Abstract
Arsenic and lead, known to have genotoxic and mutagenic effects, are ubiquitously distributed in the environment. The presence of arsenic in drinking water has been a serious health problem in many countries. Human exposure to these metals has also increased due to rapid industrialization and their use in formulation of many products. Liposuction material is a rich source of stem cells. In the present study cytotoxic and genotoxic effects of these metals were tested on adipose derived mesenchymal stem cells (AMSCs). Cells were exposed to 1-10 μg/ml and 10-100 μg/ml concentration of arsenic and lead, respectively, for 6, 12, 24 and 48 h. The cytotoxic effects were measured by neutral red uptake assay, while the genotoxic effects were tested by comet assay. The growth of cells decreased with increasing concentration and the duration of exposure to arsenic. Even the morphology of cells was changed; they became round at 10 μg /ml of arsenic. The cell growth was also decreased after exposure to lead, though it proved to be less toxic when cells were exposed for longer duration. The cell morphology remained unchanged. DNA damage was observed in the metal treated cells. Different parameters of comet assay were investigated for control and treated cells which indicated more DNA damage in arsenic treated cells compared to that of lead. Intact nuclei were observed in control cells. Present study clearly demonstrates that both arsenic and lead have cytotoxic and genotoxic effects on AMSCs, though arsenic compared to lead has more deleterious effects on AMSCs.
Keywords: Anti-proliferative activity, cytotoxic effect, comet assay, heavy metals, genotoxic effects
Introduction
Stem cells are characterized by the ability to renew themselves and to differentiate into multi-lineage of terminally differentiated cells. There are different sources of stem cells but for different applications, stem cells are preferred to be isolated where a minimally invasive procedure is required and cells are found in abundant quantities [1]. Variable number of stem cells is present in different tissues such as liver, heart, kidney, skin, bone marrow and adipose tissue etc. [2], The tissues are mainly formed from mature cells but it is now evident that stem cells also reside in tissues in specific niches [3]. Embryonic stem cells have the ability to differentiate into all types of cells of the body though the adult stem cells do not have this level of plasticity and they have multipotent to pluripotent differential potential. Earlier it was considered that stem cells can only differentiate into mature cells of the same organ but recent evidences indicate that they can also differentiate into other cell types and even into the cells of ectoderm, mesoderm and endoderm [4,5,6,7,8]. Mesenchymal stem cells (MSCs) are considered to have more plasticity and they can easily be differentiated into cells of other lineages. Though Adult MSCs can be isolated from different sources, bone marrow and adipose tissue are the main sources. Due to an increase in the rate of obesity world over and particularly in the USA, adipose tissue is now being abundantly used as a source of adipose derived mesenchymal stem cells (AMSCs). According to an estimate, nearly 400,000 liposuction surgeries nearly 400,000 liposuction surgeries are performed in the USA per year, which yield approximately 100 ml to 3000 ml of lipoaspirate tissue. Although it has great research and therapeutic applications, majority of this rich source of stem cells is wasted [9, 10].
Arsenic is ubiquitously distributed in environment. Due to its association with a number of health related problems, arsenic is considered as the top environmental health threat. According to a study in 2007 (USA Today.com, August 30, 2007), over 137 million people in more than 70 countries are probably affected by arsenic poisoning from drinking water. Exposure to inorganic arsenic leads to increased risk of malignancies, stroke, cardiovascular and chronic liver diseases [11,12,13]. Arsenic is also reported to increase the incidence of certain cancers in experimental animals [14, 15]. According to experimental findings, arsenic does not cause direct DNA damage. Instead it causes mutations indirectly by altering the DNA repair mechanism [16, 17], and has also been demonstrated to be immunotoxic [18, 19]. During pregnancy it not only has deleterious effects on the health of mother but it is also reported to cross the placental barrier and cause toxicity to fetus [20, 21].
Lead is one of the most common heavy metals in the earth crust which is also known to cause deleterious effects on human health even at low doses. Occupational exposure is a common cause of lead poisoning in adults. According to an estimate, more than 3 million workers are potentially exposed to lead in a work place in USA (http://en.wikipedia.org/wike/Lead_poisoning). Lead has long been recognized to damage the nervous, renal, hematological and cardiovascular systems[22]. Anthropogenic activities are the main source of lead exposure [23]. Humans are exposed to lead mainly by inhalation and ingestion. Once inside the body, lead is mainly stored in soft tissues of the body. The effects of lead on the human body mainly depend on the duration, route of exposure, dose and the chemical state of lead. The effects of lead are more severe on kids, the source of exposure being the use of lead paints in houses, where it causes deleterious effects on the neurons leading to low IQ levels [24].
