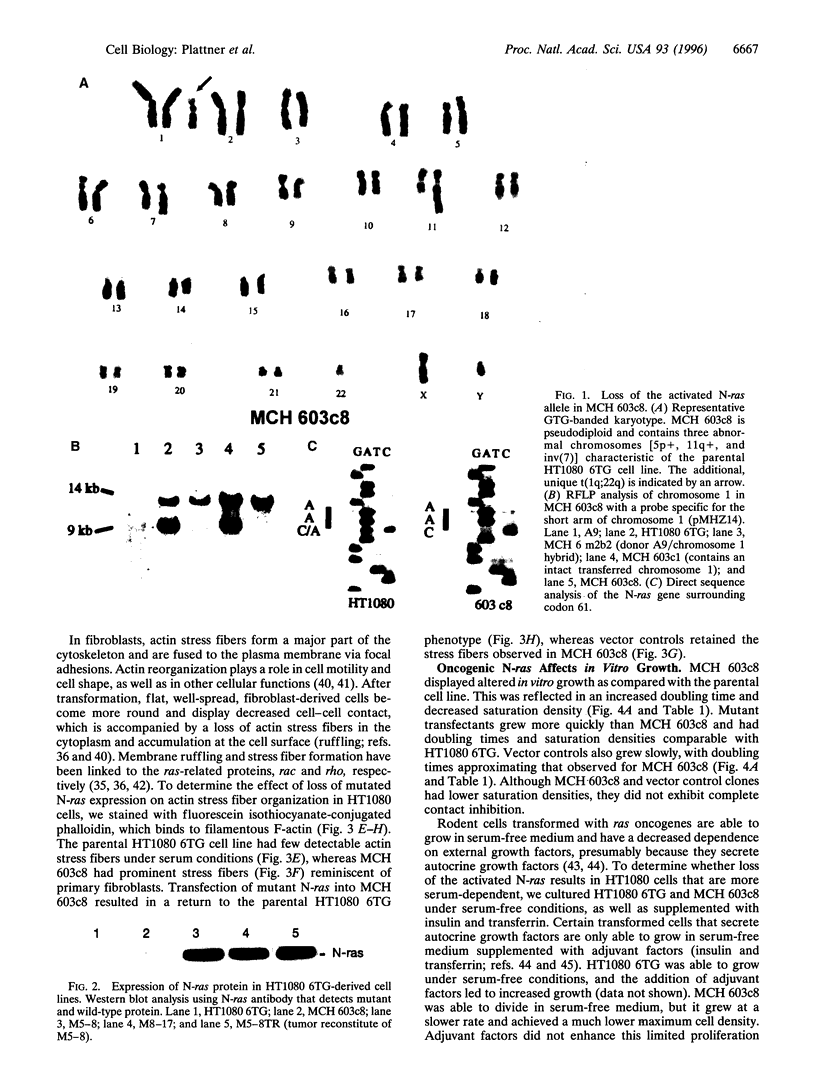
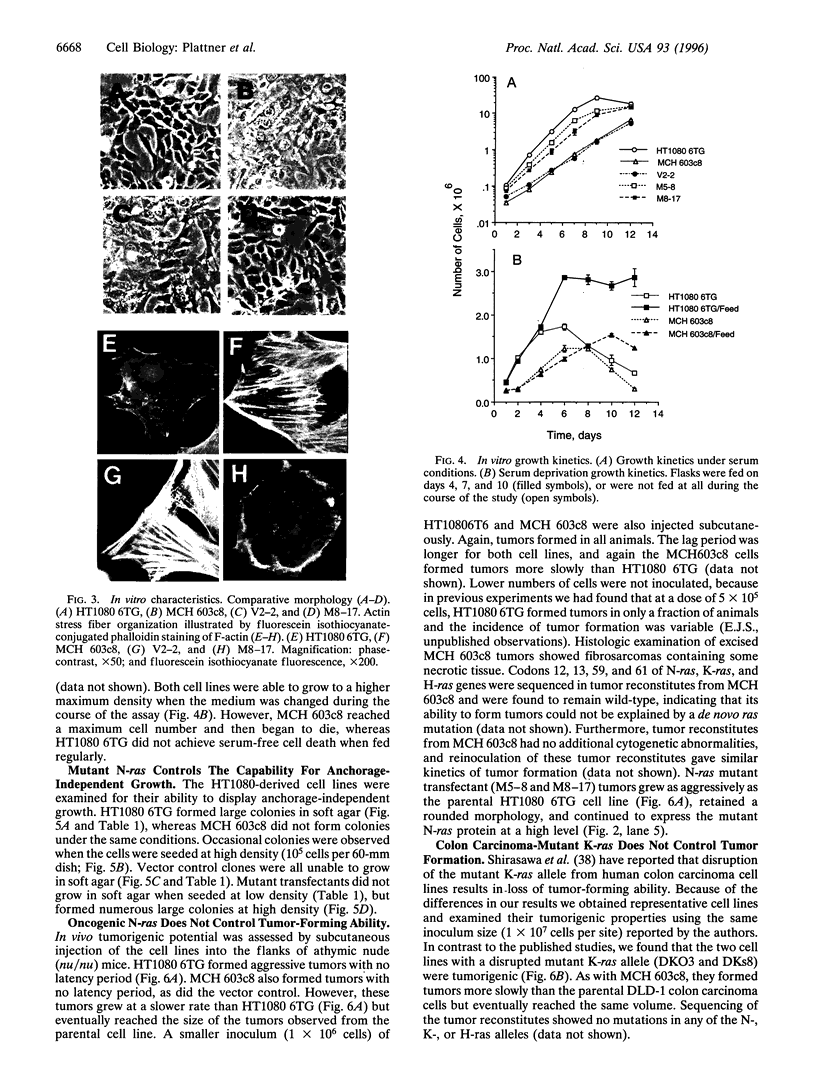
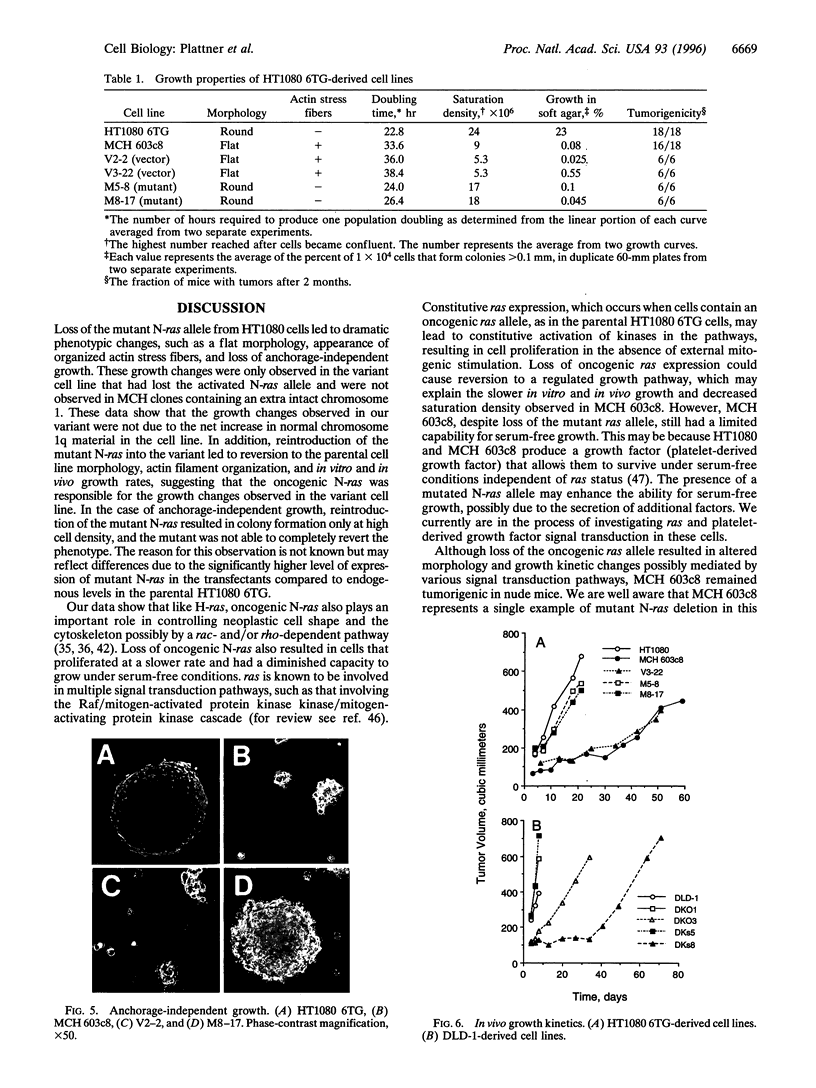
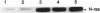Abstract
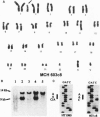
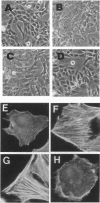
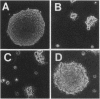
ras oncogenes are mutated in at variety of human tumors, which suggests that they play an important role in human carcinogenesis. To determine whether continued oncogenic ras expression is necessary to maintain the malignant phenotype, we studied the human fibrosarcoma cell line, HT1080, which contains one mutated and one wild-type N-ras allele. We isolated a variant of this cell line that no longer contained the mutated copy of the N-ras gene. Loss of mutant N-ras resulted in cells that displayed a less transformed phenotype characterized by a flat morphology, decreased growth rate, organized actin stress fibers, and loss of anchorage-independent growth. The transformed phenotype was restored following reintroduction of mutant N-ras. Although loss of the oncogenic N-ras drastically affected in vitro growth parameters, the variant remained tumorigenic in nude mice indicating that mutated N-ras expression is not necessary for maintenance of the tumorigenic phenotype. We confirmed this latter observation in colon carcinoma cell lines that have lost activated K-ras expression via targeted knockout of the mutant K-ras gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Casey G., Fasching C. L., Stanbridge E. J. Evidence that wild-type TP53, and not genes on either chromosome 1 or 11, controls the tumorigenic phenotype of the human fibrosarcoma HT1080. Genes Chromosomes Cancer. 1994 Apr;9(4):266–281. doi: 10.1002/gcc.2870090407. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Fernandez A., Feramisco J. R. Regulation of membrane turnover by ras proteins. Biosci Rep. 1987 May;7(5):427–434. doi: 10.1007/BF01362505. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Weissman B. E., Mark C., Stanbridge E. J. Tumorigenicity of human HT1080 fibrosarcoma X normal fibroblast hybrids: chromosome dosage dependency. Cancer Res. 1984 Aug;44(8):3471–3479. [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Peter W., Pascheberg U., Altmeier S., Fasching C., Stanbridge E. J., Fusenig N. E. Step-wise progression in human skin carcinogenesis in vitro involves mutational inactivation of p53, rasH oncogene activation and additional chromosome loss. Oncogene. 1995 Sep 7;11(5):961–969. [PubMed] [Google Scholar]
- Boukamp P., Stanbridge E. J., Foo D. Y., Cerutti P. A., Fusenig N. E. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990 May 1;50(9):2840–2847. [PubMed] [Google Scholar]
- Brown R., Marshall C. J., Pennie S. G., Hall A. Mechanism of activation of an N-ras gene in the human fibrosarcoma cell line HT1080. EMBO J. 1984 Jun;3(6):1321–1326. doi: 10.1002/j.1460-2075.1984.tb01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Davis M., Malcolm S., Hall A., Marshall C. J. Localisation of the human N-ras oncogene to chromosome 1cen - p21 by in situ hybridisation. EMBO J. 1983;2(12):2281–2283. doi: 10.1002/j.1460-2075.1983.tb01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Clark R., Wong G., Arnheim N., Milley R., McCormick F. Transient reversion of ras oncogene-induced cell transformation by antibodies specific for amino acid 12 of ras protein. Nature. 1985 Apr 18;314(6012):639–642. doi: 10.1038/314639a0. [DOI] [PubMed] [Google Scholar]
- Finney R. E., Bishop J. M. Predisposition to neoplastic transformation caused by gene replacement of H-ras1. Science. 1993 Jun 4;260(5113):1524–1527. doi: 10.1126/science.8502998. [DOI] [PubMed] [Google Scholar]
- Fournier R. E., Ruddle F. H. Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):319–323. doi: 10.1073/pnas.74.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges R. N., Mukhopadhyay T., Zhang Y., Yen N., Roth J. A. Prevention of orthotopic human lung cancer growth by intratracheal instillation of a retroviral antisense K-ras construct. Cancer Res. 1993 Apr 15;53(8):1743–1746. [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Gray G. D., Hernandez O. M., Hebel D., Root M., Pow-Sang J. M., Wickstrom E. Antisense DNA inhibition of tumor growth induced by c-Ha-ras oncogene in nude mice. Cancer Res. 1993 Feb 1;53(3):577–580. [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani-Sabet M., Funato T., Florenes V. A., Fodstad O., Scanlon K. J. Suppression of the neoplastic phenotype in vivo by an anti-ras ribozyme. Cancer Res. 1994 Feb 15;54(4):900–902. [PubMed] [Google Scholar]
