Abstract
Immunosenescence is associated to aging and among many changes in immune response is reported a reduced response to vaccination and an increase in the number of cases of autoimmunity, caused by autoantibodies known as natural antibodies whose function, according to reports, would be protection against infection and inflammation. Although immunosenescence is an irreversible process, regular moderate exercise can attenuate some aspects of the decline in the immune system. So, the aim of this study was to investigate the humoral immune response in physically active elderly individuals before and 30 days after vaccination against influenza virus. The results showed that the percentage of individuals positive for antinuclear antibodies and serum immunoglobulin M and G levels after vaccination were higher in the group that exercised regularly than in the sedentary group. We were also able to demonstrate a significant correlation between levels of natural autoantibodies and response to vaccination.
Keywords: Exercise, Influenza virus, Antinuclear autoantibodies, B cells, Elderly people
Abbreviations: IgM, immunoglobulin M; IgG, immunoglobulin G; SRCR, scavenger receptor cysteine-rich receptor; KLH, keyhole-limpet hemocyanin; ANAs, antinuclear autoantibodies; SLE, systemic lupus erythematosus; anti-dsDNA, anti-double stranded DNA antibody; BMI, body mass index
1. Introduction
The aging process is a multifactorial phenomenon characterized by a decline in physiological function [1]. The immune system, which is also affected by aging, undergoes complex changes that affect a range of cells, from hematopoietic stem cells and lymphoid progenitor cells to mature leukocytes in the mucosa or in secondary lymphoid organs [2].
Aging of the immune system, which is known as immunosenescence, is characterized by greater susceptibility to infection and cancer and increased prevalence of positive laboratory tests for autoimmune diseases [3]. These changes in the elderly are the result of alterations in both innate immunity, with a reduction in the chemotactic and phagocytic capacity of neutrophils and macrophages, and adaptive immunity. A decline in the number and function of T cells, reflected in the reduced capacity of B cells to produce high-affinity antibodies, has been described. Furthermore, in vitro, dendritic cells from elderly individuals are less able to phagocytose apoptotic debris than cells from young individuals. The elimination of apoptotic cells is fundamental to ensure normal operation of the immune system and prevent autoimmunity [4].
The response to influenza virus vaccination is an example of the different response to vaccination in the elderly and the young, the latter having been shown to gain greater protection [5]. Interestingly, the same study shows that in the elderly there is a significant increase in the levels of anti-double stranded DNA antibody (anti-dsDNA), a specific type of antinuclear antibody found mainly in individuals with systemic lupus erythematosus (SLE), but without an increase in the prevalence of the disease.
Although immune system dysfunction leads to increased production of autoantibodies, which is sometimes associated with manifestations of autoimmune disease, cancer or the presence of tissue injury, as in chronic infectious diseases, it is increasingly common for these antibodies to be detected in individuals without any apparent pathology [6,7]. Autoantibodies detected in healthy individuals are known as natural antibodies and, because they facilitate the removal of oxidized lipids and proteins as well as apoptotic cells, are associated with protection against infection and the prevention of inflammation [6].
Natural antibodies are immunoglobulins produced in the absence of an antigenic stimulus by a subset of lymphocytes known as B1 or B CD5+ cells, which are highly reactive against autoantigens [6]. They can be classified as B1a or B1b, both of which are characterized by surface markers B220low, IgDlow, IgMhigh, CD19+, CD43+, CD11b+ and CD23low. Subpopulation B1a is also characterized by expression of CD5 and CD6, which belong to the scavenger receptor cysteine-rich receptor (SRCR) family. These cells are described mainly in the peritoneal cavity of mice and the umbilical cord in humans [8].
Natural autoantibodies appear to have several crucial functions. They not only act as the first line of defense by binding to a carbon group in the membrane of pathogens and inducing complement activation, but also inhibit activation of the adaptive immune system by binding to apoptotic cells, making it easier for these to be captured by dendritic cells [9]. However, many authors still consider the presence of natural antibodies to be indicative of autoimmune disease [6].
