 0.45-mm pore-size filter (Millipore). These virus-producing cells were not used again in this study. To infect the 293 cells, the virus-containing supernatants were used the same day that they were collected; that is, the 293FT cells were infected three times, once on each day the virus-containing medium was collected. For each infection, equal amounts of supernatant containing each of the four lentiviruses were combined, and the supernatants were transferred to a culture dish containing 293FT cells and incubated at 37°C.
iPS cell generation
Twenty-four hours after the third transduction of the 293FT cells, the virus-containing medium was replaced with standard medium, which was changed daily. Five to six days after the third transduction, the 293FT cells were harvested by trypsinization, and 5 X 104 cells per 10-cm dish were plated on MEF feeder cells in Primate ES cell medium supplemented with 4 ng/ml bFGF. The medium was changed daily. Colonies with a human ES cell-like morphology (iPS cell colonies) were picked 21-25 days after transduction.
Immunofluorescence microscopy and immunostaining
To assess the extent of reprogramming, we used the Human Embryonic Stem Cell Marker Antibody Panel (R&D Systems). The cells were washed twice with phosphate-buffered saline, fixed for 20 min with 4% (w/v) paraformaldehyde, permeabilized for 60 min with phosphate-buffered saline containing 0.1% (v/v) Triton X-100, and blocked for 3 h with 20% donkey serum in phosphate-buffered saline. For immunostaining, the fixed cells were incubated with antibodies (all from R&D Systems) against the following human gene products, as indicated: alkaline phosphatase (monoclonal antibody), NANOG (polyclonal antibody), OCT4 (polyclonal antibody), SSEA-1 (monoclonal antibody), or SSEA-4 (monoclonal antibody). The cells were then washed three times with PBS containing 0.1% (v/v) Triton X-100, and then incubated with the appropriate secondary antibodies: anti-goat IgG antibody conjugated with Alexa 488 and/or anti-mouse IgG antibody conjugated with Alexa 488 (Molecular Probes). Nucleic acids were immunostained with SYTOXR Orange Nucleic Acid Stain (Molecular Probes).
Reverse-transcription (RT)-PCR
The total RNA was prepared from cells using the PureLinkTMMicro-to-Midi Total RNA Purification System (Invitrogen). The RT-PCR was performed using the SuperScriptTMIII One-Step RT-PCR System with PlatinumR Taq DNA Polymerase (Invitrogen) for human ES cell markers, at 55°C for 30 min for the reverse transcription, 94°C for 2 min to inactivate the reverse transcriptase and activate the polymerase, and then 40 cycles of 94°C for 15 s, 55°C for 30 s, 68°C for 1 min. The final extension was at 68°C for 5 min. The products were: 315 bp for OCT4, 285 bp for NANOG, 513 bp for GAPDH (7), 174 bp for STELLAR, and 150 bp for GDF3 (8).
In vitro assay (embryoid body formation)
Confluent iPS cells in a 10-cm dish were harvested by trypsinization. The iPS cells were transferred to a Poly (hydroxyethyl methacrylate-co-methyl methacrylate; HEMA-MMA)-coated dish in Primate ES cell medium. The medium was changed every other day, and the cells were maintained in floating culture for 8 days.
In vivo assay
Confluent iPS cells in a 6-well dish were harvested by trypsin treatment, collected into tubes, spun, and the pellets were suspended in HBSS. The cells were then injected into three nude mice (BALB/C, 6-weeks old) (CREA). As a control, 293FT cells were injected into three nude mice (BALB/C, 6-weeks old) (CREA). Six weeks after the injection, the tumors were dissected and fixed with 10% formalin. The pathological analyses were performed at SRL Laboratory.
Short tandem repeat analysis and karyotyping
DNA fingerprinting with short tandem repeat (STR) markers and Chromosomal G-band analyses were performed at SRL Laboratory.
