Abstract
Introduction. Amniotic membrane contains a multipotential stem cell population and is expected to possess the machinery to regulate immunological reactions. We investigated the safety and efficacy of allogeneic amniotic membrane-derived mesenchymal stromal cell (AMSC) transplantation in a porcine model of chronic myocardial ischemia as a preclinical trial.
Methods. Porcine AMSCs were isolated from amniotic membranes obtained by cesarean section just before delivery and were cultured to increase their numbers before transplantation. Chronic myocardial ischemia was induced by implantation of an ameroid constrictor around the left circumflex coronary artery. Four weeks after ischemia induction, nine swine were assigned to undergo either allogeneic AMSC transplantation or normal saline injection. Functional analysis was performed by echocardiography, and histological examinations were carried out by immunohistochemistry 4 weeks after AMSC transplantation.
Results. Echocardiography demonstrated that left ventricular ejection fraction was significantly improved and left ventricular dilatation was well attenuated 4 weeks after AMSC transplantation. Histological assessment showed a significant reduction in percentage of fibrosis in the AMSC transplantation group. Injected allogeneic green fluorescent protein (GFP)-expressing AMSCs were identified in the immunocompetent host heart without the use of any immunosuppressants 4 weeks after transplantation. Immunohistochemistry revealed that GFP colocalized with cardiac troponin T and cardiac troponin I.
Conclusions. We have demonstrated that allogeneic AMSC transplantation produced histological and functional improvement in the impaired myocardium in a porcine model of chronic myocardial ischemia. The transplanted allogeneic AMSCs survived without the use of any immunosuppressants and gained cardiac phenotype through either their transdifferentiation or cell fusion.
Keywords: Amniotic membrane, Mesenchymal stromal cell, Allogeneic cell transplantation, Chronic myocardial ischemia, Porcine model
Introduction
Despite the fact that remarkable advances in the treatment of cardiovascular disease have been achieved over the past few decades, heart disease continues to be the primary cause of death throughout the world. Because the regenerative capability of heart is not sufficient to compensate for myocardial cell loss following myocardial infarction (MI) or cardiomyopathy, such insults often lead to ventricular remodeling and result in heart failure. The concept known as “transdifferentiation,” in which somatic stem or progenitor cells differentiate into other lineages [1], and the ensuing vasculogenesis [2] have driven the investigation of stem or progenitor cell transplantation for the heart. [3] Although the underlying mechanism remains controversial, numerous clinical trials of cardiac cell therapy in patients with heart disease have been initiated using bone marrow-derived mononuclear cells, [4] peripheral blood stem cells [5], and skeletal myoblasts. [6]
Randomized controlled trials using bone marrow-derived stem cells have been intensively investigated in patients suffering from MI. Some studies have shown significant improvement in cardiac function and prevention of ventricular remodeling, [7,8] while others have demonstrated no significant increase in adverse events were recognized in almost any clinical trial for stem cell therapy compared with conventional therapy. Systematic reviews for these trials have elucidated the therapy’s safety and modest efficacy. [11,12] However, sources of stem cells, their delivery methods, the timing of implantation, and the dosage remain issues for the clinical application of cardiac stem cell therapy.
Mesenchymal stem cells (MSCs) are one of the most promising sources for stem cell therapy, [13] because they are easy to cultivate, induce angiogenesis, [14] possess pluripotent capabilities including the ability to differentiate into cardiomyocytes, [15] and release an array of cytokines that can protect the heart from insults. [16] Several clinical trials using autologous bone marrow-derived MSCs for treatment of heart disease, [17] autoimmune diseases [18] and graftversus-host disease [19] are currently ongoing. However, several reports suggest that aging and disease reduce the quantity and quality of stem cells. [20,21] Transplantation of stem cells isolated from aged patients demonstrated a lower efficacy for improving damaged myocardium than those isolated from younger patients. [22] Thus, autologous stem cell transplantation may have some limitations, especially in patients with critical illnesses such as severe heart failure. Allografts may be an alternative to autografts, although allogeneic stem cells can evoke immunological reactions. Bone marrow-derived MSCs have been reported to have immunomodulatory properties.
Several studies have shown that MSCs can be isolated from human amniotic membrane, [23,24] and human amniotic membrane-derived mesenchymal stromal cells (AMSCs) have been reported to have the potential for transdifferentiation into various cell linages, including cardiomyocytes [25] and neurons. [26] Furthermore, it is known that the amniotic membrane plays a crucial role in preventing rejection of the fetus. Maternal tolerance is maintained by several mechanisms, one of which involves non-classical major histocompatibility complex (MHC) class I molecules such as HLA-E, -F, and –G. [27] Tsuji et al. reported that xenogeneic human AMSC transplantation significantly improved cardiac function in rat MI models; they also reported that human AMSCs survived in immunocompetent rats without the use of any immunosuppressants for more than 4 weeks, and transdifferentiated into cardiomyocytes in vivo. [28].Moreover, IL-10 pretreatment, which strengthens the expression of HLA-G, affected stem cell survival in the diseased heart.
To explore the clinical application of transplantation of allogeneic AMSCs, we transplanted them into porcine hearts affected by chronic myocardial ischemia as a preclinical study. We examined whether AMSCs could survive and gain the phenotype of cardiomyocytes in allogeneic combinations without the use of immunosuppressants, and investigated their efficacy on global heart function, angiogenesis, and myocardial fibrosis.
Materials and methods
In this study, we followed “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No.86-23, revised 1985). All animal studies were performed on the National Research Institute for Child Health and Development, with ethical approval of the Institutional Review Board of the National Research Institute for Child Health and Development, Japan.
