Abstract
Recent studies have reported direct reprogramming of human fibroblasts to mature neurons by the introduction of defined neural genes. This technology has potential use in the areas of neurological disease modeling and drug development. However, use of induced neurons for large-scale drug screening and cell-based replacement strategies is limited due to their inability to expand once reprogrammed. We propose it would be more desirable to induce expandable neural precursor cells directly from human fibroblasts. To date several pluripotent and neural transcription factors have been shown to be capable of converting mouse fibroblasts to neural stem/precursor-like cells when delivered by viral vectors. Here we extend these findings and demonstrate that transient ectopic insertion of the transcription factors SOX2 and PAX6 to adult human fibroblasts through use of non-viral plasmid transfection or protein transduction allows the generation of induced neural precursor (iNP) colonies expressing a range of neural stem and pro-neural genes. Upon differentiation, iNP cells give rise to neurons exhibiting typical neuronal morphologies and expressing multiple neuronal markers including tyrosine hydroxylase and GAD65/67. Importantly, iNP-derived neurons demonstrate electrophysiological properties of functionally mature neurons with the capacity to generate action potentials. In addition, iNP cells are capable of differentiating into glial fibrillary acidic protein (GFAP)-expressing astrocytes. This study represents a novel virusfree approach for direct reprogramming of human fibroblasts to a neural precursor fate.
Keywords: Adult human fibroblast cells, induced neural precursor cells, PAX6, somatic cell reprogramming, SOX2
Introduction
Cellular differentiation and lineage commitment have traditionally been considered as robust and irreversible processes. Recent work however has demonstrated that mature somatic cells can be reprogrammed to a pluripotent embryonic stem cell-like state by forced expression of the stem cell associated genes OCT3/4, SOX2, KLF4 and/or CMYC.[1-3] Using this technology, a range of cell types have been reprogrammed including human fibroblast cells, [2,4,5] keratinocytes, [6] mesenchymal cells, [7] peripheral blood cells ,[8] melanocytes [9] and adipose stem cells. [10] In addition, it has been demonstrated that forced expression of lineage-specific transcription factors can convert fibroblasts directly to blood progenitor cells, [11] mature cardiomyocytes, [12] hepatocyte-like cells [13], neural stem/precursor-like cells [14-18] and mature neurons [19-24] without first reverting to an intermediate pluripotent state. As such, somatic cell reprogramming provides a unique platform by which to dissect the molecular mechanisms that underlie epigenetic reprogramming and lineage specification as well as the exciting opportunity to personalize human cells for both research and clinical application.
Several studies have reported direct reprogramming of human fibroblast cells to mature neurons by the introduction of defined neural genes.[19-24] Further, neuronal cell subtypes such as functional spinal motor neurons [24] or dopaminergic neurons [20,22] have been generated by over-expression of specific transcription factor combinations. Cell culture systems utilizing induced mature neurons have potential in the areas of neurological disease modeling and drug development. However, a clear limitation of postmitotic induced neurons is their inability to expand once reprogrammed. To date expandable neural stem/precursor-like cells have only been generated from mouse fibroblasts by viral transduction of pluripotent and neural transcription factors.[14-18] We therefore investigated whether adult human fibroblasts could be directly reprogrammed to become neural precursor cells through ectopic expression of specific neural transcription factors using non-viral technologies.
Different combinations of up to seven pluripotent and neural transcription factors have been identified to be capable of converting mouse fibroblasts into neural stem/precursor-like cells.[14-18] However, current knowledge regarding the key factors required to initiate neural stem/precursor fate determination in mouse and particularly in human cells is very limited. We hypothesized that genes which are involved in directing the differentiation of embryonic stem cells to the neuroectoderm lineage may also serve to reprogram human fibroblasts towards a neural stem/precursor-like state. Based on previous work, [25,26] we identified the neural genes SOX2 and PAX6 as playing a key role in the early stages of neurogenesis. SOX2 is a key factor in reprogramming cells to a pluripotent state [2,5] and is also associated with multipotent and unipotent stem cells.[27-29] PAX6 is essential for neural stem cell proliferation, multipotency and neurogenesis.[30] SOX2 and PAX6 are also expressed when embryonic stem cells undergo differentiation to a neural lineage.[25,27-32] Further, we have previously observed that neural induction of human embryonic stem cells results in mutually exclusive expression of PAX6 and the pluripotency factor OCT3/4, [25] indicating the involvement of PAX6 in neural lineage determination.
The current study provides proof-of-concept that transient expression of SOX2 and PAX6 in adult human fibroblasts generates induced neural precursor-like (iNP) colonies expressing a range of neural stem and precursor genes, and immature neural markers. Upon differentiation, iNP colonies give rise either to mature neurons capable of generating action potentials upon current injection or to astrocytes as indicated by GFAP expression. This was achieved using either non-viral plasmid transfection or recombinant protein transduction technology alleviating concerns of insertional mutation associated with the use of genome-integrating viral vectors. Furthermore, we have optimized cell culture conditions allowing us to generate iNP colonies in the absence of an animal or human feeder cell layer, an essential requirement for the transfer of reprogrammed cell lines to clinical use. This technology provides a novel and ethically viable option for obtaining neural precursor cells for neurological research and clinical application.