Results
ES cell-like colonies were generated from 293FT cells transduced with OCT4, SOX2, NANOG, and LIN28
The 293 cell line is a permanent line established from primary embryonic human kidney cells transformed with sheared human adenovirus type 5 DNA (1) The 293F cell line available from Invitrogen is a fast growing variant of the 293 line, and was originally obtained from Robert Horlick at Pharmacopeia. The 293FT line was derived from the 293F cell line and stably expresses the SV40 large T antigen under control of the human cytomegalovirus (CMV) promoter from the pCMVSPORT6TAg.neo plasmid. The SV40 large T antigen is constitutively expressed at a high level.
In this study, we attempted to generate iPS cells from these immortalized cells. We introduced lentiviruses containing human OCT4, SOX2, NANOG, and LIN28 into the 293FT cell line on 3 consecutive days (24, 48, and 72 hours after the transfection of the virus-producing cells). The transduction efficiency was more than 80% (data not shown).
Colonies with a human ES cell-like morphology first became visible 12-15 days after the transduction, under these human ES cell-supporting conditions (Fig. 1A). From 5 X 104 293FT cells, we obtained ~20 ES cell-like colonies. We performed three independent experiments and obtained the same results (data not shown). We picked the colonies 21-25 days after transduction. These ES cell-like colonies proliferated on MEF feeder cells in Primate ES cell medium containing bFGF, where they formed flat, tightly packed colonies. The cells maintained a human ES cell-like morphology, which was, importantly, different from that of the parental 293FT cells.
Figure 1.
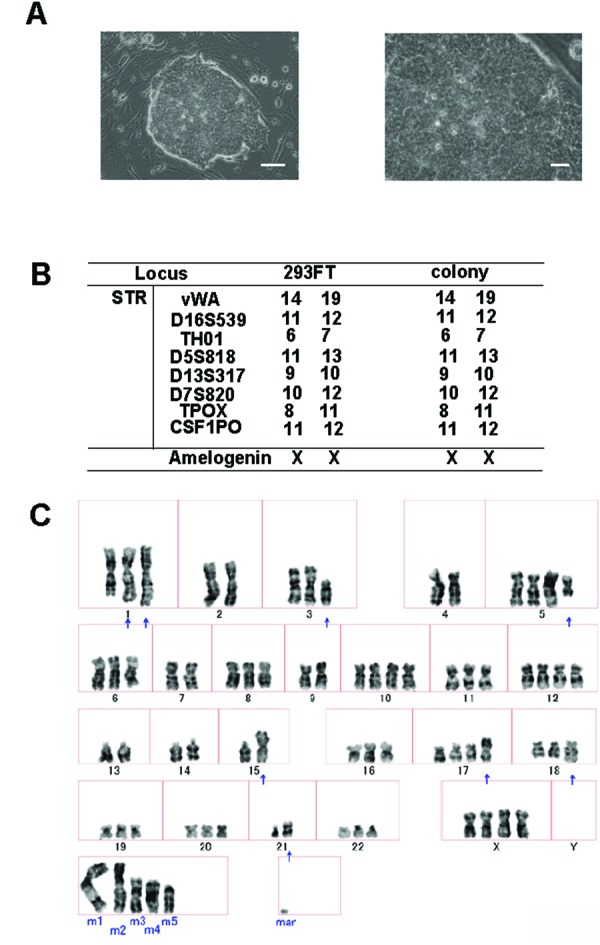
Generation of aberrant ES-like cells from 293FT cells with four transcription factors (OCT4, SOX2, NANOG, and LIN28). (A) Morphology of the aberrant ES-like cells derived from 293FT cells. Left, bars = 200 ?m. Right, bars = 60??m (B) STR analysis of 293FT cells and the aberrant ES-like cells derived from them. (C) G-banding chromosome analysis of the aberrantly reprogrammed cells derived from 293FT cells.