As the level of arsenic and lead is on the increase in developing countries, there is a great need for proper disposal of these metals because of their serious health consequences including the development of cancer. There is also a need for community education about health hazards of heavy metals. In the present study, we have investigated the cytotoxic as well as genotoxic effects of both these heavy metals on human adipose derived mesenchymal stem cells (AMSCs). Since there is no such study available on the effects of arsenic and lead on AMSCs, it becomes extremely relevant to explore the deleterious effects of both metals on stem cells.
Materials and Methods
Chemicals
Analytical grades of sodium arsenite, lead nitrate, sodium chloride, triton X-100, DMSO, NaOH, ethanol and EDTA were obtained from Sigma-Aldrich. Dulbecco’s Modified Eagle’s Minimal Essential Medium (DMEM), phosphate buffer (pH 7.4), trypsin-EDTA, penicillin, streptomycin, fetal bovine serum (FBS) were obtained from GIBCO and PAA. Sterile tissue culture flasks and sterile glass pipettes were purchased from NUCK. Lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris (pH 10), 1% N-lauryl sarcosine sodium, 1% Triton X-100 and 10% DMSO), alkaline buffer (1 mM EDTA, 300 mM NaOH) were prepared in the laboratory.
Isolation of Adipose Mesenchymal Stem Cells (AMSCs)
The fat sample (single sample) was collected from a liposuction procedure performed at the Ganga Ram Hospital, Lahore after the informed consent of a patient (29 years old female) in a vial containing 5 ml complete medium containing 0.5 ml 100X penicillin-streptomycin solution. The sample was immediately transferred to the lab for further procedures. The sample was spun at 2,000 rpm for 5 minutes to collect the fat tissue pieces. The fat pieces were washed extensively with sterile phosphate buffered saline (PBS) three times and treated with 0.01% collagenase (Type 1; Sigma Aldrich) prepared in DMEM medium for 2 h at 37°C with gentle agitation after every 10 minutes. The collagenase was inactivated with equal volume of DMEM (DMEM + 10% FBS). The mixture was centrifuged at 2000 rpm for 10 minutes. The pellet was washed once with PBS and then re-suspended in complete medium (DMEM containing glutamine, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin). The number of cells were counted by hemocytometer and added into a tissue culture flask (NUNC) followed by incubation at 37°C with 5% CO2 in a humidified environment for three days. When the cells became confluent they were trypsinized with trypsin-EDTA and sub-cultured.
Cytotoxicity Assay
The AMSCs were sub-cultured and incubated for 24h at 37°C in a humidified environment with 5% CO2 to grow the cells in a monolayer. When cells grew to 90% confluency, they were washed with phosphate buffer saline (PBS), trypsinized with 1 ml of 1X Trypsin-EDTA. The cells were counted with hemocytometer and 5 x 103 cells were added in each well of a 96 well plate for arsenic treatment and 2 x 103 cells for lead with a total volume of 200 μl of complete DMEM medium. Cells were incubated for 24h at 37 °C in a 5% CO2 incubator. The old medium was replaced by 200 μl of fresh medium containing 0-10 μg/ml arsenic and 0-100 μg/ml lead, respectively, and incubated under the same culture conditions for 6, 12, 24 and 48h. Cytotoxic effects were tested by neutral red uptake method, for which the treatment medium was aspirated and the cells were incubated with neutral red medium for 3h at 37°C. The cells were washed with PBS and images were taken. The Neutral Red de-stain solution (150 μl) was added in each well and the plates were placed on a shaker at 120 rpm for 10 min. The supernatant was used for measuring the differential absorbance at 492 nm and 630 nm using ELISA reader (Humareader plus, HUMAN). All assays were done in triplicate. Mean values and standard deviations were calculated for the data on the cytotoxic effects and then plotted on a graph.
Comet Assay
AMSCs (5x104 cells) were added in 6 well plate in 2 ml DMEM complete medium and incubated at 37°C in a humidified environment with 5% CO2 for 24 h. The medium was replaced with DMEM medium (2% FBS) containing 1 μg/ml arsenic and 10 μg/ml lead, respectively. No metal was added to the control. The cells were incubated again for 12h, washed with PBS and trypsinized with trypsin-EDTA. The number of cells was counted with heamocytometer and 5x104 cells were finally suspended in 100 μl of PBS. After this procedure, every step was carried out in indirect light. The slides were layered with 1.5% normal agarose prepared in TAE buffer. The cells in 100 μl of PBS were mixed with 400 μl of 1% low melting agarose at 37°C and 100 μl was layered over the agarose coated slide. The slides were covered with a coverslip and then placed in an incubator at 4°C for 20 minutes to solidify. The cover slip was removed and the slides were immersed in lysis solution for 1h. The slides were washed for 5 minutes in PBS and immersed in electrophoresis tank in the presence of freshly prepared alkaline buffer at room temperature. After 20 minutes, electrophoresis was done in the same buffer at 25V for 20 minutes (previously optimized). The slides were neutralized using neutralizing buffer (0.4 M Tris; pH 7) for 15 minutes. Finally the slides were fixed using absolute ethanol for 10 minutes and stored at 4°C before analysis.