Isolation and culture of porcine AMSCs
Porcine placentas were obtained from fetuses by cesarean section just before delivery. They were kept in a cold, aseptic environment and cell isolation was initiated within 24 h of surgery. Porcine amniotic membranes were peeled mechanically from the chorion, rinsed in Dulbecco’s modified phosphate-buffered saline (PBS) to remove blood, and then cut into pieces approximately 1-cm2 size. Homogeneous AMSCs were obtained by a two-step procedure: a small piece of amnion tissue was treated with 2.4U/ml dispase II (Roche Diagnostics, 4942078) at 37°C for 60 min to remove the amnion-derived epithelial cells with gentle stirring at 100 rpm. The remaining pieces were then incubated with 0.75mg/ml type-II collagenase (Worthington Biochemical, NJ, USA) at 37°C for 60 min with gentle stirring at 100 rpm. Isolated AMSCs were rinsed and seeded onto a 10 cm culture dish with 10% FBS DMEM containing Antibiotic-Antimycotic (1X). After two or three days, the cultured dishes became subconfluent in a humidified and normoxic atmosphere (20% O2) and 5% CO2 at 37ºC. The culture medium was replaced with fresh medium every 4 days. The porcine AMSCs in this study were used within 3–10 population doublings.
Green fluorescent protein-expressing porcine AMSCs
To detect the implanted AMSCs, we used green fluorescent protein (GFP)-expressing porcine AMSCs. GFP-transgenic Jinhua pigs were created by a somatic cell cloning technique [29]. GFP-expressing porcine amniotic membranes were collected after delivery of the GFP transgenic Jinhua piglets. The transgene expression of amniotic membranes derived from fetuses mated with GFP-transgenic and wild-type Jinhua pigs were examined by fluorescent microscopy. The GFP-expressing porcine AMSCs were isolated and cultured as described above, which ensured their GFP expression.
Flow cytometry analysis
Cultured porcine AMSCs were characterized by cell surface markers using flow cytometric analysis. They were stained for 60 min at 4°C with primary antibodies specific for swine antigens, washed with PBS(–), and incubated with goat antimouse IgG for 30 min at 4°C. After 3 washes with PBS(–), the stained cells were analyzed on a Cytomics FC 500 (Beckman Coulter, Inc.) and the data were analyzed with CXP analysis (Beckman Coulter, Inc.). Antibodies against CD29 (integrin-β1) (552369, BD Pharmingen), CD44 (553134, BD Pharmingen), CD45 (552292, BD Pharmingen), SLA class I (MCA2261, AbD Serotec), SLA class II DR (553642, BD Pharmingen), and SLA class II DQ (553642, BD Pharmingen) were used as primary antibodies. The GFP expression profiles of GFP-expressing porcine AMSCs were analyzed by flow cytometry as well as by analysis of cell surface markers.
Myocardial infarction and AMSC transplantation
An experimental design of this study is showed in Figure. 1A. Fifteen swine weighing 20–25 kg (Saitama Experimental Animals Supply Co., Saitama, Japan) were used. The animals were pre-anesthetized by an intramuscular injection of thiopental sodium (20 mg/kg) and mafoprazine mesylate (0.5 mg/kg). After endotracheal intubation, inhalation anesthesia was maintained with isoflurane (0%–5%) and oxygen. The endotracheal tube was connected to a volume-controlled mechanical ventilator.
Figure 1.
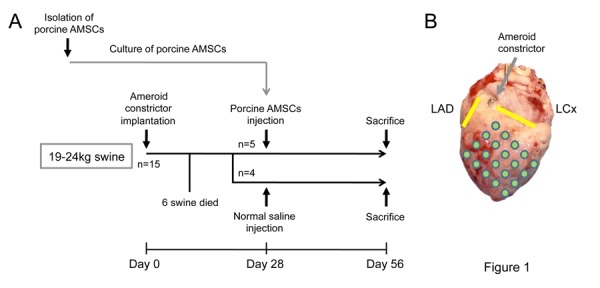
(A) Experimental design of the in vivo protocol. Abbreviation: AMSCs, amniotic membrane-derived mesenchymal stromal cells. (B) A macroscopic view of a porcine heart. An implanted ameroid constrictor is indicated by a gray arrow. Left anterior descending artery (LAD) and left circumflex artery (LCx) are shown by yellow lines. The injection spots are shown by green circles.
The swine underwent a left thoracotomy and the pericardium was dissected to expose the left circumflex (LCx) coronary artery (Figure. 1B). An ameroid constrictor (COR-2.25-SS, Research Instruments) was placed around the proximal portion of the artery, the pericardium was closed, and the thoracotomy was closed after the air had been evacuated from the thoracic cavity. The animals were treated twice with cefazolin sodium (1 g administered intravenously) at the pre-and postoperative phases.
Four weeks after inducing myocardial ischemia, AMSC transplantation was performed. Six of the 15 swine died before the second procedure. The remaining animals were assigned to transplantation (n = 5) and control (n = 4) groups. Under anesthesia, the heart was exposed via a median sternotomy. The ischemic area was visually identified and either cell suspension solution (1 ml, 1 × 106 cells) or normal saline (1 ml) was injected epicardially into the ischemic region at 16–20 locations with a 25-gauge needle (Figure. 1B). The animals were treated with cefazolin sodium intravenously in the same fashion as during the constrictor implantation, and they remained in bed for 4 weeks without any immunosuppressants. After the final estimation by echocardiography, they were sacrificed with an overdose of potassium chloride. The hearts were excised and myocardial samples were systematically collected for immunohistological analysis.