Materials & Methods
Cell culture
Human dermal fibroblast cells (HDF) isolated from the abdomen of two adult donors were obtained from Cell Applications Inc. and maintained in human HDF Growth Media (Cell Applications, Inc.). Plasmid DNA transfections and protein transduction were performed at passage 8 or earlier. Media was changed to proliferation medium comprising Neurobasal-A (Gibco), 1% D-glucose (Sigma-Aldrich), 1% Penicillin/Streptomycin/Glutamine (Gibco), 2% B27 supplement (Gibco), 0.2% EGF (PeproTech), 0.08% FGF-2 (PeproTech), 0.2% Heparin (Sigma-Aldrich) and VPA (Sigma-Aldrich) to a concentration of 1μM three days post-transfection or post-transduction. Media was changed three-times weekly, and cells were replated regularly during iNP colony formation.
Transcription factor delivery
HDF cells at 1 x 105 cells per well were seeded in polystyrene 6-well plates (Nunc - Thermo Fisher Scientific) 24 hrs prior to transfection or transduction. Media was changed to Optimem (Invitrogen) 2 hrs prior to the transfection procedure. Nonviral plasmid transfections were carried out using Lipofectamin LTX, Plus reagent (Invitrogen) and plasmid constructs encoding SOX2 , PAX6 or eGFP driven by a CMV promoter at a ratio 3:1:1 (v/v/w) and a total plasmid DNA amount of 2μg. pCMV-SPORT6 comprising PAX6 cDNA was purchased from Invitrogen. SOX2 cDNA was purchased from Addgene and cloned into pEGFP-N1 (Clontech) after removal of eGFP expression cassette from pEGFP-N1. Protein co-transductions were carried out in four transduction cycles using a mix of SOX2-TAT protein (PeproTech) and PAX6 protein (Abnova) or PE control protein (OzBioscience) with ProDeliverIn (OzBioscience) in a ratio 1:1 and a total protein amount of 5μg per transduction cycle.
Quantitative RT- PCR
Total RNA was isolated from 2.5 – 9.0 x 103 colonies of four independent iNP cell lines and two independent control cell lines by RNAeasy Mini Kit (Invitrogen), respectively. cDNA was synthesised from total RNA using Superscript reverse transcriptase (Invitrogen). Three independent duplex qPCR reactions were performed in triplicates for each independent cell line using the TaqMan system (Applied Biosystems) with ribosomal 18S rRNA as the internal standard and an equivalent of 4ng mRNA per reaction.
Immunocytochemistry
Immunocytochemical staining was carried out by standard procedures using the following human-specific primary antibodies: CD133 (Abcam), SOX2 (R&D Systems), PAX6 (Covance), NGN2 (R&D Systems), MASH1 (Chemicon), TUJ-1 (Covance), NSE (DAKO), MAP2 (Chemicon), GAD65/67 (Chemicon), TH (Chemicon), and GFAP (DAKO).
Differentiation of iNP cells.
Induced neural precursor colonies were generated by SOX2/PAX6 plasmid transfection and cultured for 6 weeks in neural precursor proliferation media medium comprising of NBA media, 2% B27, 1% D-glucose, 1% Penicillin/Streptomycin/Glutamine, 0.2% EGF, 0.08% FGF-2, 0.2% Heparin, 1μM VPA and 25ng/ml Midkine (Peprotech). For neuronal differentiation, the cells were mechanically dissociated and transferred onto poly-ornithine/laminin-coated (Sigma-Aldrich/ Invitrogen) surface following full colony formation and differentiated using a two-step protocol as previously described.[33] Briefly, cells were cultured in NBA media supplemented with 250ng/ml SHH (R&D systems), 100ng/ml DKK1 (PeproTech), 20ng/ml BDNF (PeproTech) and 10μM Y27632 (Calbiochem). After 10 days, culture conditions were changed to NBA media containing 0.5mM dibutryl-cyclic AMP (Sigma-Aldrich), 0.5μM VPA, 20ng/ml BDNF and 10μM Y27632.
For glial differentiation iNP colonies were mechanically dissociated following full colony formation and plated onto on fibronectin-coated surface. Astroglial differentiation was carried out in Glasgow minimum media (Sigma-Aldrich), supplemented with 10% FBS and 1% Penicillin/Streptomycin/Glutamine for 14 days. To promote oligodendroglial differentiation, cells were cultured in 3 stages: NBA media supplemented with 2% B27, 1% Dglucose, 1% Penicillin / Streptomycin / Glutamine, 0.08% FGF-2 and 0.1% retinoic acid (Sigma-Aldrich) for 2 days, followed by DMEM/F12 media containing 0.5% N2 supplement (Invitrogen), 10ng/mL FGF-2, 10ng/mL PDGF, 0.5mM dibutryl-cyclic AMP for 4 days and DMEM/F12, 30ng/mL 3,3',5-Triiodothyronine (Sigma-Aldrich), and 200μM Ascorbic acid (Sigma-Aldrich) for an additional 4 days.