For the analysis, the slides were stained with 50 μl of ethidium bromide solution (20 μg/ml) and images were taken using 10X objective of fluorescence microscope (Olympus BX51). Comet score15 was used to analyze comet. Five different comets were analyzed from each slide. Ten different parameters viz., Comet length, height, area, intensity, head diameter, tail length, tail area, % DNA in tail, tail movement, % DNA in head were analyzed for each comet. From the data obtained, mean values and standard deviations were calculated and plotted on a graph.
Results
AMSCs were successfully isolated from the single donor. Medium of the cells was changed every 2-3 days. When the cells reached confluency, they were sub-cultured in 1:3 ratio in other flasks. AMSCs at passage 3 were used for the determination of cytotoxic and genotoxic effects. The cells were spindle shaped and very similar in morphology to bone marrow MSCs. Some dividing cells were round in shape (Fig. 1).
Figure 1.

Morphology of AMSCs. Left: Initial growth of AMSCs in culture, Right: AMSCs at confluency.
Cytotoxic effects of metals
For the cytotoxicity test, when cells were exposed to arsenic for different time intervals, decrease in the growth was observed with an increase in concentration of arsenic. A change in morphology was observed with increase in concentration of arsenic. The cells became round at higher concentrations and did not uptake neutral red as it was done by living cells (Fig. 2). There were more drastic effects on cells when these were exposed to arsenic for longer duration which is clear from the inhibitory concentration 50 (IC50) values. IC50 was 7.9, 4.6, 4.1 and 4.8 μg/ml when cells were exposed to arsenic for 6, 12, 24 and 48h, respectively.
Figure 2.
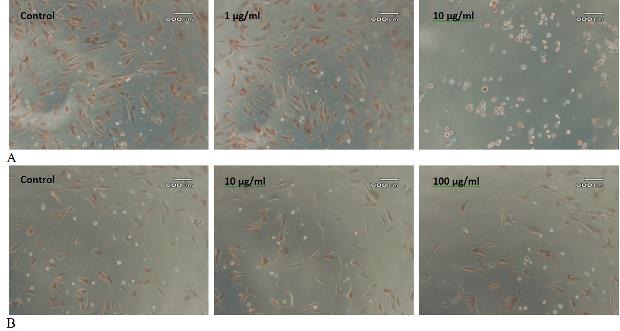
Effect of Arsenic and Lead on AMSCs. A: AMSCs cells were exposed to 1-10 μg/ml arsenic for 24h. The top panel shows control AMSCs (extreme left), cells exposed to 1 μg/ml Arsenic (middle) and 10 μg/ml Arsenic (extreme right). B: AMSCs cells were exposed to 10-100 μg/ml Lead for 24h. The bottom panel shows control AMSCs (extreme left), cells exposed to 10 μg/ml Lead (middle) and 100 μg/ml Lead (extreme right).
In the case of lead, the effect of metal was not as drastic as it was in the case of arsenic. No change in morphology of the cells was observed even at 100 μg/ml concentration and 48h of exposure. There was only 21, 14, 16 and 12 % decrease in growth, when cells were exposed to lead for 6, 12, 24 and 48h, respectively. This was a very minor decrease in growth. When cells were exposed for longer duration, there was even less decrease in the proliferation of cells which is opposite to the response of cells exposed to arsenic (Fig. 3).
Figure 3.
