Abstract
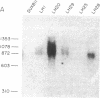
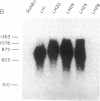
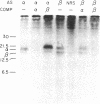
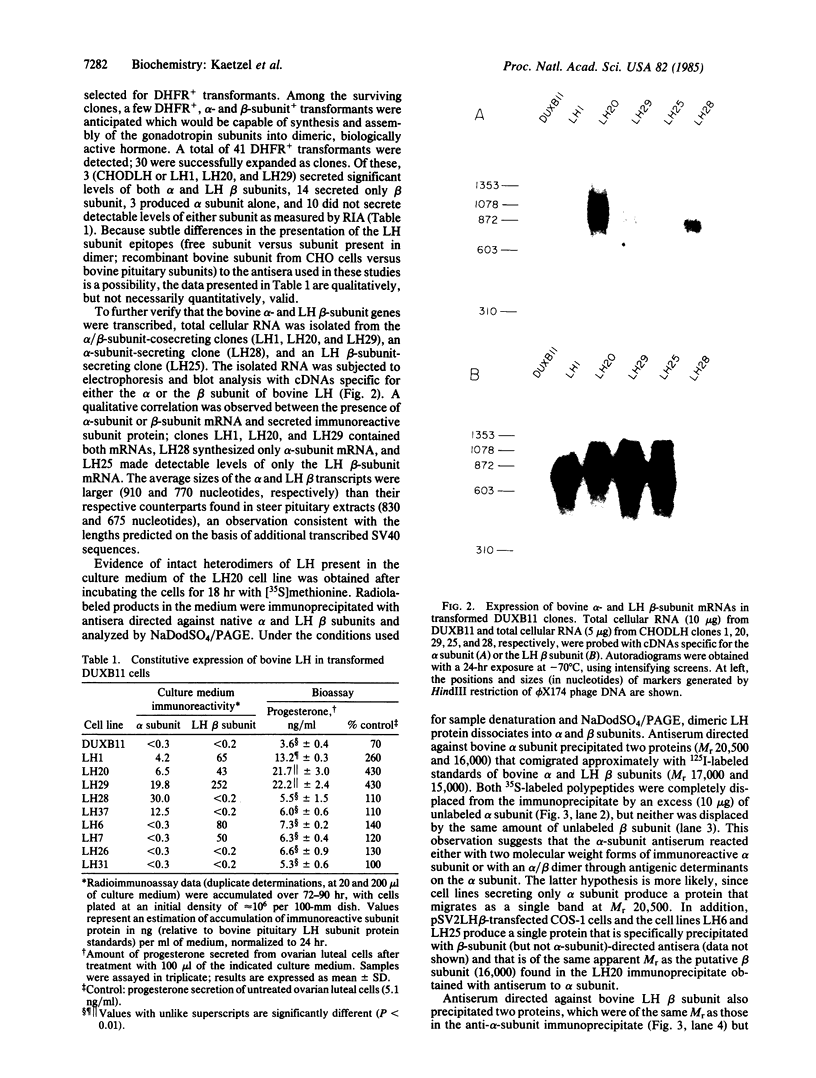
Biologically active bovine luteinizing hormone (LH) has been obtained through expression of the alpha- and LH beta-subunit genes in stably transformed clones of DUXB11, a Chinese hamster ovary cell line deficient in dihydrofolate reductase (DHFR). Expression of alpha-and LH beta-subunit mRNAs of the expected sizes (approximately 910 and 770 nucleotides, respectively) were revealed by blot analysis after electrophoresis of total cellular RNA. Furthermore, presence or absence of the gonadotropin mRNAs in several clonal lines was directly correlated with the appearance of one or both bovine LH subunits in the culture medium. Media from three clones secreting significant immunoreactive levels of both subunits also stimulated the release of progesterone in ovine luteal cells, suggesting that the secreted LH was assembled into a biologically active and glycosylated dimer. Immunoprecipitation and NaDodSO4/PAGE of [35S]methionine-labeled proteins secreted from one of the clones, CHODLH20, further confirmed the presence of an alpha/beta dimer with apparent subunit molecular weights of 20,500 and 16,000, only slightly higher than those of pituitary alpha and LH beta subunits.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke B., Warren G. Microinjection of mRNA coding for an anti-Golgi antibody inhibits intracellular transport of a viral membrane protein. Cell. 1984 Apr;36(4):847–856. doi: 10.1016/0092-8674(84)90034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetherston J., Boime I. Synthesis of bovine lutropin in cell-free lysates containing pituitary microsomes. J Biol Chem. 1982 Jul 25;257(14):8143–8147. [PubMed] [Google Scholar]
- Goodwin R. G., Moncman C. L., Rottman F. M., Nilson J. H. Characterization and nucleotide sequence of the gene for the common alpha subunit of the bovine pituitary glycoprotein hormones. Nucleic Acids Res. 1983 Oct 11;11(19):6873–6882. doi: 10.1093/nar/11.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. M., Parsons T. F., Pierce J. G. Enzymatic deglycosylation of the subunits of chorionic gonadotropin. Effects on formation of tertiary structure and biological activity. J Biol Chem. 1982 Dec 25;257(24):15059–15064. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hoshina H., Boime I. Combination of rat lutropin subunits occurs early in the secretory pathway. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7649–7653. doi: 10.1073/pnas.79.24.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Sairam M. R., Manjunath P. Inhibition of implantation and termination of pregnancy in the rat by a human chorionic gonadotropin antagonist. Endocrinology. 1983 Jul;113(1):195–199. doi: 10.1210/endo-113-1-195. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- Keutmann H. T., McIlroy P. J., Bergert E. R., Ryan R. J. Chemically deglycosylated human chorionic gonadotropin subunits: characterization and biological properties. Biochemistry. 1983 Jun 21;22(13):3067–3072. doi: 10.1021/bi00282a007. [DOI] [PubMed] [Google Scholar]