Measurement of cardiac function
Echocardiography was performed at Day 0 before implantation of an ameroid constrictor on LCx, at Day 28 before stem cell transplantation or normal saline injection, and at Day 56. Transthoracic echocardiography was performed using EnVisor C (PHILIPS Electronics, Tokyo, Japan) with a 4-MHz sector array transducer. B-mode (2D) images were acquired in the left ventricle (LV) long-axis view at the middle level of the papillary muscle. LV end-diastolic diameter (LVDd), LV end-systolic diameter (LVDs), and posterior wall thickness (PWT) were measured from M-mode images. LV ejection fraction (LVEF) was calculated as follows:
LVEF (%) = (LVEDV - LVESV)/LVEDV × 100, where LV enddiastolic volume (LVEDV) = 7.0 × LVEDd3/(2.4 + LVDd) and LV end-systolic volume (LVESV) = 7.0 × LVDs3/(2.4 + LVDs).
Electrocardiography was performed at the same times, i.e., at Days 0, 28, and 56. RR and QT intervals were measured from the electrocardiogram. Heart rate (HR) and corrected QT interval (QTc) were calculated as follows:
HR (bpm) = 1/(RR interval)
QTc (ms) = (QT interval)/√(RR interval)
Histological assessment
Four weeks after AMSC transplantation, the swine were sacrificed and their hearts obtained. Each heart was fixed with 20% buffered formalin and sliced (from the apex to the base) into 1-cm-thick serial sections in the LV transverse direction. These sections were embedded in paraffin.
The paraffin-embedded heart tissues were sectioned every 3 μm, and all sections were stained with hematoxylin–eosin (H&E) stain for histological examination. To count the blood vessels, immunohistochemical staining of the factor VIII-related antigen was performed. Each sample was observed by light microscopy (×200). Three different fields from the peri-infarct area were randomly selected for each animal, and the number of stained vascular endothelial cells in each field was counted by light microscopy. The result was expressed as the number of blood vessels per square millimeter.
Picrosirius red staining for the assessment of myocardial fibrosis was performed using a Picrosirius Red Stain Kit (Polyscientics, Inc) according to the manufacturer’s protocol. Each sample was observed by light microscopy (×100). Three digital images from different fields of the peri-infarct area were collected, and the percentage of area in red pixels in the whole heart tissue was calculated using the Photoshop software package (Adobe).
To assess cell survival, allogeneic GFP-expressing porcine AMSCs were transplanted into a porcine heart affected by chronic myocardial ischemia. Tissue sections were incubated with primary antibodies against cardiac troponin T (cTnT, MS-295-P1, NeoMarkers) or cardiac troponin I (cTnI, 4T21, HyTest Ltd.), and GFP (ab6662, Abcam or M048-3, MBL). Nuclei were stained with 4′, 6-diamidino-2-phenyindole (DAPI, 40043, Biotium, Inc.). We reconstructed 3D composite images from stacks of z-axis optical scans of each stained sample using standard laser confocal microscopy (LSM 510 META, Carl Zeiss Co., Ltd.) and Zen Image software (Carl Zeiss Co., Ltd.). The images were visualized at different rotation angles to identify colocalization of individual fluorescent staining.
Statistical analysis
All data were expressed as mean ± standard deviation, and all calculations were performed using the SPSS software package (SPSS Japan Inc., Tokyo, Japan). Changes in variables from baseline to 4 weeks after transplantation were analyzed with the paired t-test. The unpaired t-test was performed to compare LVEF, LVDd, LVDs, PWT, HR, QTc, vascular density, and myocardial fibrosis between the 2 groups. Statistical significance was determined as a p value of <0.05.
Results
We investigated the safety and efficacy of allogeneic AMSCs transplantation in a porcine model of chronic myocardial ischemia as a preclinical trial. An experimental design of this study is showed in Figure. 1 (see Materials and Methods for details).
Porcine AMSCs
Because amniotic membrane consists of both fetal membrane and maternal decidua, avascular transparent amniotic membranes were peeled off from maternal placentas and deciduas. The decidua is a part of the uterine endometrium, and the proliferation rate of human maternal endometrial cells is significantly greater than that of human AMSCs. With regard to proliferation, human and swine AMSCs were quite similar (Figure. 2A). In order to examine the homogeneity of the isolated AMSCs, a limiting dilution culture was performed and no significant difference among the colonies to be formed was recognized. The morphology of the cultured AMSCs demonstrated typical mesenchymal characteristics (Figure. 2B). The porcine AMSCs were positive for the mesenchymal cell markers CD29 and CD44 and negative for the hematopoietic cell marker CD45. The porcine AMSCs were positive for swine leukocyte antigen (SLA) class I, and were negative for SLA classes II DR and DQ (Figure. 2C-1). GFP-transgenic porcine AMSCs had the same surface profile as non-transgenic swine AMSCs. Flow cytometric analysis also revealed more than 80% GFP expression in GFP-expressing porcine AMSCs, with an extremely high intensity of GFP (Figure 2C-2). The GFP expression of GFP-expressing porcine AMSCs was also examined under fluorescent microscopy (Figure. 2D & E).
Figure 2.