Although immunosenescence is an irreversible process, certain measures can be taken to minimize its effects. Studies have shown that regularly doing moderate physical exercise can attenuate some aspects of the decline in the immune system [3] and even lead to an increase in the number and function of dendritic cells, which are crucial for induction of the adaptive immune response as they are the main antigen-presenting cells [6]. The ability of regular moderate exercise to maintain the functions of the immune system in physically active elderly individuals was demonstrated in a study of the response of young and elderly individuals to vaccination with KLH (keyhole-limpet hemocyanin), an antigen to which people are not normally exposed. As expected, the results showed that the response in young people is stronger, but that physically active elderly individuals have a better response than their sedentary counterparts in terms of production of both IgM and IgG1 [10].
Vaccination is very effective in preventing flu caused by the various types of influenza virus as well as its complications, and when the composition of the vaccine coincides with the circulating strain of the virus its effectiveness in healthy adults is between 70% and 90%, but falls to between 30% and 50% in individuals over 60 years of age [11–13]. In terms of the neutralizing antibodies (IgG and IgM) produced after influenza virus vaccination, there is a clear reduction in the response in the elderly as, in addition to being produced in smaller quantities, these antibodies have a shorter half-life and reduced antigen affinity [2].
To investigate the effect of moderate exercise training in the immune response of elderly people, we measured serum concentrations of anti-influenza virus antibodies (IgM and IgG) and serum titers of antinuclear autoantibodies (ANAs), when present, in physically active or sedentary individuals, as well as the relationship between the levels of these antibodies.
2. Material and methods
2.1. Subjects and study design
The study population consisted of 110 elderly people aged between 60 and 85 years (mean age 70.29 ± 6.89 years) living in the city of São Paulo. All the individuals were aware of the possible risks involved in the study, having given their agreement to the study protocol and signed a consent form. Both the study protocol and consent form had been approved by the UNIFESP-EPM Ethics Committee. The volunteers recruited for the study kept to their usual routine and were divided into two groups: sedentary (SE, n= 55) and physically active (PA, n= 55). The gender of all sedentary people matched to the exercised individuals. The individuals of physical activity (PA) group were recruited from a physical activity program provided by the city hall of the city of São Paulo. The sedentary group was recruited between participants of an ongoing study performed by the Department of Preventive Medicine of Federal University of São Paulo. All volunteers received the same sample of influenza virus vaccine. Laboratory tests and examination by physicians showed that none of the participants had liver, renal, cognitive, neoplastic or autoimmune diseases. The physical characteristics of the two groups are shown in Table 1.
Table 1.
Physical data (mean ± SD) and number of antinuclear autoantibody (ANA)-positive individuals.
| Variables | Sedentary | Physically active |
|---|---|---|
| Number (n) | 55 | 55 |
| Gender | ||
| Women (n) | 36 | 38 |
| Men (n) | 9 | 7 |
| Sex ratio (w/m) | 4.0 | 5.4 |
| Age (year) | 70.7 ± 7.1 | 69.6 ± 6.8 |
| Weight (kg) | 73.6 ± 13.9 | 60.4 ± 9.7* |
| Height (cm) | 158.7 ± 8.3 | 154.2 ± 7.1 |
| BMI (kg/m2) | 29.6 ± 4.9 | 25.4 ± 3.5* |
| ANAs | ||
| Positive (P) | 15 | 23 |
| Negative (N) | 40 | 32 |
p < 0.05.
2.2. Collection of the samples
Blood samples were collected from a peripheral vein before and 30 days after vaccination and placed in appropriate tubes for serum separation. After the blood had clotted, the tubes were centrifuged at 2500 rpm for 10 min to obtain 500 μL of serum, which was stored at –80 °C for later use to measure the levels of IgM and IgG produced in response to vaccination and to detect the presence of ANAs.
2.3. Detection of antinuclear autoantibodies (ANAs)
ANAs were detected by indirect immunofluorescence assay with a commercial kit (ANA/Hep-2 IgG, Hemagen—Virgo® Products Division, Columbia, USA) using human epithelial cell (HEp-2) substrate slides. Washing and addition of FITC-conjugate were carried out in an automated system (AP16 IF PLUS, Hemagen—Virgo® Products Division, Columbia, USA) using diluted sera. In accordance with the manufacturer's instructions, fluorescence patterns were defined as speckled cytoplasmic and homogeneous nuclear. ANA patterns for each sample were recorded using a fluorescence microscope (Axioskop 40, Carl Zeiss, Oberkochen, Germany) at 40× magnification. Double-blind readings were taken, and specimens were considered reactive when titers were 1:80 or higher.