Electrophysiology
Whole cell patch clamping was performed as previously described [34] on iNP–derived neurons after 13, 21 and 30 days of differentiation. Briefly, cells were bathed in artificial cerebrospinal fluid (in mM: 120 NaCl, 3 KCl, 2 CaCl2, 1.25 NaH2PO4, 2 MgSO4, 20 glucose, 26 NaHCO3). Internal solution consisted of (in mM: 120 K gluconate, 40 HEPES, 5 MgCl2, 2 NaATP, 0.3 NaGTP). Cells were visualised on an Olympus BX51W1. Electrode resistance was 5 MΩ. Action potentials were induced in current clamp mode by a 10 ms current injection using a Multiclamp 700B Commander (Molecular Devices). Series resistance was constantly monitored and results were not included if significant variation (>20%) occurred during an experiment. Data acquisition and analysis was performed using pClamp9 software.
Results
SOX2 and PAX6 convert human fibroblasts to neural precursor-like cells.
SOX2 and PAX6 are key transcription factors in the development and maintenance of neural precursor cell identity. We therefore investigated the potential of ectopic SOX2 and PAX6 expression to directly convert adult human fibroblasts to neural precursor-like cells. A homogenous population of adult human dermal fibroblasts previously used to induce pluripotency by Yamanaka and colleagues, and shown to be free of cell cross-contamination was used in this study.[2,35] Protein transduction and non-viral plasmid transfection systems were utilized to provide transient ectopic expression of SOX2 and PAX6 in adult human fibroblasts (Figure 1A). Optimization of transfection / transduction efficiencies and control experiments were performed using either an eGFP plasmid or red-fluorescent phycoerythrin (PE) protein, respectively (Figures 1B, 1C).
Figure 1.
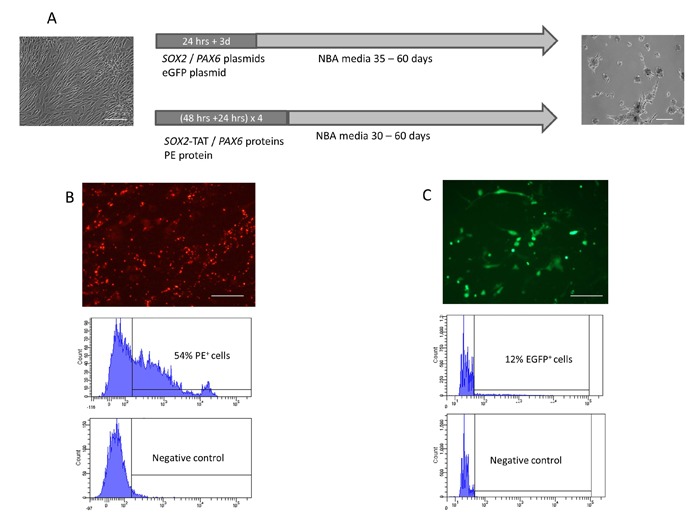
Figure 1: Timeline of protein / plasmid DNA delivery and generation of iNP cell colonies. (A) Transcription factors were introduced into fibroblast cells by either plasmid DNA transfection or protein transduction. Plasmid DNA transfections were carried out in human dermal fibroblast growth media using Lipofectamin LTX with a lipoplex incubation time of 24 hrs. Cells were subsequently cultured in human dermal fibroblast growth media for another 3 days before culture conditions were changed to NBA media to allow colony formation within 35-60 days. Protein transduction was performed in four cycles over a period of 12 days using ProDeliverin. Transductions were performed for 48 hrs in human dermal fibroblast growth media. Cells were cultured in human dermal fibroblast growth media for additional 24 hrs after transduction procedure. Transductions were carried out in four cycles of 72hrs each. (B) Phycoerythrin protein was utilised to optimize and assessed ProDeliverIn mediated protein transduction into human dermal fibroblast cells. FACS analysis revealed a protein transduction efficiency of 54%. (C) Nonviral plasmid transfection was optimized to an efficiency of 12% using pCMV-EGFP-N1 plasmid and Lipofectamine LTX. Scale bars: 100µm
Neural differentiation protocols for human embryonic stem (hES) cells suggested the use of defined neural culture conditions in the absence of a mouse embryonic fibroblast (MEF) feeder layer to drive differentiation towards a neuroectodermal fate.[36,37] Thus, fibroblasts were allowed to recover in human fibroblast growth media for 3 days after SOX2/PAX6 delivery before being transferred to specific neural precursor cell culture conditions consisting of Neurobasal A media (NBA) with B27 supplement, FGF2, EGF and valproic acid (VPA). For both SOX2/PAX6 plasmid transfection and protein transduction, we observed significant changes in fibroblast density and morphology between days 5 to 10 following transfer to neural precursor cell culture conditions. The changes occurred in a temporal order with decreasing cell density, altered morphology to a more compact shape and grouping of cells (Figure 2A). Strikingly, we observed the formation of domed and compact neurosphere-like cell colonies 4 weeks after protein delivery and 8 to 11 weeks after ectopic gene expression of SOX2 and PAX6. Colonies induced by plasmid transfection and protein transduction both appeared with a regular, round morphology with a diameter between 50 - 500μm (Figure 2B). Interestingly, fibroblasts transfected with eGFP control plasmid or transduced with PE control protein and cultured in neural culture conditions for up to 11 weeks occasionally developed cell colony structures (Figure 2C). However, colony formation in control cultures occurred significantly later, with lower efficiency and a less uniform morphology than SOX2/PAX6-induced colonies. In contrast, fibroblasts cultured in human fibroblast growth media only grew in a monolayer and showed no colony formation at any time point (Figure 2D).