- Lentz S. R., Birken S., Lustbader J., Boime I. Posttranslational modification of the carboxy-terminal region of the beta subunit of human chorionic gonadotropin. Biochemistry. 1984 Oct 23;23(22):5330–5337. doi: 10.1021/bi00317a035. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Oi V. T. Transfer and expression of immunoglobulin genes. Annu Rev Immunol. 1984;2:239–256. doi: 10.1146/annurev.iy.02.040184.001323. [DOI] [PubMed] [Google Scholar]
- Nilson J. H., Nejedlik M. T., Virgin J. B., Crowder M. E., Nett T. M. Expression of alpha subunit and luteinizing hormone beta genes in the ovine anterior pituitary. Estradiol suppresses accumulation of mRNAS for both alpha subunit and luteinizing hormone beta. J Biol Chem. 1983 Oct 25;258(20):12087–12090. [PubMed] [Google Scholar]
- Niswender G. D. Influence of the site of conjugation on the specificity of antibodies to progesterone. Steroids. 1973 Sep;22(3):413–424. doi: 10.1016/0039-128x(73)90104-9. [DOI] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Hawley T., Shulman M. J., Traunecker A., Köhler G., Hozumi N. Functional immunoglobulin M production after transfection of cloned immunoglobulin heavy and light chain genes into lymphoid cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6351–6355. doi: 10.1073/pnas.80.20.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. F., Pierce J. G. Oligosaccharide moieties of glycoprotein hormones: bovine lutropin resists enzymatic deglycosylation because of terminal O-sulfated N-acetylhexosamines. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7089–7093. doi: 10.1073/pnas.77.12.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Ramabhadran T. V., Reitz B. A., Tiemeier D. C. Synthesis and glycosylation of the common alpha subunit of human glycoprotein hormones in mouse cells. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6701–6705. doi: 10.1073/pnas.81.21.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebois R. V., Fishman P. H. Deglycosylated human chorionic gonadotropin. An antagonist to desensitization and down-regulation of the gonadotropin receptor-adenylate cyclase system. J Biol Chem. 1983 Nov 10;258(21):12775–12778. [PubMed] [Google Scholar]
- Reddy V. B., Beck A. K., Garramone A. J., Vellucci V., Lustbader J., Bernstine E. G. Expression of human choriogonadotropin in monkey cells using a single simian virus 40 vector. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3644–3648. doi: 10.1073/pnas.82.11.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons K. R., Caffrey J. L., Phillips J. L., Abel J. H., Jr, Niswender G. D. A simple method for preparing suspensions of luteal cells. Proc Soc Exp Biol Med. 1976 Jul;152(3):366–371. doi: 10.3181/00379727-152-39398. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin J. B., Silver B. J., Thomason A. R., Nilson J. H. The gene for the beta subunit of bovine luteinizing hormone encodes a gonadotropin mRNA with an unusually short 5'-untranslated region. J Biol Chem. 1985 Jun 10;260(11):7072–7077. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]