2.4. Measurement of influenza-specific IgM and IgG reactivity
Influenza-specific IgM and IgG reactivity in serum were measured by ELISA using the previously stored sera. Influenza virus vaccine diluted (0.18 μg/mL) in 0.1 M carbonate-bicarbonate buffer (pH 9.6) was used to coat the high binding microtiter plates (Corning Costar, Corning, NY) overnight at 4 °C, as described by Kohut et al. [14] The plates were then blocked with 5% non-fat milk in phosphate-buffered saline (PBS) for 2 h at 37 °C and washed three times with PBS + 0.1% Tween 20 (PBS-T). The sera were diluted 1:4000 and 1:10000 in PBS-T containing 0.25% gelatin (Sigma, St Louis, MO) (PBS-T-G) for IgM and IgG measurements, respectively, and 100 μL was incubated for 2 h at 37 °C. The plates were again washed three times with PBS-T, and 100 μL of horseradish peroxidase-conjugated goat anti-human IgM or IgG (Sigma, St Louis, MO) diluted 1:1000 in PBS-T-G was added to each well for 1 h at 37 °C. After three washes with PBS-T, 100 μL of substrate [5.5 mg of o-phenylenediamine (OPD) in 10 mL of citrate-phosphate buffer pH 4.5 plus 10 μL of 30% H2O2] was added to the wells and the plates were kept in the dark for 10 min. The reaction was stopped by adding 50 μL of 4 N H2SO4. Absorbance was read at 492 nm on a microplate reader (Labsystems Multiskan MS Plate Reader).
2.5. Exercise program
The individuals in the PA group did regular physical activity (1 h exercise sessions 4 times a week) for at least 12 months. During the study all the volunteers were supervised and conducted by an experienced instructor. The training program consisted of aerobic and resistance exercises performed in a moderate intensity. The aerobic exercises were performed between 60% and 70% of VO2max, involving at least 30 min of exercises in step, jump, coordination and rhythmic movements (sometimes dance). All the aerobic exercises performed were low impact exercises. Resistance training involved 5–10 different exercises for each muscle group (upper and lower extremity muscles, abdomen, gluteus and muscles related to postural stabilization, including dorsal and lumbar muscles). The exercises were performed slowly in two series with 10–20 repetitions each for 30 min per day, between 50% and 60% of 1 RM (repetition maximum). Resistance training was performed on 2–3 days each week and involved exercising different combinations of two muscle groups (described above) in four consecutive sessions. Functional circuit training was also included to improve aerobic capacity and muscle strength and was applied as an alternative to aerobic and resistance training.
The individuals in the SE group maintained their normal routine during the study.
2.6. Statistical analysis
Age (years), height (cm), weight (kg) and body mass index (BMI) (kg/m2) data are shown as mean and standard deviation. Serum IgM and IgG data are shown as median with the respective quartiles. Student's t-test was used to analyze differences in age, height, weight and body mass index (BMI). The Mann–Whitney test was used to determine whether the differences between pre-vaccination values (PRE) and 30 days post-vaccination values (POST) were significant in either group. The Kruskal–Wallis test with the Müller–Dunn post-test was used to identify significant differences between the two groups (SE x PA). The Chi-square test was used to determine whether the difference in the number of ANA-positive individuals between the two groups was significant. Spearman's rank correlation coefficient was used to identify a correlation between ANA and immunoglobulin levels. The significance level was set to 5% (p < 0.05).
3. Results
3.1. Presence of ANAs in the SE and PA groups
As shown in Table 1, although the number of ANA-positive individuals in the PA group was higher (41.81%) than in the SE group (27.27%), this difference was not statistically significant (p= 0.109). The members of both groups were then subdivided into positive (P) or negative (N) according to whether they were ANA positive or negative. The results for fluorescence patterns were as follows: fine nuclear speckled pattern—92.11% of the study population; speckled cytoplasmic pattern—5.26%; and homogeneous nuclear pattern—2.63%. There was a statistically significant difference in BMI between the two groups, but this was a result of the lower weight of the physically active population. Regarding to nutritional state of volunteers, all the participants were examined before being included in each program and none showed inadequate nutritional state. Besides, the nutritional habits of volunteers were not altered during the study.
3.2. Differences in specific antibody responses in the SE and PA groups
There was no statistically significant difference in IgM and IgG concentrations between the SE and PA groups before the vaccination (Fig. 1A and B). Post-vaccination antibody levels increased significantly in PA group (Fig. 1A and B) and the increase in IgM and IgG levels being statistically greater in the PA group than in the SE group (Fig. 1A and B).