Figure 2.
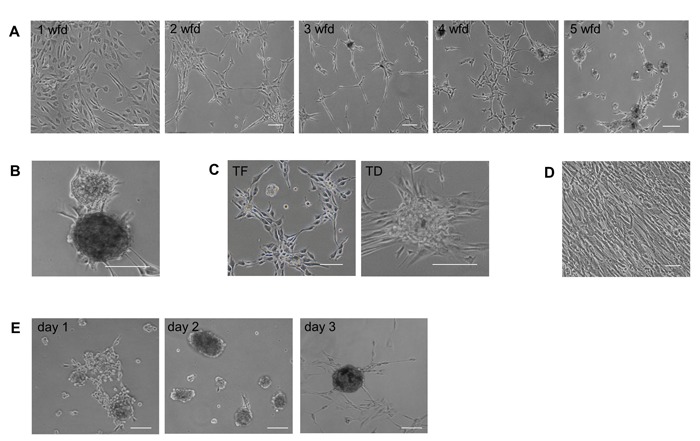
Figure 2: Generation of induced neural precursor colonies from human fibroblasts. (A) Temporal change of fibroblast morphology from 1 - 5 weeks following SOX2/PAX6 delivery (wfd). (B) Fully developed iNP colonies 6 weeks after SOX2/PAX6 delivery. (C) Fibroblast morphology 11 weeks after either eGFP plasmid transfection (TF) or PE protein transduction (TD). (D) Untreated fibroblasts maintained their morphology when cultured in human dermal fibroblast growth media. (E) Secondary iNP colonies reformed within 3 days following mechanical dissociation. Scale bars: 100 µm.
To investigate the proliferative capability of induced cell colonies, primary colonies were mechanically dissociated and replated as single cells. Within 2-3 days, individual cells generated secondary colonies with increased cell diameter (Figure 2E). Repeated dissociation and replating gave rise to tertiary and quaternary colonies. Formation and growth of uniform colonies could be increased and accelerated by regular replating of cells to an efficiency of 0.05% of the number of fibroblasts initially transfected/transduced with SOX2/PAX6. Regular cell replating also decreased the time required for full colony formation to 5 - 8 weeks following plasmid transfection.
These results demonstrate that transient ectopic delivery of SOX2 and PAX6 can direct the generation of uniform induced colonies resembling neural precursor cell neurosphere colony formation.[38,39] Furthermore, colony formation was achieved in the absence of a MEF feeder cell layer.
Induced colonies show neural precursor-like characteristics.
To assess if cell colonies induced by transient ectopic SOX2 and PAX6 expression exhibited features of neural precursor cells, we investigated the transcriptional signature and phenotypic characteristics of SOX2/PAX6 induced colonies compared to eGFP or PE controls. Total RNA from three fully developed independent colony lines was isolated at 65, 75 or 85 days respectively following plasmid transfection, and an additional colony line was isolated 27 days following protein transduction. A range of neural stem cell and precursor genes and positional markers within the neuroectodermal tube were analyzed and compared by quantitative PCR (Figure 3A). We observed that colonies transduced with SOX2 and PAX6 protein exhibited SOX2 and PAX6 mRNA expression in contrast to the PE control (Figure 3A). Due to the transient nature of protein transduction in contrast to delivery of genetic material, this observation strongly indicates the activation of endogenous SOX2 and PAX6 mRNA expression by ectopic protein transduction. Similar to protein transduction, SOX2 and PAX6 mRNA expression levels were found in independent SOX2/PAX6 induced colony lines formed at different time points after transient plasmid DNA transfection with the highest relative expression observed in colony lines isolated 65 days after transfection and gradually decreasing in colony lines isolated 75 and 85 days post transfection (Figure 3A). Quantitative PCR analysis further demonstrated elevated mRNA expression levels of the neural markers neurogenin2 (NGN2), SOX1, HOXB9, NKX6.1 and SIX3. eGFP- and PE-treated controls showed either no mRNA expression or relatively lower levels of these markers (Figure 3A). In contrast, HES1 and IRX3 mRNA levels were reduced in iNP colonies generated by SOX2/PAX6 protein transduction compared to PE control cells (Figure 3A). Transcriptional profile determination of three independent colony lines generated by plasmid transfection revealed a distinctive pattern of neural gene expression dependent on the duration following delivery of SOX2/PAX6 (Figure 3A). Colony lines analyzed 65 days following SOX2/PAX6 delivery exhibited elevated mRNA expression levels of SOX2, PAX6, SOX1, HOXB9, NKX6.1 and reduced HES1 and IRX3 expression relative to eGFP controls. Conversely, colony lines analyzed at 85 days showed a shift towards higher HES1, IRX3, SIX3 and lower PAX6, SOX2, NGN2 and SOX1 expression levels compared to the colony lines analysed 65 days post SOX2/PAX6 treatment. In accordance, colony lines examined 75 days following SOX2/PAX6 treatment displayed intermediate transcription factor expression levels. This suggests a progressive transition of neural gene expression towards a more mature phenotype over time following the initial induction of SOX2 and PAX6 expression. The gene expression pattern of the iNP colony lines was also compared to noggin-primed hES-derived neural precursor (hNP) cells (Figure 3A). Quantitative PCR analysis demonstrated higher mRNA expression levels of SOX2, PAX6, SOX1 and NGN2 in hNP cells compared to iNP cells. This may reflect either the presence of partially reprogrammed iNP cells or a population of non-reprogrammed fibroblasts present in the culture.