ES cell-like colonies derived from 293FT cells
We evaluated the ES cell-like colonies to confirm that they were derived from 293FT cells, by DNA fingerprinting using short tandem repeat markers. Comparison of the colonies with the parental 293FT cells showed that the ES cell-like colonies were indeed derived from the 293FT cells (Fig. 1B).
We also analyzed the karyotype of these ES cell-like colonies. A chromosomal G-band analysis of these cells indicated an abnormal karyotype (Fig. 1C). All of these findings indicated that the ES cell-like colonies were derived from 293FT cells and did not arise as the result of cross-contamination.
Characterization of the aberrant ES-like cells derived from 293FT cells by immunostaining. Immunolabeling shows the reactivity for alkaline phosphatase (AP) (a), NANOG (b), OCT4 (c), SSEA-1 (d), and SSEA-4 (e). Nuclei were stained with SYTOXR Orange. Bars=50?m.
ES cell-like colonies express ES cell markers
Next, we characterized the ES cell-like colonies by immunostaining. They expressed human ES cell-specific proteins, including alkaline phosphatase, NANOG, OCT4, and the surface antigen SSEA-4 (Fig. 2a-c and e), but not the stage-specific embryonic antigen (SSEA-1) (Fig. 2d). RT-PCR showed that the ES cell-like colonies expressed marker genes for undifferentiated ES cells: OCT4, NANOG, STELLAR, and GDF3 (Fig. 3).
Figure 2.
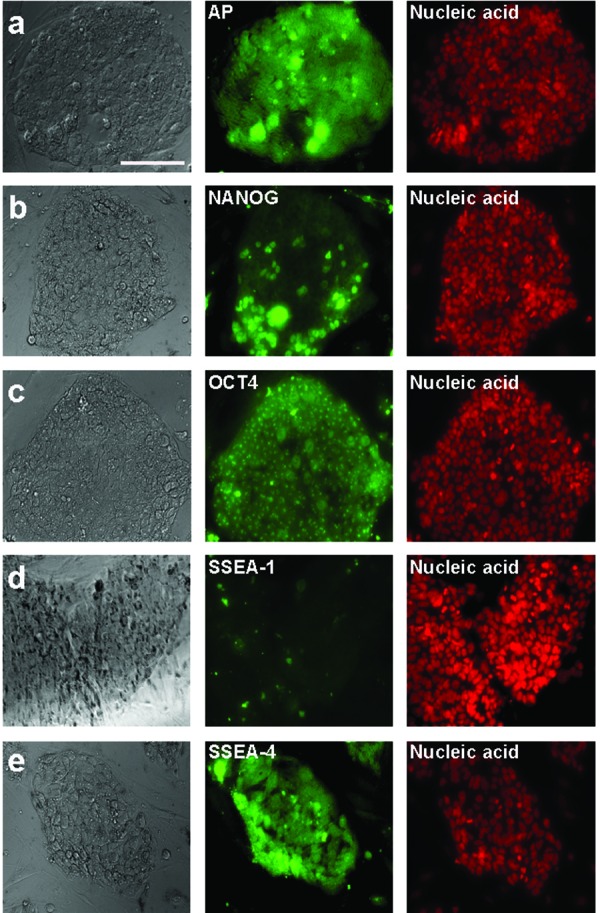
Figure 3.
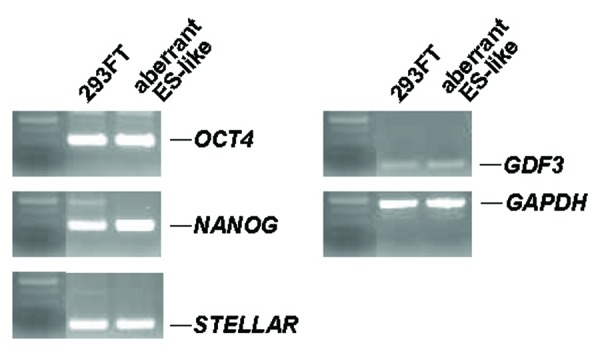
Total gene expression in aberrant ES-like cells derived from 293FT cells by RT-PCR. RT-PCR analysis was performed to detect the expression of OCT4, NANOG, STELLAR, GDF3, and GAPDH in 293FT cells and the aberrant ES-like cells derived from them. GAPDH was used as a loading control.