Fig. 1.
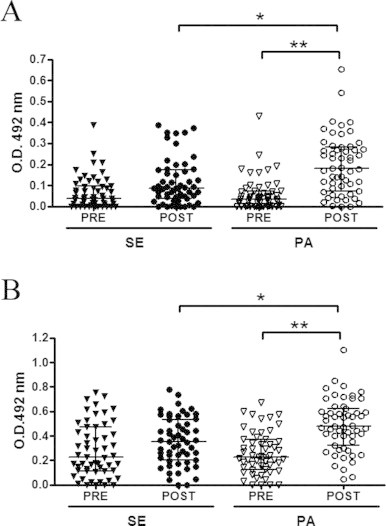
Influenza-specific IgM (A) and IgG (B) reactivity in elderly people in the sedentary (SE) and physically active (PA) groups were measured on two different occasions: before (PRE) and 30 days after (POST) influenza virus vaccination. Significance level: *p < 0.05 and **p < 0.001.
In a gender evaluation was observed that in the comparison of specific antibody response to influenza virus vaccination, the women of physical activity group showed higher levels of IgM (Fig. 2A) and IgG (Fig. 2B) post-vaccination not only in relation to values before vaccine, but also to the levels observed post-vaccination in the women of sedentary group. The specific antibody response to influenza virus vaccination of men showed that the levels of IgM (Fig. 2C) and IgG (Fig. 2D) in the physical activity group post-vaccination were significantly higher than the values before vaccine.
Fig. 2.
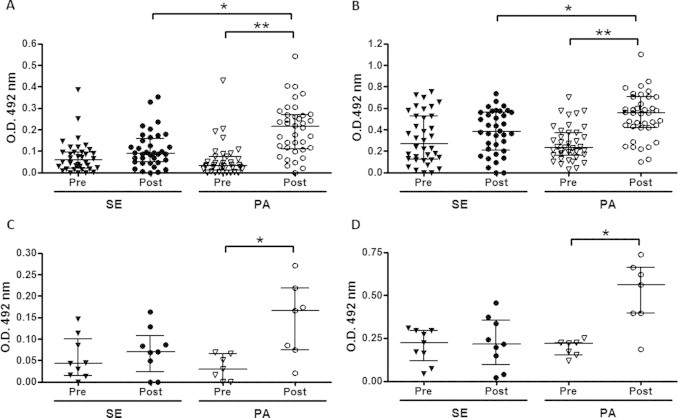
Influenza-specific IgM and IgG reactivity in women (A and B, respectively) and men (C and D, respectively) of sedentary (SE) and physically active (PA) groups were measured on two different occasions: before (PRE) and 30 days after (POST) influenza virus vaccination. Significance level: *p < 0.05 and **p < 0.001.
As at least 27% of the sedentary individuals and 42% of the physically active population in our study were ANA-positive, we divided the SE and PA groups into two subgroups (negative-N and positive-P) and investigated the relationship between this percentage and IgM and IgG levels. As shown in Fig. 3A and B, before vaccination there was no difference in IgM and IgG levels between the subgroups. After vaccination, however, IgM levels in the N subgroup of the SE group were significantly different from pre-vaccination levels (Fig. 3A). In contrast, there was a significant difference in pre- and post-vaccination immunoglobulin levels in both the N and P subgroups of the PA group (Figs. 3A and B). Serum levels of IgM and IgG were significantly higher in the P subgroup of the PA population than in the P subgroup of the SE group (Fig. 3A and B).
Fig. 3.
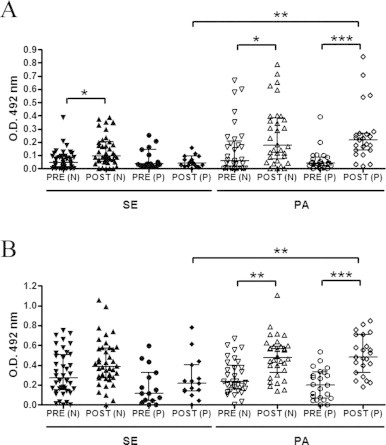
Influenza-specific IgM (A) and IgG (B) reactivity were measured in elderly individuals in the sedentary (SE) and physically active (PA) groups, each divided into negative (N) and positive (P) subgroups, on two different occasions: before and 30 days after influenza virus vaccination. Significance level: *p < 0.05, **p < 0.01 and ***p < 0.001.