Figure 3.
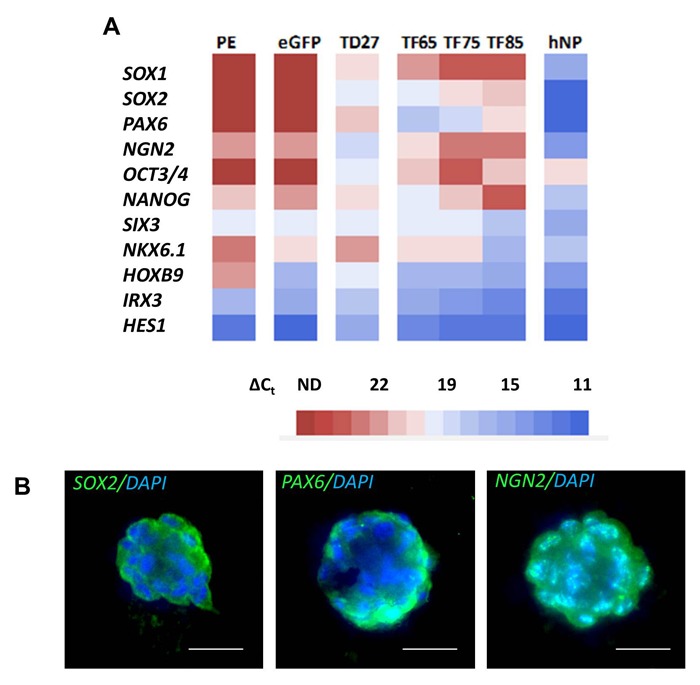
Figure 3: Characterization of induced neural precursor cells. (A) Gene expression profiles of independent iNP colony lines generated by protein transduction (TD) or plasmid transfection (TF) at different time points post transcription factor delivery compared to eGFP transfection (eGFP), PE transduction (PE) and hESC-derived neural precursor cell (hNP) controls. Heatmap represents the average cycle threshold values normalized to internal ribosomal 18S rRNA control (∆Ct) obtained from three independent qPCR reactions, respectively. (B) Expression of SOX2, PAX6 and NGN2 in iNP colonies. Scale bars: 25 µm.
It has been shown that the putative pluripotent marker OCT3/4 is expressed in neural stem cells and that expression of NANOG is partially regulated by a SOX2-OCT3/4 complex.[40-42] Thus, we assessed if expression of SOX2 in iNP colonies is associated with the expression of OCT3/4 and/or NANOG. OCT3/4 mRNA levels were found to be up-regulated in iNP colony lines isolated 65 days after plasmid transfection and in colony lines generated by protein transduction when compared to controls. However, analysis of iNP colony lines isolated 75 days following SOX2/PAX6 gene delivery revealed no increase in OCT3/4 expression (Figure 3A). Similar results were found for NANOG, with an increase in NANOG mRNA expression only observed in iNP colony lines isolated at 65 or 75 days post transfection and in protein-treated colony lines when compared to controls (Figure 3A).
To further assess the expression of neural precursor markers, immunocytochemical analysis was conducted. Positive staining of iNP colonies for SOX2, PAX6 and NGN2 confirmed qPCR results at the protein level (Figure 3B). However, irregular expression patterns were observed in a small number of colonies which may also suggest partial reprogramming. Combined, these results demonstrate that transient expression of SOX2 and PAX6 in human fibroblasts induce cell colonies that display the transcriptional and phenotypic signature of neural precursor cells.
Induced neural precursor colonies have the capacity to differentiate into mature neurons and astrocytes.
To investigate the capacity of iNP colonies to differentiate into mature neurons and glial cells, we tested several differentiation conditions. We have previously demonstrated efficient differentiation of neural precursor cells derived from the subventricular zone of the adult rat brain using standard neural differentiation media.[43,44] As such, induced neural precursor colonies generated by plasmid transfection were cultured in standard neural differentiation media for two weeks. Immunocytochemical analysis demonstrated coexpression of the pro-neural markers MASH1 and NGN2 (Figure 4A) and enduring expression of SOX2 and PAX6 (Figure 4B) following two weeks of differentiation. However, iNP colonies did not differentiate into either neurons or glia as indicated by a lack of staining for the markers MAP2, GFAP and RIP, and remained in their colony assemblage.
Figure 4.