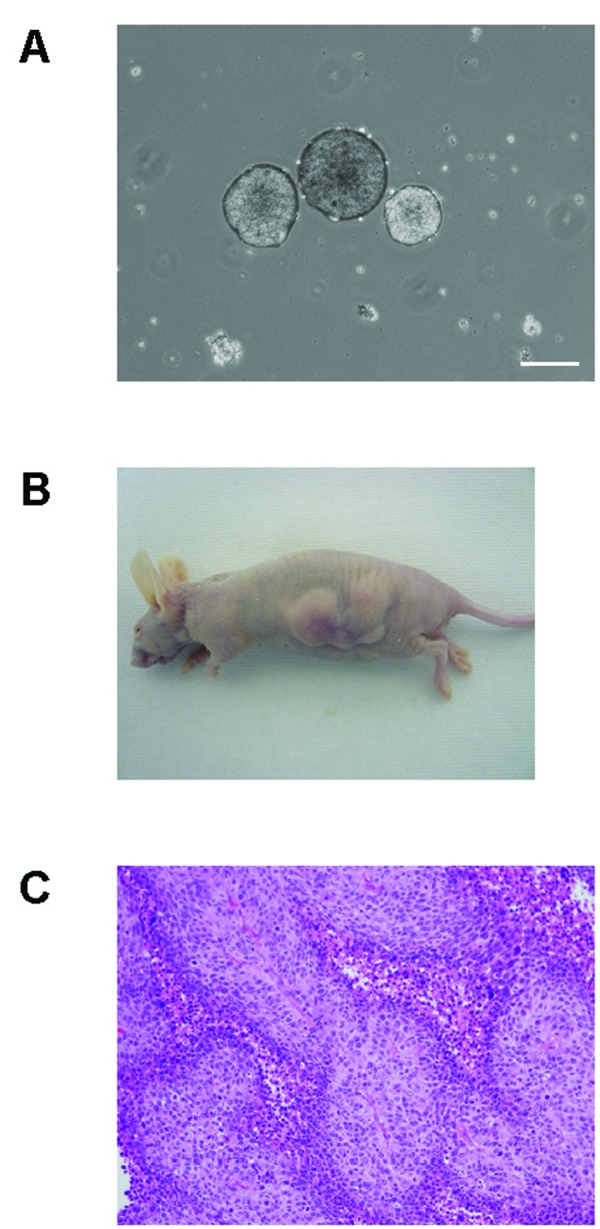
Embryoid body and teratoma formation. Teratomas containing immature neural and keratin-containing epidermal tissue (ectoderm) formed from the aberrant ES-like cells derived from 293FT cells. (A) Embryoid bodies formed from the ES-like cells after 8 days in a floating culture, bars = 200 ?m. (B) Nude mice, 6 weeks after the injection of the 293FT-derived ES-like cells. (C) Hematoxylin and eosin staining of sections of tumors generated by the 293FT-derived ES-like cells.
Embryoid body formation from ES-like cell colonies
The formation of embryoid bodies is one of the easiest procedures for eliciting the in vitro differentiation of ES cells. We investigated whether the ES cell-like colonies were pluripotent in vitro, by making embryoid bodies. After 8 days in a floating culture, the ES-like cells had formed small free-floating aggregates (Fig. 4A).
Figure 4.
Tumor formation from ES cell-like colonies
We next investigated whether the ES cell-like colonies were pluripotent in vivo, by transplanting them into the dorsal flanks of nude mice. Six weeks later, we observed tumor formation (Fig. 4B) in two of the three mice tested. Histological inspection showed that the tumors contained immature neural and keratin-containing epidermal tissues (ectoderm) (Fig. 4C). We could not detect cells of other tissue types in these tumors. As a control, we also injected 293FT cells into three nude mice, and observed them for tumor formation. Two of the mice developed tumors, which contained immature neural and keratin-containing epidermal cells, like the ES cell-like-derived tumors (data not shown).