- Khosravi-Far R., Der C. J. The Ras signal transduction pathway. Cancer Metastasis Rev. 1994 Mar;13(1):67–89. doi: 10.1007/BF00690419. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Mosser S. D., deSolms S. J., Giuliani E. A., Pompliano D. L., Graham S. L., Smith R. L., Scolnick E. M., Oliff A., Gibbs J. B. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science. 1993 Jun 25;260(5116):1934–1937. doi: 10.1126/science.8316833. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Wilson F. R., Mosser S. D., Giuliani E., deSolms S. J., Conner M. W., Anthony N. J., Holtz W. J., Gomez R. P., Lee T. J. Protein farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Montano X., Jimenez A. Intracellular expression of the monoclonal anti-ras antibody Y13-259 blocks the transforming activity of ras oncogenes. Cell Growth Differ. 1995 May;6(5):597–605. [PubMed] [Google Scholar]
- Nagasu T., Yoshimatsu K., Rowell C., Lewis M. D., Garcia A. M. Inhibition of human tumor xenograft growth by treatment with the farnesyl transferase inhibitor B956. Cancer Res. 1995 Nov 15;55(22):5310–5314. [PubMed] [Google Scholar]
- Pantazis P., Pelicci P. G., Dalla-Favera R., Antoniades H. N. Synthesis and secretion of proteins resembling platelet-derived growth factor by human glioblastoma and fibrosarcoma cells in culture. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2404–2408. doi: 10.1073/pnas.82.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Paterson H., Reeves B., Brown R., Hall A., Furth M., Bos J., Jones P., Marshall C. Activated N-ras controls the transformed phenotype of HT1080 human fibrosarcoma cells. Cell. 1987 Dec 4;51(5):803–812. doi: 10.1016/0092-8674(87)90103-6. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Landegent J., Collins C., Fuscoe J., Segraves R., Lucas J., Gray J. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pironin M., Clément G., Benzakour O., Barritault D., Lawrence D., Vigier P. Growth in serum-free medium of NIH3T3 cells transformed by the EJ-H-ras oncogene: evidence for multiple autocrine growth factors. Int J Cancer. 1992 Jul 30;51(6):980–988. doi: 10.1002/ijc.2910510624. [DOI] [PubMed] [Google Scholar]