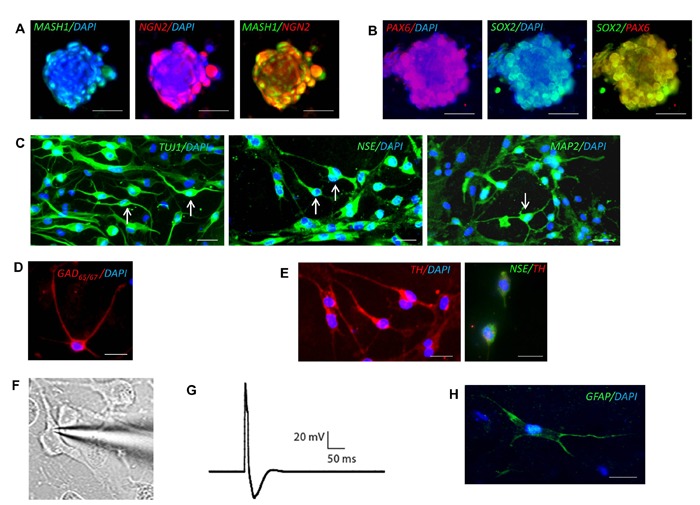
Figure 4: Neuronal and astroglial differentiation of induced neural precursor cells. Co-expression of (A) MASH1 and NGN2 and (B) SOX2 and PAX6 in differentiating iNP colonies. (C) iNP-derived neurons express the neuronal markers TUJ1, NSE, and MAP2. Arrows indicate cells exhibiting a typical neuronal morphology. (D) iNP-derived neurons express GAD65/67 or (E) tyrosine hydroxylase (TH) with NSE co-expression. (F) Image of iNP-derived neuron undergoing patch clamp recording after 30 days of differentiation. (G) Representative trace of action potential generated in response to current injections from iNP-derived neuron after 30 days of differentiation. (H) Astrocytes derived from iNP cells express the astroglial marker GFAP. Scale bars: 25 µm.
Several studies suggest the use of sonic hedgehog (SHH) to efficiently regulate neuronal differentiation of hES-derived neurospheres.[33,45] Thus, we examined whether a modified two-staged differentiation protocol which has previously been demonstrated to generate mature neurons from iPSCs[33] could drive efficient neuronal differentiation of iNP cells. Induced neural precursor colonies were mechanically dissociated and cultured in neural differentiation media supplemented with SHH, DKK1, BDNF and Y27632 for 10 days. The cells were subsequently exposed to dibutryl-cyclic AMP, VPA, BDNF and Y27632. After 13 days of differentiation a proportion of iNP cells developed a neuronlike morphology with tangential and radial processes. Continued differentiation up to 30 days resulted in an increase in the proportion of iNP cells exhibiting a typical neuronal morphology and the generation of a more mature phenotype with the appearance of multiple arborizing dendrites and a single axon from the cell soma. Further confirmation of neuronal differentiation was demonstrated by robust expression of the neuronal markers TUJ-1, NSE and MAP2 (Figure 4C). A population of iNP-derived neurons also expressed the GABAergic marker GAD65/67 (Figure 4D) or the catecholaminergic marker tyrosine hydroxylase (TH) (Figure 4E). To determine whether iNP-derived neurons exhibit excitable membrane properties similar to mature neurons, whole cell patch clamping was performed. Initial recordings performed after 13 or 21 days of differentiation revealed the presence of functionally immature cells. The average resting membrane potentials were depolarized (-39.8 ± 4.3 mV, n = 6 cells and -38.5 ± 3.3 mV, n = 11 cells respectively) and no action potentials could be evoked by current injection. However, after 30 days differentiation functionally mature neurons were able to fire single action potentials upon current injection (Figures 4F, 4G). Moreover, the average resting membrane potential had shifted to significantly more hyperpolarised potentials (-50.8 ± 2.9 mV, n = 15 cells) compared to recordings performed at days 13 and 21 (p < 0.05). This was accompanied by a significant increase in membrane capacitance (from 164.5 ± 48.0 pF to 869.3 ± 334.2 pF; p < 0.05) consistent with significant increases in the amount of neuronal membrane as the cells develop a more mature neuronal morphology.
To determine the glial differentiation potential of iNP colonies, we mechanically dissociated iNP cells and cultured them in astroglial or oligodendroglial differentiation media, respectively. We observed a small proportion (< 1%) of cells expressing the astrocytic marker GFAP and exhibiting an astrocyte-like morphology after 14 days of differentiation (Figure 4H). However, no cells were found expressing the oligodendrocyte markers O4 or RIP when cultured under oligodendroglial conditions (data not shown).
Discussion
The current study demonstrates a method to directly convert adult human fibroblasts into neural precursor cells. This was achieved by exogenous application of the transcription factors SOX2 and PAX6. In order to avoid genome interference, non-viral plasmid transfection or protein transduction techniques were chosen for ectopic expression of SOX2 and PAX6. The time period required for fibroblasts to form iNP colonies was comparable with previous studies using protein transduction and plasmid transfection to generate iPSCs, indicating a significant impact of methodology on the reprogramming process.[46,47] In contrast, the efficiency of iNP colony generation of 0.05% of the initial transfected/transduced fibroblast cells was considerably higher than the induction of iPSCs using non-viral methods.[46,47]
Others have recently demonstrated the direct generation of neural stem and precursor cells from either embryonic [14-17] or adult [18] mouse fibroblast cells. This was achieved by viral transduction of either the four pluripotent transcription factors OCT3/4, SOX2, KLF4, and C-MYC in a transient manner [15], or viral transduction of up to eleven neural stem cellassociated factors.[16-18] In two other studies the protooncogene BMI1 was identified as a key factor in reprogramming mouse fibroblasts into either induced neural stem or precursor cells.[17,18] Interestingly, Han and colleagues [14] observed morphological transformation of mouse embryonic fibroblasts 2-3 weeks after viral delivery of SOX2 and PAX6. However, the cells were not analyzed or cultured to a stage later than 3 weeks post transduction. Further, a recent study has demonstrated the reprogramming of mouse embryonic fibroblasts and human fetal foreskin fibroblasts into multipotent neural stem-like cells following retroviral transduction of SOX2 when cultured on a mitomycin C-treated MEF feeder layer.[48] This is the first study to demonstrate the generation of neural stem-like cells from human fibroblasts and supports the findings presented in the current study. Using both SOX2 and PAX6, we now extend these findings to the generation of iNP cells from adult human fibroblasts by non-viral delivery without the requirement of a MEF feeder layer.