Discussion
In this study, we showed that the transduction of four transcription factors (OCT4, SOX2, NANOG, and LIN28) into 293FT cells caused the transformed cells to produce ES-like cells with aberrant properties. The morphology of these 293FT-derived induced pluripotent stem (iPS) cells was clearly different from that of the 293FT cells, and similar to that of ES cells. In sum, the attempted reprogramming of these immortalized cells produced aberrant ES-like cells, not fully reprogrammed iPS cells.
In 2006, mouse embryonic and adult fibroblasts were reported to acquire properties similar to those of ES cells after being transformed by retrovirally introduced genes encoding four transcription factors, OCT4, SOX2, KLF4, and c-Myc (9). The authors called these cells iPS cells. iPS cells are similar to ES cells in morphology, proliferation, expression of some ES cell marker genes, and the formation of teratomas. In 2007, iPS cells were generated from human fibroblasts (3,4,6).
The generation of iPS cells requires complete nuclear reprogramming. It is still unclear how cells undergo nuclear reprogramming to become iPS cells. Three types of iPS cells have now been generated by the present iPS cell technology. The first type, obtained by direct reprogramming, consists of fully reprogrammed iPS cells that are comparable to ES cells. Such fully reprogrammed iPS cells can form mature teratomas. The second type, partially reprogrammed iPS cells, can self-renew and undergo in-vitro differentiation. The third type of iPS cells includes aberrantly reprogrammed cells that can self-renew and are refractory to differentiation (10). Thus, the incomplete reprogramming of somatic cells into iPS cells, as in the second case, could still result in differentiation-competent cells that can give rise to a desired cell type.
Another problem of iPS technology is the presence of transgenes in iPS cells. Most iPS cells are generated from somatic cells by retrovirus- or lentivirus-mediated gene transduction, which results in the transgenes being integrated into the host cell genome. The transgenes are largely silenced in the iPS cells, but their reactivation could lead to tumorigenesis (11). Leaky transgene expression might also inhibit iPS cells from completely differentiating and maturating, leading to a greater risk of immature teratoma formation (10).
Many reports have stated that full reprogramming should be followed up by the long-term observation of chimeric mice and their progeny, to evaluate the safety of the technology (10-13). Some recent publications suggest that the examination of germline transmission and teratoma formation may not be absolutely required for partial and aberrantly reprogrammed cells. In addition, the pluripotency of human iPS cells should be judged by their embryoid body and teratoma formation.
iPS technology is expected to be useful for drug discovery, disease models, and regenerative medicine in the near future. Therefore, it is important to understand the mechanism of complete reprogramming. The creation of aberrantly reprogrammed cells in addition to fully reprogrammed cells could be useful for examining this mechanism. Here we made aberrant, differentiation-refractory ES-like cells from 293FT cells. We suggest that the immature neural and keratin-containing epidermal tissue (ectoderm) formation was due to the 293 cells transforming into human neuronal lineage cells. Alternatively, the SV40 large T antigen in the 293FT cells can cause chromosomal aneuploidy and might have resulted in the immature tumors. A recent study confirmed that the large T antigen enhances the reprogramming efficiency by up to 70-fold, but it also showed that the large T viral sequence was integrated into the genome and increased chromosomal aneuploidy (14). Indeed, three decades of investigation into the SV40-encoded 708-amino acid large T antigen has and continues to contribute to our understanding of tumor transformation and other biological processes (15). Interestingly, the transforming activity of the SV40 virus activity is partly due to the 172-amino acid small t antigen, which, like the large T antigen, is also encoded by the SV40 early region. It will be of great interest to determine whether the variation in the cellular targets of T observed in cellular transformation are also important in the T-mediated enhancement of iPS cell production.
