Abstract
Natural killer (NK) cells have been known to enhance the host immune responses against cancer. NK cell number and cytotoxicity in patients with cancer is often low. Therefore, we developed a large-scale ex vivo NK cell expansion method without feeder layers and introduced NK cell-based autologous immune enhancement therapy (AIET). In this paper, we discuss the epidemiological data that show the relationship between NK activity and cancer incidence, monitoring of NK cell number and activity, anti-cancer activities of NK cells in vitro and in vivo and the effects of the combination of expanded NK cells with monoclonal antibody drugs on cancers through antibody-dependent cellular cytotoxicity. Finally, we also present the clinical cases of NK cell-based AIET and the effect of AIET on advanced stage of pancreatic cancer and on various advanced cancers refractory to conventional therapies. NK cell-based AIET might be a useful strategy in the multidisciplinary approach to cancer.
Keywords: NK cell, immunotherapy, antibody-dependent cellular cytotoxicity (ADCC), pancreatic cancer, palliative therapy
Introduction
Cancer is still a leading cause of death in the world. In Japan, the number of cancer patients is increasing every year since 1950s. Among mortality due to various diseases, the mortality due to cancer occupies the first position since 1980s[1]. Now, one in two among the population develops cancer and one third dies from cancer in Japan. Half of the cancer patients died within five years under standard treatment [2]. A new strategy expected to improve the survival rate of cancer patients is needed.
As standard treatments, surgical therapy, radiotherapy and/or chemotherapy are available for treating cancer at present. To eliminate cancer, the immune system in our body works together with such standard treatments. When the host immune system does not work, chemotherapy and radiotherapy cannot kill the cancer cells efficiently [3]. It is important to improve the host immune system for the efficient treatment of cancer. Therefore, we have introduced autologous immune enhancement therapy (AIET) based on immune cells, especially NK cells, for cancer treatment.
Which immune cells are used in AIET for cancer treatment?
For AIET using immune cells, the process involves separation of the lymphocytes from the peripheral blood of the patient followed by selective immune cell expansion using the expansión kit (BINKIT, Biotherapy Institute of Japan) without feeder layers and then infusion of the expanded-activated NK cells [4-6].
To enhance immune activities using AIET, we can use many types of immune cells such as NK cells, NKT cells, γδT cells, αβT cells, cytotoxic T cells (CTL) and dendritic cells (DC). In the multidisciplinary approach to treating cancer, different types of cells can be used. However, we have several reasons for selecting NK cells as the base of AIET. First, the epidemiological data show the relationship between NK activity and cancer incidence. Secondly, we can measure NK cell number and activity in patient’s blood to monitor the immune status of patients to evaluate the effect of AIET. Thirdly, experimental results show that expanded NK cells can kill cancers efficiently. Finally, NK cells can enhance the effects of monoclonal antibody (mAb) drugs on cancers through antibody-dependent cellular cytotoxicity (ADCC).
Epidemiological indications of NK cell importance
There are clear indications of significant roles of natural immunological host defence in preventing the development of cancer. A prospective cohort study among 3625 residents of a Japanese general population for 11 years shows that medium and high NK activity is associated with reduced cancer incidence, whereas low activity is associated with increased cancer incidence [7].
In patients with cancer and viral infection, NK cell function has been shown to be impaired [8]. We have also showed that NK activity of peripheral blood mononuclear cells (PBMCs) was significantly lower in patients with breast cancer than that of healthy individuals [9].
These data suggest an important role of NK cells for host defence mechanisms against cancer.
Monitoring of NK cell number and activity in blood
After infusing ex vivo expanded NK cells, it is possible to monitor immunological parameters such as lymphocyte number, NK cell number and NK activity in blood. When these NK cells were infused into healthy volunteers, not only NK cells but also T and B cells accumulated in the peripheral blood, suggesting that ex vivo expanded NK cells can induce immune reactions of a host receiving NK cell-based AIET [4]. Furthermore, after injection of the cells, NK cell activities also increased in the peripheral blood without any adverse effects [8]. However, the response patterns of lymphocyte phenotype vary among cancer patients after AIET because cancer patients have various level of immune suppressive status. Based on the pattern of immune status, it will be possible to choose the proper immune cells and the interval of AIET to adjust the immune status of each patient for tailor-made treatment.