In the current study, iNP colonies exhibited elevated expression of the neural stem and pro-neural markers SOX1, SOX2, PAX6, NGN2, HOXB9 and NKX6.1, with downregulation of HES1 and IRX3 mRNA after SOX2/PAX6 protein delivery or 65 days following plasmid transfection. Due to the transient nature of both the plasmid transfection and protein transduction procedures utilized in this study, we were able to determine the effect of endogenous SOX2 and PAX6 mRNA expression in the resultant iNP cells. Interestingly, temporal analysis of mRNA expression revealed an inverse shift of expression with reduced relative mRNA levels of PAX6, SOX2, NGN2 and SOX1 and concurrent elevation of HES1, IRX3, SIX3 expression in independent colonies analyzed at 75 and 85 days compared to 65 days following SOX2/PAX6 delivery. PAX6 has previously been demonstrated to regulate NGN2 expression in a concentration-dependent manner [49], reflected by the temporal correlation of PAX6 and NGN2 expression observed in iNP colonies. Further, down-regulation of the transcriptional repressor HES1, with opposing functions to PAX6, is in accordance with PAX6 up-regulation and PAX6/HES1 regulation of neurogenesis. [30] Elevated mRNA expression of the putative pluripotent markers OCT3/4 and NANOG was also detected with gradually decreasing levels in cells from 65 to 75 days following SOX2/PAX6 delivery. OCT3/4 and NANOG expression in iNP cells was in agreement with studies demonstrating that SOX2 and OCT-3/4 work cooperatively to regulate their own transcription by a positive feedback loop, and that OCT3/4 and SOX2 in turn activate NANOG expression.[40,50] Interestingly, 85 days post SOX2/PAX6 transfection OCT3/4 levels were found to be increased whereas NANOG and SOX2 expression levels were decreased compared to 75 days post transfection.
To prove the capacity for iNP cells generated by SOX2/PAX6 delivery to form mature neurons, we tested several differentiation conditions and assessed the expression of mature neuronal markers as well as the electrophysiological properties of differentiated iNP cells. Mature neurons were able to be derived from iNP colonies by a differentiation protocol which included SHH and BDNF. Differentiated cells showed a distinctive neuronal morphology including multiple arborizing dendrites and expression of the neuronal markers TUJ1, NSE and MAP2 with a population of iNP-derived neurons expressing TH or GAD65/67. Most importantly, iNPderived neurons demonstrated the ability to fire action potentials in response to current injection. Measurement of the electrical properties of iNP-derived neurons at different time points during differentiation confirmed a gradual maturation process over a period of 30 days. In addition, iNP cells were able to give rise to a small population of astrocytes as demonstrated by staining for the astrocytic marker GFAP. However, we were not able to detect oligodendroglial differentiation. Lujan and colleagues [16] also observed a lack of oligodendrocyte differentiation from neural precursor cells which were generated from mouse embryonic fibroblasts by viral delivery of the two transcription factors SOX2 and FOXG. Oligodendroglial differentiation was only achieved by addition of BRN2. It would be interesting to further assess whether a combination of BRN2, PAX6 and Sox2 can also successfully convert human fibroblasts to iNP cells capable of oligodendrocyte differentiation.
Conclusion
The current study provides proof-of-concept that transient expression of the neural transcription factors SOX2 and PAX6 directly converts adult human fibroblasts into neural precursor-like cells. The use of either non-viral plasmid transfection or recombinant protein transduction alleviates concerns of insertional mutation associated with the use of genome-integrating viral vectors. Further studies will be necessary to thoroughly optimize the efficiency for human iNP cell generation and neural differentiation. Such studies will facilitate the application of this method for future use for in vitro disease modeling, drug screening and as a ready source of human autologous neural precursor cells for cell replacement therapy.