Two previous reports showed that when permeabilized 293T cells are treated for 1 hour with an extract of undifferentiated human NCCIT EC cells or mouse ES cells, but not of control cell types such as Jurkat, some of the surviving 293T cells stably transition into a pluripotent phenotype (16,17). Even after >24 passages, the clones of these primed 293T cells express hundreds of EC/ES-specific genes, such as OCT4 and NANOG, concomitant with the downregulation of 293T-specific genes and differentiation markers (16,17). However, when T was absent, no evidence of somatic cell priming or reprogramming in any human cell type was obtained in these reports. An interesting question is how much the presence of T in the 293T cells contributes to the observed reprogramming by protein extracts and whether loading the cells with purified T protein would enhance the programming of cell types without requiring integration of the T coding sequence. One study indicates that the sustained presence of T is probably critical to the enhanced reprogramming of human fibroblasts, but that the persistence of T may also contribute an increased level of abnormal karyotypes (14). In this study, although 293FT cells assumed a pluripotent phenotype following their transduction with OCT4, SOX2, NANOG, and LIN28, their ES-like phenotype was aberrant. We speculate that the large T antigen expressed in the 293FT cells contributed to the aberrant phenotype.
We also found that the ES-like cells did not form mature teratomas, and might resemble cancer stem cells in this respect. Thus, induced cancer stem cell models might be derived from 293FT cells. In sum, iPS cell technology will lead to a better understanding of nuclear reprogramming, and iPS cells offer a promising cell source for clinical applications in the future. The generation and examination of aberrantly reprogrammed cells should reveal useful information about iPS cells.
Acknowledgements
This work was supported by a research grant from the Suzuki Urological Foundation and a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS). H.K. supervised the entire project.
References
- (1).Graham FL, Smiley J, Russell WC, Nairn R.Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59-74 [DOI] [PubMed] [Google Scholar]
- (2).Shaw G, Morse S, Ararat M, Graham FL.Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. Faseb J. 2002;16:869-71 [DOI] [PubMed] [Google Scholar]
- (3).Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S.Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-72 [DOI] [PubMed] [Google Scholar]
- (4).Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA.Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-20 [DOI] [PubMed] [Google Scholar]
- (5).Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K.Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ.Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141-6 [DOI] [PubMed] [Google Scholar]
- (7).Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R.Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481-5 [DOI] [PubMed] [Google Scholar]
- (8).Ezeh UI, Turek PJ, Reijo RA, Clark AT.Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 2005;104:2255-65 [DOI] [PubMed] [Google Scholar]
- (9).Takahashi K, Yamanaka S.Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-76 [DOI] [PubMed] [Google Scholar]
- (10).Yamanaka S.A fresh look at iPS cells. Cell. 2009;137:13-7 [DOI] [PubMed] [Google Scholar]
- (11).Okita K, Ichisaka T, Yamanaka S.Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-7 [DOI] [PubMed] [Google Scholar]
- (12).Meissner A, Wernig M, Jaenisch R.Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177-81 [DOI] [PubMed] [Google Scholar]
- (13).Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R.In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-24 [DOI] [PubMed] [Google Scholar]
- (14).Mali P, Ye Z, Hommond HH, Yu X, Lin J, Chen G, Zou J, Cheng L.Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26:1998-2005 [DOI] [PubMed] [Google Scholar]
- (15).Ahuja D, Saenz-Robles MT, Pipas JM.SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729-45 [DOI] [PubMed] [Google Scholar]
- (16).Taranger CK, Noer A, Sorensen AL, Hakelien AM, Boquest AC, Collas P.Induction of dedifferentiation, genomewide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Mol Biol Cell. 2005;16:5719-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Freberg CT, Dahl JA, Timoskainen S, Collas P.Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol Biol Cell. 2007;18:1543-53 [DOI] [PMC free article] [PubMed] [Google Scholar]