3.3. Correlation between pre- and post-vaccination antibody responses as measured by ANA in the SE and PA groups
Spearman's rank correlation coefficient did not show a significant correlation between ANA titers and the ratio of pre- to post-vaccination antibody levels [IgM (r = −0.19, p > 0.05)—Fig. 4A and IgG (r = −0.31, p > 0.05)—Fig. 4B] in individuals in the SE group. However, the same analysis in the PA group revealed a significant correlation for both IgM [r = 0.42, p < 0.05 (Fig. 5A)] and IgG [r = 0.44, p < 0.05 (Fig. 5B)].
Fig. 4.
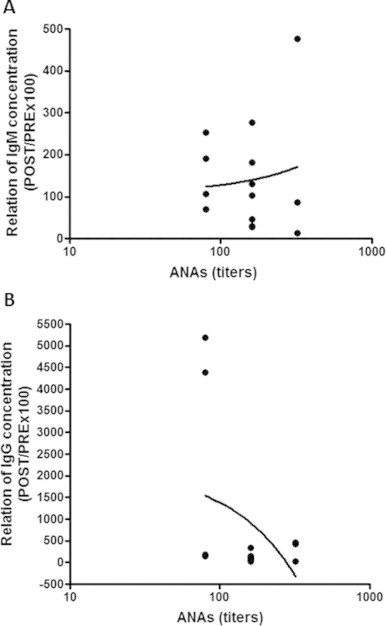
No correlation between ANA titers (expressed in log10) and the ratio of post- to pre-vaccination influenza-specific IgM (A) and IgG (B) reactivity (POST/PRE x 100) in the sedentary individuals. Significance level: *p < 0.05.
Fig. 5.
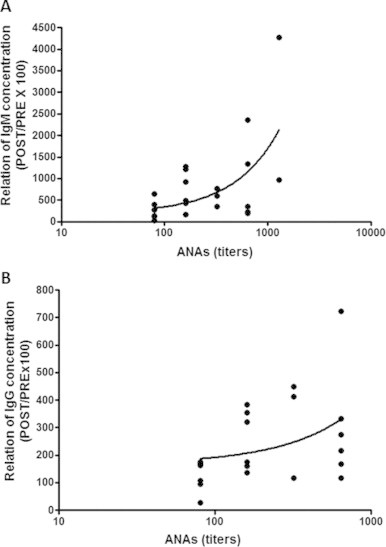
Significant correlation between ANA titers (expressed in log 10) and the ratio of post- to pre-vaccination influenza-specific IgM (A) and IgG (B) reactivity (POST/PRE x 100) in physically active individuals. Significance level: *p < 0.05.
4. Discussion
The process of immunosenescence causes both morphological and functional changes in the immune system [15,16]. One of the main features of immunosenescence in the elderly is the loss of the ability to recognize foreign antigens and the body's own antigens, leading to an increase in autoantibody levels. Other characteristics of immunosenescence include a reduction in naive T-cells, an accumulation of memory cells and a change in cytokine profile from Th1 to Th2 [15].
Despite the elevated levels of autoantibodies in this population, the supposition that the incidence of autoimmune diseases is greater in the elderly remains to be confirmed. Indeed, various studies have reported higher autoantibody levels without any clinical manifestation of autoimmune disease [15,16]. This finding is the subject of considerable debate among researchers and is also evidence that the mechanisms that lead to the development of autoimmune disease are not fully understood.
Various authors have suggested that the higher incidence of autoantibodies in the elderly may be related to their greater exposure to exogenous factors, such as recurrent viral infections or increased consumption of different medications in old age [5,16]. Others suggest that this increase is a consequence of the presence of and increase in the synthesis of natural autoantibodies, a class of immunoglobulins synthesized without any external stimulus that have been suggested to have a protective role [6].