In vitro and in vivo activities of ex vivo expanded NK cells
The expression of activating receptors on the expanded NK cells, and production of IFN-γ and TNF-α from the NK cells is up-regulated [6]. The cytotoxicity of expanded NK cells against various cancer cells is higher compared with that of freshly isolated NK cells [4,6]. Although freshly isolated NK cells have only low cytotoxicity against cancer stem cells, expanded NK cells efficiently kill cancer stem cells [6,10-12]. NK cells have many activating and inhibitory receptors, which recognize specific ligands as expressed on target cells [4]. A balance of the activating and inhibitory signals through these receptors from the ligands on target cells regulates the effector function of NK cells. NK cell-activating receptors are expressed not only by NK cells but also αβT cells and γδT cells. When we compared the cytotoxic activity of ex vivo expanded NK cells against various cancer cell lines with that of ex vivo expanded αβT cells or γδT cells, the NK cells always showed higher cytotoxicity compared with the αβT cells or the γδT cells (13).
These highly cytotoxic, expanded NK cells can be adoptively transferred into the body as NK cell-based AIET.
In our studies using the NOG/SCID γc (null) (NOG) mouse model, expanded NK cells were shown to reduce the size of malignant tumors and the volume of ascites also inhibiting lung metastasis of cancer cells [14,15].
These results suggest usefulness of NK cell-based AIET for cancer treatment.
ADCC of NK cells
ADCC has recently been identified as one of the critical factors for the clinical efficacy of anticancer antibodies, in which NK cells are the major effectors of ADCC. ADCC is triggered following the binding of the antibody Fc region to the Fcγ receptor (FcγR) on effector cells. Most of the NK cells express CD16 (FcγRIII), a receptor that binds to the Fc region of IgG1 and IgG3 isotypes [16].
When combined with mAb drugs, a synergistic cytotoxicity of the ex vivo expanded NK cells against target cells as well as higher IFN-γ and TNF-α production are observed [6]. Many therapeutic mAbs are being developed such as rituximab [17,18], trastuzumab [19,20,21], cetuximab [22], and alemtuzumab [23], and the effects of these mAbs on cancer increase when combined with ex vivo expanded NK cells.
We reported a suggestive case of the effect of the expanded NK cells combined with a mAb drug. The HER-2-positive tumor was refractory to several agents, including anti-HER-2 therapy, trastuzumab, and lapatinib. After re-induction of trastuzumab in combination with NK cell-based AIET, tumor markers decreased, and finally a synergistic effect of taxane and capecitabine led to treatment response (Figure 1) [5]. The results suggest that NK cell-based AIET in combination with mAb drugs is a promising cancer immunotherapy.
Figure 1.
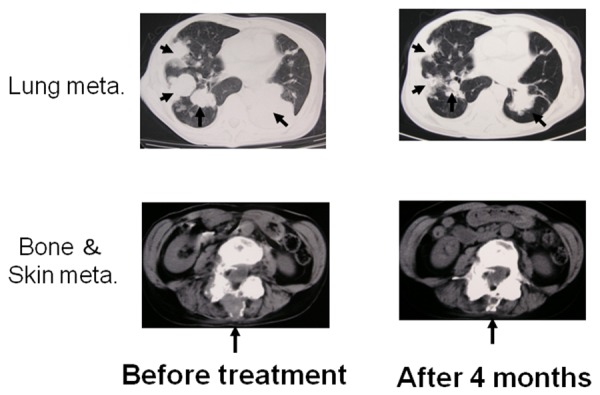
An effect of NK cell-based autologous immune enhancement therapy (AIET) combined with a mAb drug. Images of a 55-year-old female patient with metastasis of lung, bone, and skin of breast cancer (invasive ductal carcinoma) post operation. After receiving informed consent from the patient, autologous activated NK cells (2 x 109 cells/injection) were transfused into the patients at 2-week intervals. After failing of most of chemotherapy including trastuzumab, a synergistic effect of NK cell-based AIET, chemotherapy (trastuzumab, taxane, capecitabine) and hyperthermia led to treatment response [5].