- Qiu R. G., Chen J., Kirn D., McCormick F., Symons M. An essential role for Rac in Ras transformation. Nature. 1995 Mar 30;374(6521):457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Okamoto M., Sato C., Sugio K., Soejima J., Iwama T., Ikeuchi T., Tonomura A., Miyaki M., Sasazuki T. Loss of constitutional heterozygosity in colorectal tumors from patients with familial polyposis coli and those with nonpolyposis colorectal carcinoma. Cancer Res. 1989 Aug 15;49(16):4402–4406. [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Leipzig G. V., Sameshima J. H., Stanbridge E. J. Selective transfer of individual human chromosomes to recipient cells. Mol Cell Biol. 1985 Jan;5(1):140–146. doi: 10.1128/mcb.5.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. F., Phillips J. A., 3rd, Migeon B. R. DNA restriction endonuclease analysis for localization of human beta- and delta-globin genes on chromosome 11. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4563–4565. doi: 10.1073/pnas.76.9.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L., Ma Z., Rands E., Kohl N. E., Gibbs J. B., Oliff A., Rosen N. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995 Nov 15;55(22):5302–5309. [PubMed] [Google Scholar]
- Shirahata S., Rawson C., Loo D., Chang Y. J., Barnes D. ras and neu oncogenes reverse serum inhibition and epidermal growth factor dependence of serum-free mouse embryo cells. J Cell Physiol. 1990 Jul;144(1):69–76. doi: 10.1002/jcp.1041440110. [DOI] [PubMed] [Google Scholar]
- Shirasawa S., Furuse M., Yokoyama N., Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993 Apr 2;260(5104):85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Fry D. G., Maher V. M., McCormick J. J. Transformation of diploid human fibroblasts by transfection of N-ras-oncogenes. Carcinogenesis. 1989 Apr;10(4):635–640. doi: 10.1093/carcin/10.4.635. [DOI] [PubMed] [Google Scholar]