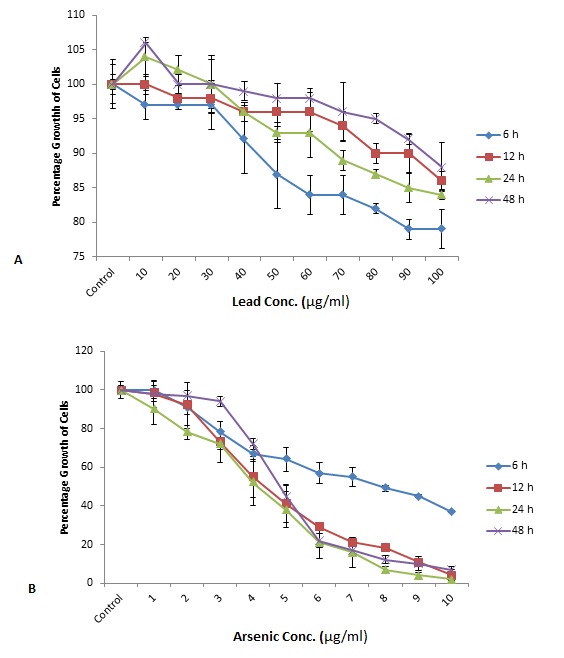
Effect of different concentrations of Arsenic (1-10 μg/ml) and Lead (10-100 μg/ml), exposed for different periods of time viz., 6, 12, 24 and 48h on the percentage growth of AMSCs. AMSCs were more tolerant to lead when exposed for longer duration (48h), whereas there was increase in proliferation when compared to cells exposed for shorter time (6h). Moreover, lead concentration administered for a longer period of time also supported the proliferation of cells. The number of cells after lead treatment was greater than control cells.
Genotoxic Effects of Metals
AMSCs were exposed to arsenic and lead for a specific time interval and comet assay was performed to measure the effect of these metals on the DNA. The morphology of AMSCs nucleus was observed under fluorescent microscope. There was no damage to the nucleus in control cells and they appeared round in shape (Fig. 4). In arsenic and lead treated AMSCs varying degrees of DNA damage was observed; comets were formed with different tail lengths (Fig. 4). The comet length was greater in arsenic treated samples, compared to that of lead treatment (Fig. 5). The score of comet parameters of control and both metal treated samples are given in Table 1, which clearly show the DNA damage in both the types of metal treated cells. The damage in the arsenic treated cells was greater than in the cells kept as control.
Figure 4.
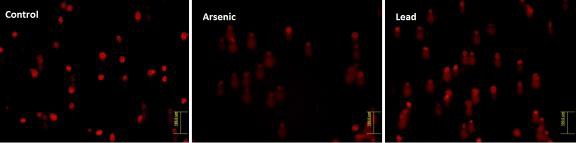
Images of control (extreme left), Arsenic-treated (middle) and Lead-treated (extreme right) MSCs after comet assay. Comet assay slides were stained with ethidium bromide and images were taken under fluorescent microscope.
Figure 5.
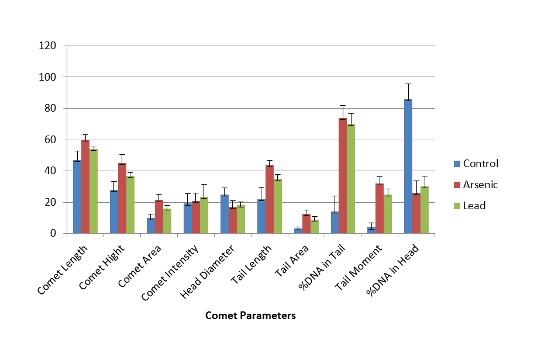
Parameters of comet assay in control (extreme left), Arsenic-treated (middle) and Lead- treated (extreme right) AMSCs. Error bars indicate the standard deviations from the means.
Table 1.
Evaluation of DNA damage by comet assay after arsenic and lead treatment to AMSCs.
| Comet parameters | Control (n=3) | Arsenic-Treated (n=3) | P value | Control (n=3) | Lead-treated (n=3) | P value |
| Comet length | 47 ± 5.6 | 60 ± 3.2 | 0.001 | 47 ± 5.6 | 54 ± 1.5 | 0.001 |
| Comet height | 28 ± 5.2 | 45 ± 5.5 | 0.001 | 28 ± 5.2 | 37 ± 1.8 | 0.001 |
| Comet area | 982 ± 269 | 2134 ± 392 | 0.001 | 982 ± 269 | 1592 ± 205 | 0.001 |
| Comet intensity | 19117± 6363 | 20867± 4996 | 0.001 | 19117± 6363 | 23326± 7953 | 0.001 |
| Head diameter | 25 ± 4.3 | 17 ± 3.8 | 0.001 | 25 ± 4.3 | 18 ± 2.2 | 0.001 |
| Tail length | 22 ± 7.7 | 44 ± 2.9 | 0.001 | 22 ± 7.7 | 35 ± 2.7 | 0.001 |
| Tail area | 318 ± 92 | 1247 ± 239 | 0.001 | 318 ± 92 | 872 ± 218 | 0.001 |
| %DNA in tail | 14 ± 9.8 | 74 ± 7.8 | 0.001 | 14 ± 9.8 | 70 ± 6.6 | 0.001 |
| Tail movement | 3.8 ± 3.1 | 32 ± 4.3 | 0.001 | 3.8 ± 3.1 | 25 ± 3.5 | 0.001 |
| %DNA in head | 86 ± 9.8 | 26 ± 7.8 | 0.001 | 86 ± 9.8 | 30 ± 6.6 | 0.001 |
Discussion
Lot of research work has been done to investigate the effects of arsenic and lead on different cell lines, animal models and humans. After the discovery of the stem cells, several reports appeared on the effect of different compounds on stem cells. Yadav et al. [25] and Sharifi et al. [26] investigated the effects of arsenic and lead on bone marrow MSCs. Since adipose tissue is one of the richest sources of stem cells and potentially can have many therapeutic applications, we started growing these cells and studied the effects of arsenic and lead on AMSCs.