Clinical cases
Successful clinical studies of NK cell-based AIET has been reported for metastatic renal cell carcinoma and brain tumors [24,25]. However, the efficacies of the AIET have not been well established because it has been difficult to culture sufficient numbers of highly active NK cells. However we developed a feasible and safe method for the large scale ex vivo expansion of NK cells and it is now available as a cultivation kit (BINKIT). The clinical effects of AIET using this NK cell expanded method have been presented for some cancers, including lung cancer, lung metastasis of renal cell carcinoma, breast cancer, ovarian cancer, and cervical cancer [4,5,13,26,27].
To improve the effects of expanded NK cells on treatment against cancer, we are also working on combination immunotherapy using expanded T cells and monocyte derived DC with expanded NK cells. The principle behind combining NK cells and CTL is a dual advantage approach combining the innate immune system and adaptive immune system against cancer. The functional cross-talk between NK cells and DC activate both innate and adaptive immune system synergistically [4].
Moreover, we evaluated the benefit in survival of AIET in combination with hyperthermia to patients with advanced stages of pancreatic cancer (Figure 2).
Figure 2.
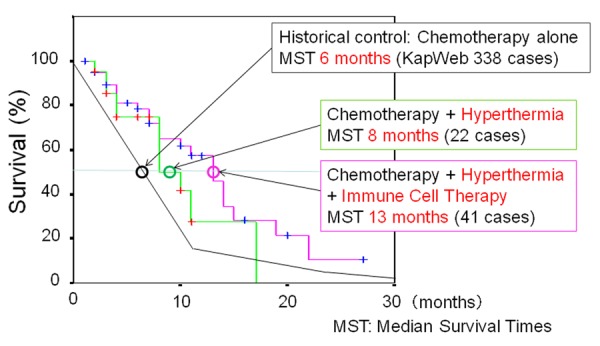
A retrospective analysis of the survival time in patients with stage IV pancreatic cancer who received hyperthermia and/or AIET. All our 63 patients received hyperthermia and chemotherapy. After receiving informed consent from the patients, 41 out of 63 patients received NK cell-based AIET and some patients also received T cell- and DC-based AIET. The survival time of patients who received AIET was compared with those of our 22 patients receiving hyperthermia without AIET and the 338 patients receiving conventional treatment, mostly chemotherapy alone in a data of Survival statistics of Japanese association of Clinical Cancer Centers by Kaplan-Meier method of KapWeb [28] from 18 core medical centers for cancer in Japan in 2004.
We retrospectively analyzed the survival time in a group of sixty-three patients with stage IV pancreatic cancer. We used a data of Survival statistics of Japanese association of Clinical Cancer Centers by Kaplan-Meier method of KapWeb[28] as a survival rate of the patients receiving conventional treatment which is based on the data of 338 cases with stage IV pancreatic cancer collected from 18 core medical centers for cancer in Japan in 2004. All our 63 patients received hyperthermia and chemotherapy.
Forty one out of 63 patients received NK cell-based AIET and some patients also received T cell and DC-based AIET. The median survival time was 13 months for 41 patients treated with AIET, and 8 months for 22 patients treated without AIET. Both of these data were better than those seen with conventional treatments, mostly chemotherapy alone for approximately 6 months in KapWeb. In addition, more than 50 percent of patients who received AIET lived for 13 months after the first diagnosis. The results suggest that the AIET combined with hyperthermia and chemotherapy was effective against advanced pancreatic cancer.
We have also reported the clinical effects of NK cell-based AIET when combined with hyperthermia as palliative treatment on advanced stages of cancer [13]. Fifty-two patients with advanced cancers in organs like lung, breast, colon, prostate, liver, kidney, ovary etc., refractory to conventional therapies were treated with a combination of NK cell and CTL-based AIET and hyperthermia with low-dose chemotherapy. After three month of treatment, in 18 out of 52 patients (35%), objective responses was observed including one complete response (CR) and 17 partial response (PR), evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (Figure 3a). Sixteen patients had stable disease (SD), whereas 18 had progressive disease (PD). Disease control rate was 66% including CR, PR and SD. After treatment for six months, the objective responses and disease control rate were 25% and 52%, respectively (Figure 3b). No adverse reaction over grade 1 (defined according to the Common Toxicity Criteria of the National Cancer Institute) was observed. The result suggests that NK cell and CTL based AIET combined with hyperthermia with low-dose chemotherapy was effective even in advanced cancers which were refractory to conventional therapeutic modalities.
Figure 3.