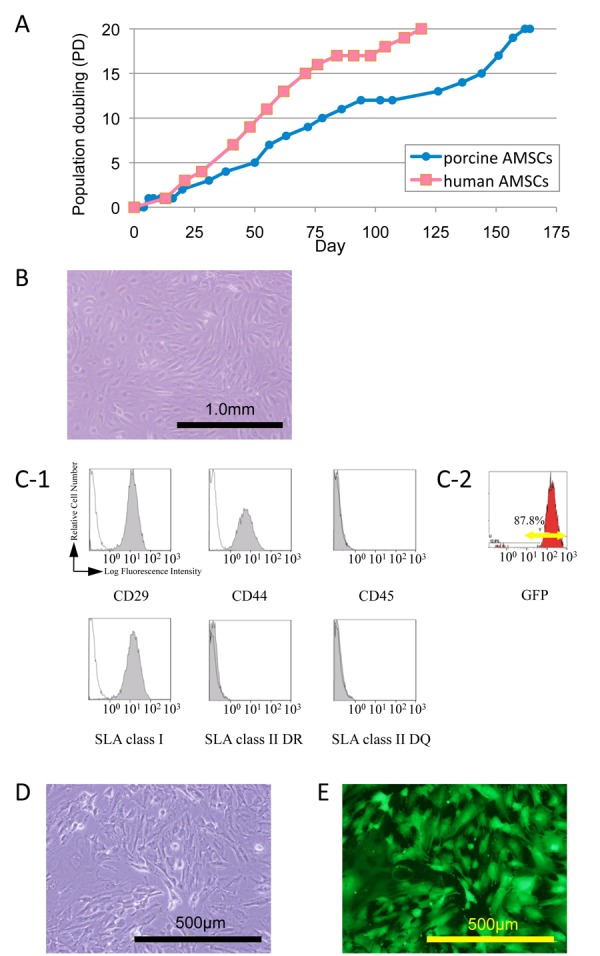
Characterization of porcine amniotic membrane-derived mesenchymal stromal cells (AMSCs). (A) The growth curves of porcine AMSCs used in this experiment compared to human AMSCs from a previous paper (ref. 28). (B) Phase contrast microscopic view of porcine AMSCs. (C-1) Flow cytometric analysis of porcine AMSCs using antibodies for CD29, CD44, CD45, swine leukocyte antigen (SLA) class I, SLA class II DR, and SLA class II DQ. Black lines and shaded areas indicate reactivity of antibodies for isotype controls and that of antibodies for cell surface markers, respectively; (C-2) Representative flow cytometrical GFPexpressing pattern in GFP-expressing porcine AMSCs. (D, E) Microscopic view of porcine GFP-expressing AMSCs.
Intramyocardial AMSC transplantation
A swine MI model was induced by ameroid constrictor (see Materials and methods), because of the most widely used swine model of MI. [30, 31] Fifteen swine were assigned to this experimental group, and six died following the implantation of an ameroid constrictor. One animal died from ventricular fibrillation just after implantation. In the remaining animals, lethal arrhythmia might have occurred before the second surgery because postmortem pathology did not show any large myocardial infarctions or ventricular remodeling. No animals died after AMSC transplantation or normal saline injection.
The average AMSC dose per animal was 1.08 ± 0.11 × 106 cells, which was maintained throughout the experimental procedures. The distribution of epicardial injection sites was from 2 cm left of the left anterior descending coronary artery to the posterolateral region, and the number of injections, which depended on the size and geometry, ranged from 16 to 20. Nine animals that completed this experiment showed no morbidity including wound infection or arrhythmia.
Functional assessment of the infarcted heart
Four weeks after AMSC transplantation, echocardiography showed a significant improvement in LVEF (10.0% ± 5.2%, p = 0.013). In contrast, there was no improvement in LVEF in the control group (-1.2% ± 2.6%, p = 0.435). The magnitude of LVEF improvement after porcine AMSC transplantation was significantly larger than that after normal saline injection (p = 0.006) (Figure. 3A). In the control group, echocardiography showed a significant increase in LVDd for 4 weeks (5.8 ± 1.9mm, p = 0.009). In the transplantation group, there were no remarkable changes in LVDd after porcine AMSC transplantation (-1.5 ± 5.8 mm, p = 0.587). The absolute change in LVDd in the control group was significantly large compared with that in the transplantation group (p = 0.047) (Figure. 3B). The absolute change of LVDs in control group was also significant larger than that in transplantation group (Transplantation vs. control = -3.5 ± 3.6 mm vs. 4.5 ± 1.3 mm, p=0.004) (Fig. 3C). PWT in the control group showed no significant difference after normal saline injection (6.6 ± 2.2 mm vs 7.5 ± 1.6 mm, p = 0.370). On the other hand, it was significantly increased at the final estimation in the transplantation group (5.1 ± 1.0 mm vs 9.8 ± 1.9 mm, p = 0.016) (Figure. 3D). The absolute changes of HR and QTc showed no significant difference between the two groups (HR: 1.5 ± 17.3 bpm vs -9.2 ± 8.9 bpm, p = 0.365; QTc: 4.2 ± 19.0 ms vs 11.3 ± 9.5 m, p = 0.574) (Figures. 3E, F).
Figure 3.

Global functional effects of an infarcted myocardium after transplantation. (A) The left ventricular ejection fraction (LVEF), (B) the left ventricular end-diastolic diameter (LVDd), (C) the left ventricular end-systolic diameter (LVDs), and (D) posterior wall thickness (PWT) at baseline and 4 weeks after transplantation were measured by echocardiography. The absolute change in each measurement is summarized and shown on the right. LVEF was significantly improved in the transplantation group. In control group, the increments of LVDd and LVDs were larger than in transplantation group. PWT was significantly increased after AMSC transplantation. (E, F) The absolute changes of heart rate (HR) and correct QT interval (QTc) did not demonstrate the significant difference in both groups.