In our study we observed that elderly individuals who exercised regularly had a better response to influenza virus vaccination, a finding that is in agreement with the literature [14]. It has been known for over a decade that regular moderate exercise contributes to improved immune function in the elderly by increasing cell-mediated immunity (T and B lymphocytes as well as NK cells) [17,18] and producing a more favorable cytokine profile [3,19], thus reducing inflammation [20] and improving the response to immunization [14,21,22]. Nevertheless, we failed to find any reference in the literature to the role of natural autoantibodies or a possible relationship between these antibodies in physically active individuals and their response to vaccination. The relationship between the response to influenza virus vaccination and autoantibody levels is a major source of concern among physicians because of the fear that vaccination might increase the risk of autoimmune responses or diseases. Toplak et al. [23] showed that influenza virus vaccination in general not only failed to change the percentage of healthy adults with positive autoantibodies but also increased the levels of autoantibodies in apparently healthy adults by only 15%. In another study, Huang et al. [5] showed that specific antibody response to influenza virus vaccination in older women was lower than that in their young counterparts but that anti-dsDNA antibody response was significantly higher in elderly women than in young women.
ANAs are among the most commonly found non-organ-specific natural autoantibodies in the elderly [16]. Our finding of higher number of ANA positivity in physically active elderly individuals is very important, as none of the participants had an autoimmune disease, neoplasia or any other disease that could explain this finding. An explanation for the increased number of ANA positivity in active group is that it could be associated with an increase in natural autoantibody production, as shown by Elphick et al. [24,25], who demonstrated not only that there were more B1 cells in the peritoneal cavity of mice subjected to physical exercise, but also that serum concentrations of natural antibodies were higher in young adult rats subjected to physical exercise than in sedentary rats. Although it has not been shown that B1 cells could be responsible for the increased production of autoantibodies in elderly individuals who exercise regularly, it is reasonable to suppose that a close relationship exists.
There is not yet sufficient evidence to conclude that these autoantibodies have a protective effect; we can only assert that they do not have a harmful effect. However, the greater prevalence of these antibodies in active elderly individuals raises questions about their role in immunity, in particular in the protection conferred by vaccination. In the physically active group there was a significant increase in the production of immunoglobulins in response to vaccination, mainly in comparison to ANA-positive individuals of sedentary group. Thus, although a causal relationship cannot yet be established in the present study and is currently being investigated by our group, a correlation between the findings of this study can be made. Indeed, improved response to vaccination [26] and increased production of natural antibodies [24,25,27] in response to physical exercise have already been reported in the literature.
Our findings are important because they show that exercise improved the response of two distinct populations of B cells. However, an important question raised by our findings is if there is a synergism between the specific and non-specific antibody responses or if exercises have global influence in the humoral immune response.
In view of the mechanisms responsible for the different behavior of the immune system in young and elderly individuals, it is reasonable to suggest that autoantibody expression may be an important protective factor in the elderly. Further research is required to establish the potential of exercise training for increasing specific and non-specific antibody responses in elderly people.
Acknowledgements
This study was supported by Fundação de Amparo à Pesquisa de São Paulo (FAPESP), São Paulo, Brazil under reference number 2010/50025-1.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS Journal. 2009;276:5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Reviews in Molecular Medicine. 2007;9:1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- 3.Drela N, Kozdron E, Szczypiorski P. Moderate exercise may attenuate some aspects of immunosenescence. BMC Geriatrics. 2004;4:8. doi: 10.1186/1471-2318-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clinicaland Experimental Immunology. 2008;152:448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YP, Gauthey L, Michel M, Loreto M, Paccaud M, Pechere JC, Michel JP. The relationship between influenza vaccine-induced specific antibody responses and vaccine-induced nonspecific autoantibody responses in healthy older women. Journals of Gerontology. 1992;47:M50–M55. doi: 10.1093/geronj/47.2.m50. [DOI] [PubMed] [Google Scholar]