In the present study we successfully isolated AMSCs from the liposuction material. After collagenase digestion, the fat cells float in the medium making it easy to separate them from the AMSCs by centrifugation. AMSCs attach to the plastic surface and grow like bone marrow MSCs. Initially it was hard to obtain pieces of fat tissues and to isolate cells but the procedure has been greatly simplified by liposuction and this procedure also does not greatly alter the viability of the cells [27,28].
However when the liposuction is done with the assistance of ultrasound, the number of recovered AMSCs is reduced [29]. The recovery of AMSCs from adipose tissue is improved by manipulating the centrifugation speed and it is reported that 1200 x g is the optimal speed to isolate AMSCs [30].
When we studied the cytotoxic effects of arsenic, the arsenic caused cell death in a dose and time dependent manner. When cells were exposed for 6h, there was only 63% decrease in proliferation of cells at 10 μg/ml concentration of arsenic, while at 12, 24 and 48h, at the same concentration of arsenic there was almost 100% cell death observed and the results were comparable with these time intervals. It was also observed that with an increase in the concentration of the arsenic from 1 to 10 μg/ml there was a gradual decrease in the proliferation of the cells (Fig. 3A). The cytotoxic effects of lead on AMSCs were different compared to arsenic. There was not much decrease in proliferation of cells even at a higher lead concentration of 100 μg/ml and as opposite to arsenic there was less decrease in the proliferation of cells when cells were exposed to lead for longer durations (48h).
In addition it was also observed that at a low concentration, lead stimulates the proliferation of cells and cell proliferation rate was even better than that of the control (Fig. 3B). According to different studies, lead is toxic to cells and causes cell death in a dose and time dependent manner and IC50 was achieved at 49.0 ± 18.0, 37.5 ± 9.2 and 3.5 ±0.7 μg/mL upon 24, 48 and 72h of exposure in case of HepG2 cells [31]. In another study lead has been shown to be highly toxic for HepG2 cells and also to immune cells, where it alters the morphology of cells and also inhibits the adherence [32, 33]. In our study no such results were obtained and lead proved to be less toxic for AMSCs and IC50 could not be achieved even at 100 μg/ml concentration of lead after 48 h of exposure. There is a need to further investigate as to why lead did not show much toxicity for AMSCs. The increase in proliferation of cells at low lead concentration also seems to be unusual. Tchounwou et al. (2004) observed the same phenomenon while working on HepG2 cells. Another study also supports these results in which low concentration of lead increased the proliferation of Th2 cells [34].
According to different reports arsenic impairs important biological functions such as angiogenesis, differentiation, apoptosis and proliferation of cells [35, 36]. When arsenic enters the cells it mainly affects the mitochondria and changes the mitochondrial trans-membrane potential. This results in induction of reactive oxygen species which causes damage to vital biomolecules [37, 38]. The possible mechanism of arsenic induced apoptosis in cells is due to change in trans-membrane potential of mitochondria and production of reactive oxygen species [39].
In the present study we tested the genotoxic effects of arsenic and lead by single cell gel electrophoresis assay or comet assay. DNA damage was induced by both arsenic and lead which was observed by formation of comet due to fragmentation of DNA. The DNA was observed after staining with ethidium bromide under the fluorescence microscope. There was no DNA damage in the control cells (un-treated) in which the nucleus remained intact and no comet was formed but comet were formed in both the types of metal treated cells. This proves that both arsenic and lead have genotoxic effect on AMSCs. More DNA damage was observed in the arsenic treated AMSCs as there was increase in length of comet, comet tail, percentage of DNA in tail etc. (Table 1).
As opposed to cytotoxic effect, the result of genotoxic effects were almost comparable in both arsenic and lead treated cells.