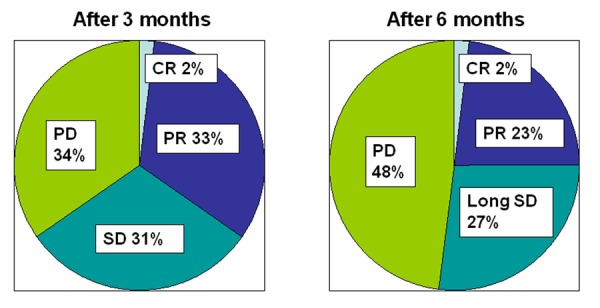
The clinical effects of NK cell-based AIET when combined with hyperthermia as palliative treatment on advanced stages of cancer [13]. Fifty-two patients with advanced cancers in various organs, refractory to conventional therapy were treated with a combination of NK cell-based AIET and hyperthermia with low-dose chemotherapy. The response of treatment was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. After three month of treatment, the objective responses and disease control rate were 35% and 66%, respectively. After treatment for six months, the objective responses and disease control rate were 25% and 52%, respectively. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease.
Conclusion
These data suggest that AIET is a safe adjunct treatment modality of tackling cancer and may induce effective immune responses in cancer patients and help improve the prognosis. To determine more suitable target cancers for NK cell-based AIET, more clinical data of the AIET must be collected. It is important to make appropriate evaluations of the cell quality including their safety as the quality and quantity of immune cell cultures differ among institutes. To prepare the good clinical grade NK cells and other immune cells for AIET, we emphasize the necessity for optimization of the culture method for each patient based on proper evaluation of ex vivo expanded immune cells.
Abbreviations
- NK cells:
Natural Killer cells
- CTL:
Cytotoxic T cells
- AIET:
Autologous Immuen Enhancement Therapy
- ADCC:
Antibody Dependant Cellular Cytotoxicity
- mAb:
Monoclonal Antibody
- PBMCs:
Peripheral Blood Mononuclear cells
- FcγR :
Fcγ Receptor
- CR:
Complete Response
- PR:
Partial Response
- RECIST:
Response Evaluation Criteria in Solid Tumors
- SD:
Stable Disease
- PD:
Progressive Disease
References
- 1.http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai11/dl/gaikyou23.pdf
- 2.http://ganjoho.jp/data/professional/statistics/odjrh3000000hwsa-att/mcij2003-2005_report.pdf
- 3.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira J-P, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andr F, Delaloge S, Tursz T, Kroemer G, Zitvogel L.Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 2007; 13(9): 1050-1059 [DOI] [PubMed] [Google Scholar]
- 4.Terunuma H, Deng X, Dewan Z, Fujimoto S, Yamamoto N.Potential role of NK cells in the induction of immune responses: Implications for NK cell-based immunotherapy for cancers and viral infections. Int Rev Immunol. 2008; 27(3): 93-110 [DOI] [PubMed] [Google Scholar]
- 5.Takada M, Terunuma H, Deng X, Dewan MZ, Saji S, Kuroi K, Yamamoto N, Toi M.Refractory lung metastasis from breast cancer treated with multidisciplinary therapy including an immunological approach. Breast Cancer. 2011; 18(1): 64-67 [DOI] [PubMed] [Google Scholar]
- 6.Deng X, Terunuma H, Nieda M, Xiao W, Nicol A.Synergistic cytotoxicity of ex vivo expanded natural killer cells in combination with monoclonal antibody drugs against cancer cells. International Immunopharmacology. 2012; 14(4): 593-605 [DOI] [PubMed] [Google Scholar]
- 7.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K.Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000; 356(9244): 1795-1799 [DOI] [PubMed] [Google Scholar]
- 8.Whiteside TL, Vujanovic NL, Herberman RB.Natural killer cells and tumor therapy. Curr Top Microbiol Immunol. 1998; 230: 221-244 [DOI] [PubMed] [Google Scholar]
- 9.Dewan MZ, Takada M, Terunuma H, Deng X, Ahmed S, Yamamoto N, Toi M.Natural killer activity of peripheral-blood mononuclear cells in breast cancer patients. Biomed Pharmacother. 2009; 63(9): 703-706 [DOI] [PubMed] [Google Scholar]
- 10.Pietra G, Manzini C, Vitale M, Balsamo M, Ognio E, Boitano M, Queirolo P, Moretta L, Mingari MC.Natural killer cells kill human melanoma cells with characteristics of cancer stem cells. Int Immunol. 2009; 21(7): 793-801 [DOI] [PubMed] [Google Scholar]
- 11.Wu A, Wiesner S, Xiao J, Ericson K, Chen W, Hall WA, Low WC, Ohlfest JR.Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007; 83(2): 121-31 [DOI] [PubMed] [Google Scholar]