Histological assessment
Macroscopic images showed the lateral wall thickness in the transplantation group had recovered compared with the control group (Figure. 4A, Supplementary Figure. 1). Histology sections were examined by an experienced pathologist. Transmural infarctions were consistently observed in all hearts. In order to quantify the vascularity and the area of fibrosis, immunohistochemistry was performed for von Willebrand factor and picro-sirius red staining was performed. There was no difference in vascular density between the transplantation group and the control group (transplantation: control = 105.9±32.2 /mm3: 112.7±22.7 /mm3, p = 0.523) (Fig. 4B). In contrast, picro-sirius red staining demonstrated that percentage of fibrosis was significantly reduced in the transplantation group as compared with the control group (transplantation: control = 3.0±1.5%: 6.1±3.6%, p=0.015) (Figure. 4C).
Figure 4.
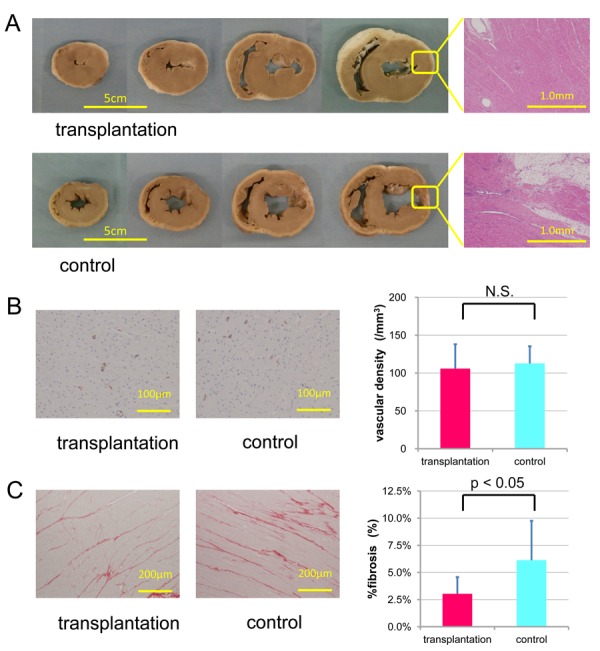
Macroscopic images of impaired myocardium-transplanted porcine AMSCs and their histological evaluation. (A) Macroscopic images in the transplantation (upper) and control (lower) groups. The injected areas were pointed by yellow arrows. (B) Vascular density was measured by immunohistochemical staining of factor VIII-related antigen. (C) Fibrosis was measured by picrosirius red staining. The absolute change in each measurement was summarized and shown on the right. There was no significant difference between the transplantation and control group in vascular density. However, the percentage of fibrosis of the transplantation group was significantly lower than that of the control group.
Porcine AMSCs-injected hearts did not show tumor formation 4 weeks after cell transplantation. Moreover, ectopical differentiation such as bone, cartilage, or storage of excessive extracellular matrices was never found in any of the hearts that received AMSCs at Day 56.
Cell survival assessment
To monitor the injected cells, GFP-expressing AMSCs were transplanted (Supplementary Figure. 2A) and tissue sections of the recipient hearts were made immediately and 4 weeks after cell transplantation. Immediately after transplantation, GFP-positive cells were observed in the tissue sections around scars caused by the syringe needle (Supplementary Figure. 2B). It was difficult to find injection scars 4 weeks after transplantation; however allogeneic GFP-expressing AMSCs were identified in the peri-infarcted region at Day 56 following allogeneic transplantation. AMSCs could survive for 4 weeks without any immunosuppressant. There was no cellular infiltration indicating immunological reactions was observed around the area of grafted cells . GFP-expressing AMSCs clearly expressed cardiac troponin I and T as cardiac markers (Figure. 5), although whether the new cardiac phenotype was obtained through transdifferentiation or cell fusion has not yet been determined.
Figure 5.
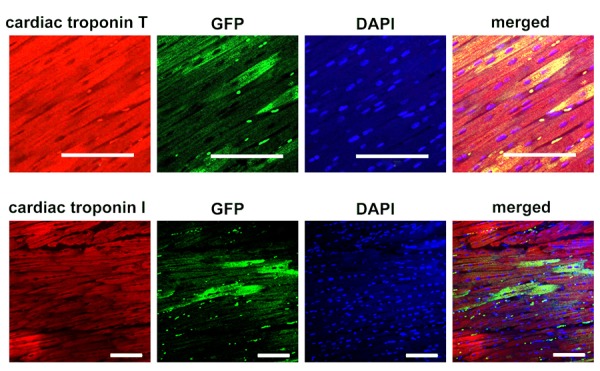
Survival of allogeneic green fluorescent protein (GFP)-expressing amniotic membrane-derived mesenchymal stromal cells (AMSCs). Immunohistochemical staining with cardiac troponin I (cTnI, upper right) and cardiac troponin T (cTnT, lower right). The second row from left indicates the GFP expression, and the third row is staining with 4,6-diamino-2-phyenylindole (DAPI) to show the nucleus. Left row demonstrate the merged view of right row with the second row from left, indicating the donor cells with expressing cardiac structural proteins. Bars indicate 100 μm.
Discussion
Since a multipotential stem cell population is found in the amnionic membrane, we investigated the safety and efficacy of allogeneic AMSC transplantation in a porcine model of chronic myocardial ischemia. Echocardiography revealed a significant improvement of LV function after allogeneic AMSC transplantation compared with that after normal saline injection. Histologically, allogeneic AMSC transplantation significantly decreased fibrosis, and GFP-expressing allogeneic AMSCs with cardiac structural proteins survived in the porcine ischemic heart without the use of any immunosuppressants for at least 4 weeks.