After ingestion, arsenic is readily absorbed by the digestive system and then it circulates in the blood and reaches various tissues and organs of the body. In the tissues and organs it causes damage at a cellular level. Arsenic is considered to be a genotoxic heavy metal. According to studies it causes damage to DNA; not directly but as a co-mutagen [40, 41]. It mainly causes DNA damage by inhibiting DNA repair machinery that results in different alterations in the genome [42]. In addition it also interacts directly with cell cycle regulatory proteins and causes arrest in cell cycle and result in apoptosis [29, 43]. Arsenic not only causes DNA damage in in vitro conditions but also in vivo [44]. Lead has the tendency to share electron, this way it can form covalent attachment with macromolecules [45]. Lead is also reported to deplete cellular glutathione level due to its avidity for sulfhydryl groups. This results in an increase in the level of lipid peroxidation which accelerates lipofuscinogenesis, this leads to DNA damage [46]. Some studies report lead as a weak genotoxic agent. The possible mechanism of genotoxicity could be oxidative stress in the exposed tissues and organs [47, 48]. In addition it is also reported that lead causes impairment in the DNA synthesis and also cause chromosomal aberrations [49, 50].
In the present study, the results demonstrate that arsenic have strong effects on proliferation of cells and results in the change in cell morphology and causes cellular death in AMSCs. The effect of lead is not as severe as those of arsenic. There was gradual decrease in growth of cells with an increase in arsenic concentration and the time of exposure. Contrary to the effect of arsenic there was increase in the proliferation of cells at longer exposure and lower concentration of lead also increased the proliferation of cells. The morphology of cells changed at higher arsenic concentration; there was shrinkage in size of cells as they became round in shape and also increased cell death. The results of comet assay proved that both arsenic and lead have genotoxic effects on AMSCs and both cause DNA damage. More DNA damage was reported in arsenic treated cells. The results indicated that arsenic and lead are toxic heavy metals and they could be a great health hazard in the future if proper measures are not taken for their use and disposal.
Abbreviations
- AMSCs:
Adipose derived Mesenchymal Stem Cells
- DMEM:
Dulbecco’s modified Eagle’s Minimal Essential Medium
- DMSO:
Dimethylsulfoxide
- MSC:
Mesenchymal stem cells
References
- 1.Gimble JM.Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003; 3(5):705–13 [DOI] [PubMed] [Google Scholar]
- 2.Wei G, Schubiger G, Harder F, Muller AM.Stem cells plasticity in mammals and transdetermination in Drosophila: common theme? Stem Cells. 2000; 18(6):409–14 [DOI] [PubMed] [Google Scholar]
- 3.Woodbury D, Reynolds K, Black IB.Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci. 2002; 69(6):908–17 [DOI] [PubMed] [Google Scholar]
- 4.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal Ginard BM, Silvestri F, Leri A, Beltrami CA.Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001; 344(23):1750–7 [DOI] [PubMed] [Google Scholar]
- 5.Kotton DN, Fine A.Derivation of lung epithelium from bone marrow cells. Cytotherapy 2003; 5(2):169–73 [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR.Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411):143–7 [DOI] [PubMed] [Google Scholar]
- 7.Prockop DJ.Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276(5309):71–4 [DOI] [PubMed] [Google Scholar]
- 8.Terskikh AV, Easterday MC, Li L, Hood L, Kornblum HI, Geschwind DH, Weissman IL.From hematopoiesis to neuropoiesis: evidence of overlapping genetic programs. Proc Natl Acad Sci U S A. 2001; 98(14):7934–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray GA.Medical consequences of obesity. J Clin Endocrinol Metab. 2004; 89(6):2583–9 [DOI] [PubMed] [Google Scholar]
- 10.Katz AJ, Llull R, Hedrick MH, Futrell JW.Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999; 26(4):587–603, viii [PubMed] [Google Scholar]