- 12.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora F, Moretta L, Moretta A, Corte G, Bottino C.NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009; 182(6): 3530-9 [DOI] [PubMed] [Google Scholar]
- 13.Terunuma H, Deng X, Toki A, Yoshimura A, Nishino N, Takano Y, MIE Nieda M, Sasanuma J, Teranishi Y, Watanabe K.Effects of Hyperthermia on the Host Immune System: From NK Cell-Based Science to Clinical Application. Thermal Med. 2012; 28 (1): 1-9 [Google Scholar]
- 14.Dewan MZ, Terunuma H, Takada M, Tanaka Y, Abe H, Sata T, Toi M, Yamamoto N.Role of natural killer cells in hormone-independent rapid tumor formation and spontaneous metastasis of breast cancer cells in vivo. Breast Cancer Res Treat. 2007; 104(3): 267-275 [DOI] [PubMed] [Google Scholar]
- 15.Dewan MZ, Terunuma H, Toi M, Tanaka Y, Katano H, Deng X, Abe H, Nakasone T, Mori N, Sata T, Yamamoto N.Potential role of natural killer cells in controlling growth and infiltration of AIDS-associated primary effusion lymphoma cells. Cancer Sci. 2006; 97(12): 1381-1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulica A, Morel P, Metes D, Herberman RB.Ig-binding receptors on human NK cells as effector and regulatory surface molecules. Int Rev Immunol. 2001; 20(3-4): 371-414 [DOI] [PubMed] [Google Scholar]
- 17.Berinstein NL, Grillo-López AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, Green D, Rosenberg J, McLaughlin P, Shen D.Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1998; 9(9): 995-1001 [DOI] [PubMed] [Google Scholar]
- 18.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H.Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcyRIIIa gene. Blood. 2002; 99(3): 754-758 [DOI] [PubMed] [Google Scholar]
- 19.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A.Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004; 10(17): 5650-5655 [DOI] [PubMed] [Google Scholar]
- 20.Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L.Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008; 6: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kute TE, Savage L, Stehle JR, Jr, Kim-Shapiro JW, Blanks MJ, Wood J, Vaughn JP.Breast tumor cells isolated from in vitro resistance to trastuzumab remain sensitive to trastuzumab anti-tumor effects in vivo and to ADCC killing. Cancer Immunol Immunother. 2009; 58(11):1887-1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maréchal R, De Schutter J, Nagy N, Demetter P, Lemmers A, Devière J, Salmon I, Tejpar S, Van Laethem JL.Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer. 2010; 10: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siders WM, Shields J, Garron C, Hu Y, Boutin P, Shankara S, Weber W, Roberts B, Kaplan JM.Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leuk Lymphoma. 2010; 51(7): 1293-1304 [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Farace F, Angevin E, Charpentier F, Nitenberg G, Triebel F, Hercend T.Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL-2. Eur. J. Cancer. 1994; 30A(8): 1078-1083 [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, Ohno T.Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004; 24(3b): 1861-1871 [PubMed] [Google Scholar]
- 26.Manjunath SR, Ramanan G, Dedeepiya VD, Terunuma H, Deng X, Baskar S, Senthilkumar R, Thamaraikannan P, Srinivasan T, Preethy S, Abraham SJ.Autologous immune enhancement therapy in recurrent ovarian cancer with metastases: a case report. Case Reports in Oncology. 2012; 5(1):114-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Premkumar S, Dedeepiya VD, Terunuma H, Senthilkumar R, Srinivasan T, Reena HC, Preethy S, Abraham SJK.Cell Based Autologous Immune Enhancement Therapy (AIET) after Radiotherapy in a Locally Advanced Carcinoma of the Cervix. Case Reports in Oncological Medicine 2013 (2013) Article ID 903094, http://dx.doi.org/10.1155/2013/903094 [DOI] [PMC free article] [PubMed]
- 28.https://kapweb.chiba-cancer-registry.org/web/general/top.aspx