The amniotic membrane comprises the epithelium, compact layer, amniotic mesoderm, and spongy layer [32]. The amniotic epithelial and mesenchymal layers originate from different embryological sites; the former is derived from the inner cell mass and the latter from the extraembryonic mesoderm. We acquired enriched AMSCs using 2 step process similar to that which has been established for human amniotic membrane. [32] Our isolated porcine AMSCs expressed the mesenchymal markers CD29 and CD44, but did not express the hematopoietic marker CD45. These results suggest that the porcine AMSCs used in this study could be a counterpart to the human AMSCs. AMSCs were chosen rather than amnion epithelium cells (AECs) because of their great capability for proliferation. Human and porcine AMSCs can expand prolifically, with 10 or more population doublings within 2 months (total cell number: more than 1 × 109/one term amnion), but AECs can only proliferate for less than 5 population doublings (data not shown). Because stem cell therapy may require repetitive administration in a clinical setting, AMSCs may be superior to AECs.
The efficacy of cardiac regeneration by stem cell transplantation relies on successful functional integration of the graft into the host heart. There is still controversy over whether cardiac differentiation of implanted stem or progenitor cells occurs by way of transdifferentiation [3,33] or stem cell fusion, [34,35] and little information is available regarding the contribution of transdifferentiation and stem cell fusion to the acquisition of a cardiac phenotype in vivo. [36] Tsuji et al. demonstrated that the GFP- and cardiac markerco expressing cells observed possessed human but not rat chromosome following GFP-labeled human AMSCs transplantation into rat hearts. [28] A recent study demonstrated that in vitro acquisition of cardiac futures in human cord blood-derived CD34+ progenitor cells could be the result of fusion events with cardiac myocytes. [37] Cellfusion-mediated reprogramming might be involved in tissue/organ regeneration, where cell-to-cell communication could lead to pluripotent reprogramming of the differentiated state of a cell [38] or lineage reprogramming (direct conversion) to a different differentiated state. We showed that transplanted GFP-positive cells expressed cTnT and cTnI, and showed a clear striation pattern in a porcine ischemic heart model. In this study, cells positive for GFP and cardiac-specific markers could have been formed by either transdifferentiation or cell fusion. The more doublepositive cells survived in damaged hearts, the more functional recovery was recognized. [28] It remains to be seen whether cellular reprogramming might contribute to organ repair, in addition to the effects of paracrine factors [39] or microvesicles (exosomes). [40]
The ability of stem or progenitor cell transplantation to ameliorate organ damage might be explained by angiogenesis and restoration of the extracellular matrices. Angiogenesis may lead to increased perfusion, which results in the salvage of hibernating cardiomyocytes. Despite the fact that bone marrow-derived mononuclear cells and MSCs have been able to exert reparative effects via this phenomenon, neither our small [28] nor large animal studies have demonstrated any significant increase in angiogenesis. These results suggest that AMSC transplantation might also be effective in non-ischemic cardiomyopathy because the mechanism of AMSC grafting dows not seem to be related to perfusion improvement. On the other hand, the extracellular matrices could be restored following AMSC transplantation, which in turn may facilitate donor cell incorporation and donor-to-host cell communication. An increase in interstitial collagen content causes the myocardium to become stiffer, while a reduction in collagen content leads to a dilated left ventricle. In our study, the fibrosis content in extracellular matrices was normalized after cell transplantation, and did not decrease to less than that of the normal heart. The restoration of the balance between collagen production and degradation has been also recognized following implantation of a left ventricular assist device, especially in hearts that could be later weaned off of it. [41] Adequate extracellular matrices should be a crucial hallmark for the repair process in the diseased heart.
MSCs can be isolated from bone marrow, adipose tissue, amniotic fluid, menstrual blood, and the umbilical cord as well as from amniotic membrane, and possess low immunogenicity. However, xenogeneic AMSCs were shown to be unable to survive, even with immunosuppressants [42], and human menstrual blood-derived MSCs were rejected in immunocompetent rats. [43] In contrast, human AMSCs did not induce either allogeneic or xenogeneic lymphocyte proliferation responses, and AMSC transplantation in swine and rats resulted in human microchimerism in various organs and tissues. [44] In line with these results, human AMSC transplantation into immunocompetent rats was shown to be able to ameliorate cardiac function of infarcted heart without the use of immunosuppressants. [28] Therefore, compared with other cells such as myoblasts, bone marrow-derived mononuclear cells and mesenchymal stem cells, the advantages of the cell therapy using AMSCs are; 1: amniotic membrane can be obtained at every delivery (because it is usually considered as medical waste, it is an easily accessible cellular source without ethical problems; 2: AMSCs can be transdifferentiated into cardiomyocytes in vitro and vivo and the cardiomyogenic transdifferentiation efficiency of AMSCs is significantly higher than that of marrow-derived mesenchymal stem cells; 3: xenografted AMSCs transdifferentiated into cardiomyocytes and survived. It is known that amniotic membrane plays an important role in preventing rejection of the fetus from the maternal immune system, [27] and HLA-E, -F, and -G, which are non-classical MHC class I molecules (class Ib) [45], could be involved in fetomaternal tolerance. In particular, a number of immunomodulatory functions have been ascribed to HLA-G. [46] The expression of HLA-G increased substantially in amnion-derived cells when they were stimulated in mixed lymphocyte reaction. [47] In our small animal experiment, HLA-G expression of human AMSCs was strongly enhanced by pretreatment with IL-10 and IFN-gamma [28], and it was also reported that HLA-G expression may promote survival of cardiac allografts through induction of Tregs. Moreover, HLAG is thought to facilitate the recruitment of HLA-E, and the interactions of these molecules with CD94/NKG2 on NK cells contribute to the inhibition of NK cells. [48] We previously demonstrated that stable engraftment of human placental artery-derived endothelial cells in xenogeneic animals could be attributed to HLA-E expression. [49] The non-classical MHC system might allow AMSCs to survive in allogeneic organ for a longer period of time. In the present study, it is unclear to what extent these non-classical MHC class I molecules contributed to the long-term engraftment of allogeneic porcine AMSCs. The porcine functional homologs of HLA-E, -F, and -G have not been established, although SLA-6, -7, and -8 are the genomic homologs of HLA-E, -F, and -G, respectively. [50] Further studies are needed to elucidate the relationship between allogeneic porcine AMSC transplantation and non-classical MHC class I molecules.