References
- 1.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008; 26: 101-106 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131: 861-872 [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126: 663-676 [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007; 448: 318-324 [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318: 1917-1920 [DOI] [PubMed] [Google Scholar]
- 6.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boue S, Izpisua Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008; 26: 1276-1284 [DOI] [PubMed] [Google Scholar]
- 7.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008; 134: 877-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, Kim K, Miller JD, Ng K, Daley GQ. Generation of induced pluripotent stem cells from human blood. Blood. 2009; 113: 5476-5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utikal J, Maherali N, Kulalert W, Hochedlinger K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci. 2009; 122: 3502-3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, Wu JC. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009; 106: 15720-15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010; 468: 521-526 [DOI] [PubMed] [Google Scholar]
- 12.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011; 13: 215-222 [DOI] [PubMed] [Google Scholar]
- 13.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011; 475: 390-393 [DOI] [PubMed] [Google Scholar]
- 14.Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Scholer HR. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012; 10: 465-472 [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011; 108: 7838-7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012; 109: 2527-2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon JH, Heo JS, Kim JS, Jun EK, Lee JH, Kim A, Kim J, Whang KY, Kang YK, Yeo S, Lim HJ, Han DW, Kim DW, Oh S, Yoon BS, Scholer HR, You S. Reprogramming fibroblasts into induced pluripotent stem cells with Bmi1. Cell Res. 2011; 21: 1305-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian C, Ambroz RJ, Sun L, Wang Y, Ma K, Chen Q, Zhu B, Zheng JC. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med. 2012; 12: 126-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011; 9: 113-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011; 476: 224-227 [DOI] [PubMed] [Google Scholar]
- 21.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011; 476: 220-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011; 108: 10343-10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfisterer U, Wood J, Nihlberg K, Hallgren O, Bjermer L, Westergren-Thorsson G, Lindvall O, Parmar M. Efficient induction of functional neurons from adult human fibroblasts. Cell Cycle. 2011; 10: 3311-3316 [DOI] [PubMed] [Google Scholar]
- 24.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011; 9: 205-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson KC, Jamshidi P, Daly R, Hearn MT, Pera MF, Dottori M. Wnt3a regulates survival, expansion, and maintenance of neural progenitors derived from human embryonic stem cells. Mol Cell Neurosci. 2007; 36: 408¬415 [DOI] [PubMed] [Google Scholar]
- 26.Denham M, Dottori M. Signals involved in neural differentiation of human embryonic stem cells. Neurosignals. 2009; 17: 234-241 [DOI] [PubMed] [Google Scholar]
- 27.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003; 6: 1162-1168 [DOI] [PubMed] [Google Scholar]
- 28.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003; 39: 749-765 [DOI] [PubMed] [Google Scholar]
- 29.Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005; 15: 7-13 [DOI] [PubMed] [Google Scholar]
- 30.Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet. 2009; 5: e1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gajovic S, St-Onge L, Yokota Y, Gruss P. Retinoic acid mediates Pax6 expression during in vitro differentiation of embryonic stem cells. Differentiation. 1997; 62: 187-192 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Yang Y, Lavaute TM, Li XJ, Ayala M, Bondarenko GI, Du ZW, Jin Y, Golos TG, Zhang SC. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010; 7: 90-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang N, An MC, Montoro D, Ellerby LM. Characterization of Human Huntington's Disease Cell Model from Induced Pluripotent Stem Cells. PLoS Curr. 2010; 2: RRN1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheyne JE, Grant L, Butler-Munro C, Foote JW, Connor B, Montgomery JM. Synaptic integration of newly generated neurons in rat dissociated hippocampal cultures. Mol Cell Neurosci. 2011; 47: 203-214 [DOI] [PubMed] [Google Scholar]
- 35.Takayama N, Nishimura S, Nakamura S, Shimizu T, Ohnishi R, Endo H, Yamaguchi T, Otsu M, Nishimura K, Nakanishi M, Sawaguchi A, Nagai R, Takahashi K, Yamanaka S, Nakauchi H, Eto K. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010; 207: 2817-2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001; 19: 1129-1133 [DOI] [PubMed] [Google Scholar]
- 37.Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007; 25: 1511-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall GP, Reynolds BA, Laywell ED. Using the neurosphere assay to quantify neural stem cells in vivo. Curr Pharm Biotechnol. 2007; 8: 141-145 [DOI] [PubMed] [Google Scholar]
- 39.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006; 419: 3-23 [DOI] [PubMed] [Google Scholar]
- 40.Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005; 280: 24731-24737 [DOI] [PubMed] [Google Scholar]
- 41.Chin JH, Shiwaku H, Goda O, Komuro A, Okazawa H. Neural stem cells express Oct-3/4. Biochem Biophys Res Commun. 2009; 388: 247-251 [DOI] [PubMed] [Google Scholar]
- 42.Zangrossi S, Marabese M, Broggini M, Giordano R, D'Erasmo M, Montelatici E, Intini D, Neri A, Pesce M, Rebulla P, Lazzari L. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells. 2007; 25: 1675-1680 [DOI] [PubMed] [Google Scholar]
- 43.Chen K, Hughes SM, Connor B. Neural progenitor cells derived from the adult rat subventricular zone: characterization and transplantation. Cell Transplant. 2007; 16: 799-810 [DOI] [PubMed] [Google Scholar]
- 44.Vazey EM, Chen K, Hughes SM, Connor B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington's disease. Exp Neurol. 2006; 199: 384-396 [DOI] [PubMed] [Google Scholar]
- 45.Hu BY, Zhang SC. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol Biol. 2010; 636: 123-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008; 322: 949-953 [DOI] [PubMed] [Google Scholar]
- 47.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009; 4: 381-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct Reprogramming of Mouse and Human Fibroblasts into Multipotent Neural Stem Cells with a Single Factor. Cell Stem Cell. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scardigli R, Baumer N, Gruss P, Guillemot F, Le Roux I. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development. 2003; 130: 3269-3281 [DOI] [PubMed] [Google Scholar]
- 50.Rizzino A. Sox2 and Oct-3/4: a versatile pair of master regulators that orchestrate the self-renewal and pluripotency of embryonic stem cells. Wiley Interdiscip Rev Syst Biol Med. 2009; 1: 228-236 [DOI] [PMC free article] [PubMed] [Google Scholar]