- 11.WHO Arsenic and arsenic compound. In: Environmental Health Criteria, vol. 224, 2001World Health Organization, Geneva. [Google Scholar]
- 12.IARC Some drinking water disinfectants and contaminants, including arsenic. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans, vol. 84 International Agency for Research on Cancer, Lyon, France, 2004, pp. 209–214 [Google Scholar]
- 13.Yoshida T, Yamauchi H, Fan Sun G.Chronic health effects in people exposed to arsenic via the drinking water: dose–response relationships in review. Toxicol Appl Pharmacol. 2004; 198(3):243–52 [DOI] [PubMed] [Google Scholar]
- 14.Waalkes MP, Liu J, Ward JM, Diwan BA.Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology 2004; 198(1-3):31–8 [DOI] [PubMed] [Google Scholar]
- 15.Waalkes MP, Liu J, Ward JM, Powell DA, Diwan BA.Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer Res. 2006; 66 (3): 1337–45 [DOI] [PubMed] [Google Scholar]
- 16.Andrew AS, Karagas MR, Hamilton JW.Decreased DNA repair gene expression among individuals exposed to arsenic in United States drinking water. Int J Cancer. 2003; 104(3):263–8 [DOI] [PubMed] [Google Scholar]
- 17.Andrew AS, Burgess JL, Meza MM, Demidenko E, Waugh MG, Hamilton JW, Karagas MR.Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ Health Perspect. 2006; 114(8):1193–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson R, Vega L, Trouba K, Bortner C, Germolec D.Arsenic-induced alterations in the contact hypersensitivity response in Balb/c mice. Toxicol Appl Pharmacol. 2004; 198(3): 434–43 [DOI] [PubMed] [Google Scholar]
- 19.Sakurai T, Ohta T, Tomita N, Kojima C, Hariya Y, Mizukami A, Fujiwara K.Evaluation of immunotoxic and immunodisruptive effects of inorganic arsenite on human monocytes/macrophages. Int Immunopharmacol. 2006; 6 (2): 304–315 [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Xi S, Li X, Lu C, Li G, Xu Y, Qu C, Niu Y, Sun G.Arsenic speciation transported through the placenta from mother mice to their newborn pups. Environ. Res. 2006; 101 (3): 349–355 [DOI] [PubMed] [Google Scholar]
- 21.Waalkes MP, Liu J, Diwan BA.Transplacental arsenic carcinogenesis in mice. Toxicol. Appl. Pharmacol. 2007; 222 (3): 271–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ATSDR, Agency for Toxic Substances and Disease Registry. Toxicological profile for Lead. U.S. Department of Health and Human Services, Atlanta, GA, 2007 [Google Scholar]
- 23.Patrick L.Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev. 2006; 11(1):2–22 [PubMed] [Google Scholar]
- 24.Schmidt CW.Poisoning young minds. Environ Health Perspect. 1999; 107(6):A302-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav S, Shi Y, Wang F, Wang H.Arsenite induces apoptosis in human mesenchymal stem cells by altering Bcl-2 family proteins and by activating intrinsic pathway. Toxicol Appl Pharmacol. 2010; 244(3): 263–72 [DOI] [PubMed] [Google Scholar]
- 26.Sharifi AM, Ghazanfari R, Tekiyehmaroof N, Sharifi MA.Investigating the effect of lead acetate on rat bone marrow-derived mesenchymal stem cells toxicity: role of apoptosis. Toxicol Mech Methods. 2011; 21(3): 225–30 [DOI] [PubMed] [Google Scholar]
- 27.Moore JH, Kolaczynski JW, Morales LM, Considine RV, Pietrzkowski Z, Noto PF, Caro JF.Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaine. Aesthetic Plast Surg. 1995; 19(4):335–9 [DOI] [PubMed] [Google Scholar]
- 28.Lalikos JF, Li YQ, Roth TP, Doyle JW, Matory WE, Lawrence WT.Biochemical assessment of cellular damage after adipocyte harvest. J Surg Res. 1997; 70(1):95–100 [DOI] [PubMed] [Google Scholar]
- 29.Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C, Rousselot P, Robledo, Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, Ritt MJ, van Milligen FJ.Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006; 8(2):166–77 [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K.Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006; 208(1):64–76 [DOI] [PubMed] [Google Scholar]
- 31.Tchounwou PB, Yedjou CG, Foxx DN, Ishaque AB, Shen E.Lead-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG2) cells. Mol Cell Biochem. 2004; 255(1-2):161–70 [DOI] [PubMed] [Google Scholar]