Conclusions
Because amniotic membrane can be obtained at each delivery and is usually considered medical waste, it is an easily accessible cellular source whose acquisition involves no ethical problems. In the present study, we observed neither tumor nor teratoma formation in porcine AMSC-transplanted hearts. In addition, neither ectopic differentiation nor extracellular matrix storage was observed throughout the experiment. Taken together, these results indicate that human AMSCs might be an ideal cell source for cardiac cell therapy, and they hold promise as an “off-the-shelf” product.
Supplementary Information


Acknowledgments
We would like to thank Kawarasaki T (Swine and Poultry Research Center, Shizuoka Prefectural Livestock Institute; School of Agriculture, Tokai University) for providing GFP transgenic porcine amniotic membrane. We thank Kawano N and Yamazaki-Inoue M for their help with animal husbandry, Ota M for helping with FACS analysis of AMSCs, and Sajima Y and Yachida M for their help with histology preparation, and Saito K and Suehiro Y for secretarial work. We thank Miyata H for advising statistical analyses.
Abbreviations
- AMSCs =
amniotic membrane-derived mesenchymal stromal cells
- DAPI =
4,6-diamino-2-phyenylindole
- GFP =
green fluorescent protein
- HLA =
human leukocyte antigen
- LCx =
left circumflex
- LV =
left ventricle
- LVDd =
left ventricular end-diastolic diameter
- LVDs =
left ventricular end-systolic diameter
- LVEDV =
left ventricular end-diastolic volume
- LVEF =
left ventricular ejection fraction
- LVESV =
left ventricular end-systolic volume
- MHC =
major histocompatibility complex
- MI =
myocardial infarction
- MSC =
mesenchymal stem cell
- PWT =
posterior wall thickness
- SLA =
swine leukocyte antigen
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR.Multilineage potential of adult human mesenchymal stem cells. Science 1999284: 143-147 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T.Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 19995: 434-438 [DOI] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P.Bone marrow cells regenerate infarcted myocardium. Nature 4102001: 701-705 [DOI] [PubMed] [Google Scholar]
- 4.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kogler G, Wernet P, Muller HW, Kostering M.Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol 200546: 1651-1658 [DOI] [PubMed] [Google Scholar]
- 5.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM.Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002106: 3009-3017 [DOI] [PubMed] [Google Scholar]
- 6.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP.Myoblast transplantation for heart failure. Lancet 2001357: 279-280 [DOI] [PubMed] [Google Scholar]
- 7.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM.Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006355: 1210-1221 [DOI] [PubMed] [Google Scholar]
- 8.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H.Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004364: 141-148 [DOI] [PubMed] [Google Scholar]
- 9.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de WF.Autologous bone marrow-derived stem-cell transfer in patients with STsegment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 2006367: 113-121 [DOI] [PubMed] [Google Scholar]
- 10.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K.Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 2006355: 1199-1209 [DOI] [PubMed] [Google Scholar]
- 11.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW.Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol 200750: 1761-1767 [DOI] [PubMed] [Google Scholar]
- 12.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM.Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 200829: 1807-1818 [DOI] [PubMed] [Google Scholar]
- 13.Gojo S, Umezawa A.Plasticity of mesenchymal stem cells--regenerative medicine for diseased hearts. Hum Cell 200316: 23-30 [DOI] [PubMed] [Google Scholar]
- 14.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S.Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 2004287: H2670-H2676 [DOI] [PubMed] [Google Scholar]
- 15.Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, Hata J, Umezawa A.In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res 2003288: 51-59 [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi C, Yamagishi M, Yamahara K, Hagino I, Mori H, Sawa Y, Yagihara T, Kitamura S, Nagaya N.Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun 2008374: 11-16 [DOI] [PubMed] [Google Scholar]
- 17.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr., Reisman MA, Schaer GL, Sherman W.A randomized, double-blind, placebocontrolled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 200954: 2277-2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW.Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 201059: 1662-1669 [DOI] [PubMed] [Google Scholar]
- 19.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, Monroy R, Uberti J.Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant 200915: 804-811 [DOI] [PubMed] [Google Scholar]
- 20.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A.Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 200545: 1449-1457 [DOI] [PubMed] [Google Scholar]
- 21.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C.Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 200545: 1441-1448 [DOI] [PubMed] [Google Scholar]
- 22.Zhuo Y, Li SH, Chen MS, Wu J, Kinkaid HY, Fazel S, Weisel RD, Li RK.Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg 2010139: 1286-94, 1294 [DOI] [PubMed] [Google Scholar]
- 23.Moore RM, Silver RJ, Moore JJ.Physiological apoptotic agents have different effects upon human amnion epithelial and mesenchymal cells. Placenta 200324: 173-180 [DOI] [PubMed] [Google Scholar]
- 24.Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A, Wengler GS, Parolini O.Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med 20071: 296-305 [DOI] [PubMed] [Google Scholar]