- 6.Lleo A, Invernizzi P, Gao B, Podda M, Gershwin ME. Definition of human autoimmunity–autoantibodies versus autoimmune disease. Autoimmunity Reviews. 2010;9:A259–A266. doi: 10.1016/j.autrev.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Tan EM. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Annals of the New York Academy of Sciences. 1997;815:1–14. doi: 10.1111/j.1749-6632.1997.tb52040.x. [DOI] [PubMed] [Google Scholar]
- 8.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. Journal of Clinical Investigation. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elluru SR, Vani J, Delignat S, Bloch MF, Lacroix-Desmazes S, Kazatchkine MD. Modulation of human dendritic cell maturation and function by natural IgG antibodies. Autoimmunity Reviews. 2008;7:487–490. doi: 10.1016/j.autrev.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. Journal of Applied Physiology. 2004;97:491–498. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- 11.Muszkat M, Friedman G, Schein MH, Naveh P, Greenbaum E, Schlesinger M. Local SIgA response following administration of a novel intranasal inactivated influenza virus vaccine in community residing elderly. Vaccine. 2000;18:1696–1699. doi: 10.1016/s0264-410x(99)00509-5. [DOI] [PubMed] [Google Scholar]
- 12.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. Journal of the American Medical Association. 1994;272:1661–1665. [PubMed] [Google Scholar]
- 13.Kaiser L, Couch RB, Galasso GJ, Glezen WP, Webster RG, Wright PF. First international symposium on influenza and other respiratory viruses: summary and overview: Kapalua, Maui, Hawaii, December 4–6, 1998. Antiviral Research. 1999;42:149–175. doi: 10.1016/S0166-3542(99)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 15.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmunity Reviews. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Casals M, Garcia-Carrasco M, Brito MP, Lopez-Soto A, Font J. Autoimmunity and geriatrics: clinical significance of autoimmune manifestations in the elderly. Lupus. 2003;12:341–355. doi: 10.1191/0961203303lu383ed. [DOI] [PubMed] [Google Scholar]
- 17.Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL. Effects of high- vs moderate-intensity exercise on natural killer cell activity. Medicine and Science in Sports and Exercise. 1993;25:1126–1134. [PubMed] [Google Scholar]
- 18.Shinkai S, Konishi M, Shephard RJ. Aging, exercise, training, and the immune system. Exercise Immunology Review. 1997;3:68–95. [PubMed] [Google Scholar]
- 19.Pedersen BK, Bruunsgaard H, Jensen M, Krzywkowski K, Ostrowski K. Exercise and immune function: effect of ageing and nutrition. Proceedings of the Nutrition Society. 1999;58:733–742. doi: 10.1017/s0029665199000968. [DOI] [PubMed] [Google Scholar]
- 20.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators of Inflammation. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haaland DA, Sabljic TF, Baribeau DA, Mukovozov IM, Hart LE. Is regular exercise a friend or foe of the aging immune system? A systematic review. Clinical Journal of Sport Medicine. 2008;18:539–548. doi: 10.1097/JSM.0b013e3181865eec. [DOI] [PubMed] [Google Scholar]
- 22.Simpson RJ, Guy K. Coupling aging immunity with a sedentary lifestyle: has the damage already been done?—A mini-review. Gerontology. 2010;56:449–458. doi: 10.1159/000270905. [DOI] [PubMed] [Google Scholar]
- 23.Toplak N, Kveder T, Trampus-Bakija A, Subelj V, Cucnik S, Avcin T. Autoimmune response following annual influenza vaccination in 92 apparently healthy adults. Autoimmunity Reviews. 2008;8:134–138. doi: 10.1016/j.autrev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Elphick GF, Greenwood BN, Campisi J, Fleshner M. Increased serum nIgM in voluntarily physically active rats: a potential role for B-1 cells. Journal of Applied Physiology. 2003;94:660–667. doi: 10.1152/japplphysiol.00547.2002. [DOI] [PubMed] [Google Scholar]
- 25.Elphick GF, Wieseler-Frank J, Greenwood BN, Campisi J, Fleshner M. B-1 cell (CD5+/CD11b+) numbers and nIgM levels are elevated in physically active vs. sedentary rats. Journal of Applied Physiology. 2003;95:199–206. doi: 10.1152/japplphysiol.01054.2002. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto Y, Ueki S, Kasai T, Takato J, Shimanuki H, Honda H. Effect of exercise, aging and functional capacity on acute secretory immunoglobulin A response in elderly people over 75 years of age. Geriatrics and Gerontology International. 2009;9:81–88. doi: 10.1111/j.1447-0594.2008.00502.x. [DOI] [PubMed] [Google Scholar]
- 27.Veljkovic M, Dopsaj V, Stringer WW, Sakarellos-Daitsiotis M, Zevgiti S, Veljkovic V. Aerobic exercise training as a potential source of natural antibodies protective against human immunodeficiency virus-1. Scandinavian Journal of Medicine and Science in Sports. 2010;20:469–474. doi: 10.1111/j.1600-0838.2009.00962.x. [DOI] [PubMed] [Google Scholar]