- 32.Tully DB, Collins BJ, Overstreet JD, Smith CS, Dinse GE, Mumtaz MM, Chapin RE.Effects of arsenic, cadmium, chromium and lead on gene expression regulated by a battery of 13 different promoters in recombinant HepG2 cells. Toxicol Appl Pharmacol. 2000; 168(2):79–90 [DOI] [PubMed] [Google Scholar]
- 33.Sengupta M, Bishayi B.Effect of lead and arsenic on murine macrophage response. Drug Chem Toxicol. 2002; 25(4):459–72 [DOI] [PubMed] [Google Scholar]
- 34.Heo Y, Parsons PJ, Lawrence DA.Lead differentially modifies cytokine production in vitro and in vivo. Toxicol Appl Pharmacol. 1996; 138(1):149–57 [DOI] [PubMed] [Google Scholar]
- 35.Han YH, Kim SZ, Kim SH, Park WH.Arsenic trioxide inhibits growth of As4.1 uxtaglomerular cells via cell cycle arrest and caspase-independent apoptosis. Am J Physiol Renal Physiol. 2007; 293(2):F511–20. [DOI] [PubMed] [Google Scholar]
- 36.Hyun Park W, Hee Cho Y, Won Jung C, Oh Park J, Kim K, HyuckIm Y, Lee MH, Ki Kang W, Park K.Arsenic trioxide inhibits the growth of A498 renal cell carcinoma cells via cell cycle arrest or apoptosis. Biochem Biophys Res Commun. 2003; 300(1):230–5 [DOI] [PubMed] [Google Scholar]
- 37.Haga N, Fujita N, Tsuruo T.Involvement of mitochondrial aggregation in arsenic trioxide (As2O3)-induced apoptosis in human glioblastoma cells. Cancer Sci. 2005; 96(11):825–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park WH, Jung CW, Park JO, Kim K, Kim WS, Im YH, Lee MH, Kang WK, Park K.Trichostatin inhibits the growth of ACHN renal cell carcinoma cells via cell cycle arrest in association with p27, or apoptosis. Int J Oncol. 2003; 22(5):1129–34. [PubMed] [Google Scholar]
- 39.Kim HR, Kim EJ, Yang SH, Jeong ET, Park C, Kim SJ, Youn MJ, So HS, Park R.Combination treatment with arsenic trioxide and sulindac augments their apoptotic potential in lung cancer cells through activation of caspase cascade and mitochondrial dysfunction. Int J Oncol. 2006; 28(6):1401–8 [PubMed] [Google Scholar]
- 40.Lee TC, Lee KC, Tzeng YJ, Huang RY, Jan KY.Sodium arsenite potentiates the clastogenicity and mutagenicity ofDNA crosslinking agents. Environ Mutagen. 1986; 8(1):119–28 [DOI] [PubMed] [Google Scholar]
- 41.Li JH, Rossman TG.Inhibition of DNA ligase activity by arsenite: a possible mechanism of its comutagenesis. Mol Toxicol. 1989; 2(1):1–9 [PubMed] [Google Scholar]
- 42.Li JH, Rossman TG.Mechanism of comutagenesis of sodium arsenite with N-methyl-N-nitrosourea. Biol Trace Elem Res. 1989; 21:373–81 [DOI] [PubMed] [Google Scholar]
- 43.Li JP, Yang JL.Cyclin B1 proteolysis via p38 MAPK signaling participates in G2 checkpoint elicited by arsenite. J Cell Physiol. 2007; 212(2):481–8 [DOI] [PubMed] [Google Scholar]
- 44.Ostrosky-Wegman P, Gonsebatt ME, Montero R, Vega L, Barba H, Espinosa J, Palao A, Cortinas C, García-Vargas G, del Razo LM, Cebrian M.Lymphocyte proliferation kinetics and genotoxic findings in a pilot study on individuals chronically exposed to arsenic in Mexico. Mutat Res. 1991; 250(1-2):477–82 [DOI] [PubMed] [Google Scholar]
- 45.Zelikoff JT, Li JH, Hartwig A, Wang XW, Costa M, Rossman TG.Genetic toxicology of lead compounds. Carcinogenesis. 1988; 9(10):1727–32 [DOI] [PubMed] [Google Scholar]
- 46.Valverde M, Trejo C, Rojas E.Is the capacity of lead acetate and cadmium chloride to induce genotoxic damage due to direct DNA-Metal interaction? Mutagenesis. 2001; 16(3):265–70 [DOI] [PubMed] [Google Scholar]
- 47.Skoczynska A.Lipid peroxidation as a toxic mode of action for lead and cadmium. Med Pr. 1997; 48(2):197–203 [PubMed] [Google Scholar]
- 48.Valverde M, Fortoul TI, Díaz-Barriga F, Mejía J, del Castillo ER.Genotoxicity induced in CD-1 mice by inhaled lead: differential organ response. Mutagenesis. 2002; 17(1):55–61 [DOI] [PubMed] [Google Scholar]
- 49.Johnson FM.The genetic effect of environmental lead. Mutat Res. 1998; 410(2):123–40 [DOI] [PubMed] [Google Scholar]
- 50.Rojas E, Herrera LA, Pourier LA, Ostrosky-Wegman P.Are metals dietary carcinogens? Mutat Res. 1999; 443(1-2):157–81 [DOI] [PubMed] [Google Scholar]