- 25.Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T.Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation 200579: 528-535 [DOI] [PubMed] [Google Scholar]
- 26.Sakuragawa N, Kakinuma K, Kikuchi A, Okano H, Uchida S, Kamo I, Kobayashi M, Yokoyama Y.Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J Neurosci Res 200478: 208-214 [DOI] [PubMed] [Google Scholar]
- 27.Guleria I, Sayegh MH.Maternal acceptance of the fetus: true human tolerance. J Immunol 2007178: 3345-3351 [DOI] [PubMed] [Google Scholar]
- 28.Tsuji H, Miyoshi S, Ikegami Y, Hida N, Asada H, Togashi I, Suzuki J, Satake M, Nakamizo H, Tanaka M, Mori T, Segawa K, Nishiyama N, Inoue J, Makino H, Miyado K, Ogawa S, Yoshimura Y, Umezawa A.Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res 2010106: 1613-1623 [DOI] [PubMed] [Google Scholar]
- 29.Kawarasaki T, Uchiyama K, Hirao A, Azuma S, Otake M, Shibata M, Tsuchiya S, Enosawa S, Takeuchi K, Konno K, Hakamata Y, Yoshino H, Wakai T, Ookawara S, Tanaka H, Kobayashi E, Murakami T.Profile of new green fluorescent protein transgenic Jinhua pigs as an imaging source. J Biomed Opt 200914: 054017. [DOI] [PubMed] [Google Scholar]
- 30.Hughes GC, Post MJ, Simons M, Annex BH.Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol 2003, 94: 1689-701 [DOI] [PubMed] [Google Scholar]
- 31.Caillaud D, Calderon J, Réant P, Lafitte S, Dos Santos P, Couffinhal T, Roques X, Barandon L.Echocardiographic analysis with a two-dimentional strain of chronic myocardial ischemia induced with ameroid constrictor in the pig. Interact Cardiovasc Thorac Surg., 201010: 689-93 [DOI] [PubMed] [Google Scholar]
- 32.Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC.Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 200826: 300-311 [DOI] [PubMed] [Google Scholar]
- 33.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S.Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 2003107: 1024-1032 [DOI] [PubMed] [Google Scholar]
- 34.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC.Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004428: 668-673 [DOI] [PubMed] [Google Scholar]
- 35.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE.Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 200410: 494-501 [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET.Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation 2004110: 3803-3807 [DOI] [PubMed] [Google Scholar]
- 37.Avitabile D, Crespi A, Brioschi C, Parente V, Toietta G, Devanna P, Baruscotti M, Truffa S, Scavone A, Rusconi F, Biondi A, D'Alessandra Y, Vigna E, Difrancesco D, Pesce M, Capogrossi MC, Barbuti A.Human cord blood CD34+ progenitor cells acquire functional cardiac properties through a cell fusion process. Am J Physiol Heart Circ Physiol 2011300: H1875-H1884 [DOI] [PubMed] [Google Scholar]
- 38.Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, Christov C, Baudin X, Auber F, Yiou R, Dubois-Rande JL, Rodriguez AM.Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 201129: 812-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltais S, Tremblay JP, Perrault LP, Ly HQ.The paracrine effect: pivotal mechanism in cell-based cardiac repair. J Cardiovasc Transl Res 20103: 652-662 [DOI] [PubMed] [Google Scholar]
- 40.Ratajczak MZ.The emerging role of microvesicles in cellular therapies for organ/tissue regeneration. Nephrol Dial Transplant 201126: 1453-1456 [DOI] [PubMed] [Google Scholar]
- 41.Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E, Lehmkuhl HB, Knosalla C, Hetzer R.Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. Eur Heart J 201132: 1148-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiavegato A, Bollini S, Pozzobon M, Callegari A, Gasparotto L, Taiani J, Piccoli M, Lenzini E, Gerosa G, Vendramin I, Cozzi E, Angelini A, Iop L, Zanon GF, Atala A, De Coppi P, Sartore S.Human amniotic fluidderived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol 200742: 746-759 [DOI] [PubMed] [Google Scholar]
- 43.Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, Kiyono T, Kyo S, Shimizu T, Okano T, Sakamoto M, Ogawa S, Umezawa A.Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 200826: 1695-1704 [DOI] [PubMed] [Google Scholar]
- 44.Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, Albertini A, Zorzi F, Cavagnini A, Candotti F, Wengler GS, Parolini O.Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation 200478: 1439-1448 [DOI] [PubMed] [Google Scholar]
- 45.Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Fernandez-Vina M, Geraghty DE, Holdsworth R, Hurley CK, Lau M, Lee KW, Mach B, Maiers M, Mayr WR, Muller CR, Parham P, Petersdorf EW, Sasazuki T, Strominger JL, Svejgaard A, Terasaki PI, Tiercy JM, Trowsdale J.Nomenclature for factors of the HLA system, 2010. Tissue Antigens 201075: 291-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunt JS, Langat DL.HLA-G: a human pregnancy-related immunomodulator. Curr Opin Pharmacol 20099: 462-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A.Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol 200869: 321-328 [DOI] [PubMed] [Google Scholar]
- 48.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE.Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol 2003171: 1376-1384 [DOI] [PubMed] [Google Scholar]
- 49.Cui CH, Miyoshi S, Tsuji H, Makino H, Kanzaki S, Kami D, Terai M, Suzuki H, Umezawa A.Dystrophin conferral using human endothelium expressing HLA-E in the non-immunosuppressive murine model of Duchenne muscular dystrophy. Hum Mol Genet 201120: 235-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho CS, Lunney JK, Ando A, Rogel-Gaillard C, Lee JH, Schook LB, Smith DM.Nomenclature for factors of the SLA system, update 2008. Tissue Antigens 200973: 307-315 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials




