Abstract
After scald burn-injury, the intestinal immune system responds to maintain immune balance. In this regard CD4+T cells in Gut-Associated Lymphoid Tissues (GALT), like mesenteric lymph nodes (MLN) and Peyer's patches (PP) respond to avoid immune suppression following major injury such as burn. Therefore, we hypothesized that the gut CD4+T cells become dysfunctional and turn the immune homeostasis towards depression of CD4+ T cell-mediated adaptive immune responses. In the current study we show down regulation of mucosal CD4+ T cell proliferation, IL-2 production and cell surface marker expression of mucosal CD4+ T cells moving towards suppressive-type. Acute burn-injury lead to up-regulation of regulatory marker (CD25+), down regulation of adhesion (CD62L, CD11a) and homing receptor (CD49d) expression, and up-regulation of negative co-stimulatory (CTLA-4) molecule. Moreover, CD4+CD25+ T cells of intestinal origin showed resistance to spontaneous as well as induced apoptosis that may contribute to suppression of effector CD4+ T cells. Furthermore, gut CD4+CD25+ T cells obtained from burn-injured animals were able to down-regulate naïve CD4+ T cell proliferation following adoptive transfer of burn-injured CD4+CD25+ T cells into sham control animals, without any significant effect on cell surface activation markers. Together, these data demonstrate that the intestinal CD4+ T cells evolve a strategy to promote suppressive CD4+ T cell effector responses, as evidenced by enhanced CD4+CD25+ T cells, up-regulated CTLA-4 expression, reduced IL-2 production, tendency towards diminished apoptosis of suppressive CD4+ T cells, and thus lose their natural ability to regulate immune homeostasis following acute burn-injury and prevent immune paralysis.
Keywords: Immune homeostasis, Immune suppression, Peyer's patches, Mesentric lymph nodes, CD4+CD25+ T cells, Apoptosis
1. Introduction
Burn-injury leads to immunosuppression [1]. This deficit in host immune defenses is attributed to T cell immune suppression, including depletion of T cells [2], decrease in T cell proliferation [3], Th17 cells produced cytokines [4], deficiencies in pro-inflammatory cytokine production [5], induction of T cell anergy [6], dysfunctional dendritic cells [7], nitric oxide production by macrophages [8], TLR4 co-stimulation [9], nuclear factor-kB (NF-kB) and activated protein 1 (AP-1) alterations [10], and Fas-mediated apoptosis [11]. Despite all this published information little is known about the precise role of gut-associated lymphoid tissue CD4+T cells. We have previously shown that T cells and neutrophils both contribute to immune suppression observed following day-3 post-burn [7,12–15]. We have also observed that immunosuppression following burn injury leads to loss of intestinal mucosal barrier thus allowing bacteria to translocate and subsequently appear in intestinal lymph nodes such as mesenteric lymph nodes (MLN) and even circulation, culminating in septicemia and death [15]. Apart from neutrophil associated factors causing intestinal tissue injury and loss of gut barrier function, we also reported a role of CD4+ T cells and antigen presenting cells (APC). The current study examining the role of CD4+ T cells of intestinal origin (GALT) is a progression of our previous published studies [7,12]. In an established scald burn injury model we documented that intestinal CD4+ T cells obtained from mesenteric lymph nodes (MLN) have suppressed IL-2 production and proliferation, accompanied by substantial deletion via apoptosis [12]. The data has started to appear delineating the role of CD4+ T cells in burn-injury as well as other injury conditions [6]. However, all these published studies in burn model so far have looked at CD4+ T cells of splenic and/or draining lymph nodes. Nobody to our knowledge has specifically examined the gut-associated lymphoid tissue (GALT)-CD4+ T cells, specifically originating from Peyer's patches and mesenteric lymph nodes. In the current study we elaborated the functional and phenotypic characterization of GALT-CD4+ T cells, the adoptive transfer of burn-injured-associated CD4+ T cells and their ability to modulate immune response of naïve CD4+ T cells of the recipient animals. Our data showed that acute burn-injury altered the function of GALT-CD4+ T cells, including cell proliferative responses, cell surface marker expression and IL-2 sensitivities. Furthermore, burn-injury also decreased the sensitivity of GALT-CD4+CD25+ T cells to camptothecin-associated, as well as spontaneous apoptosis. Finally, the ability of burn-induced GALT-CD4+CD25+ T cells to transfer their depressive effects to sham rats following adoptive transfer over 1-3 days postburn. We conclude, GALT-derived CD4+ T cells, theoretically known to be of tolerant-type being at the fore front of antigenic challenge, both from commensals and gut antigens; following burn-injury, tend to modulate towards losing their normal regulatory function, and play a role in breach of the normal immune-homeostasis towards an unwanted immune-paralysis.
2. Materials and methods
2.1. Scald burn-injury
Male Sprague Dawley rats, ∼2 months old, weighing 200–250 g (Harlan, Indianapolis, IN) were used in this investigation. All animal housing, experimentation and treatment were based on previously published protocols approved by Chicago State University Animal Care and Use Committee (IACUC) committee and in accordance with NIH guidelines. For adoptive transfer experiments male Lewis rats (250–300 g) were used. All rats were acclimatized in the animal facility for at least one week before being subjected to any treatment and were given free access to water and standard lab rat chow ad libitum. The criteria stated here are based on previous published protocols of the acute burn injury model (see references Fazal et al. [12–15]). As pain therapy, buprenorphine (2 mg/kg) intra-peritoneal were given to rats 30 min prior to burn injury for preemptive analgesia, and every 8 h till ready for sacrifice and/or terminally euthanized. Major burn injury protocols have shown to be associated with full thickness 3rd degree skin burns over ∼30% of the total body surface associated with fairly consistent inflammation-linked immune as well as gut barrier abnormalities. Moreover, almost all rats survive this injury/trauma and mortality rate is <5% over 3-days. Scald burn destroys pain receptors and most rats handle this scald well and are able to move around, eat and drink normally during the three-day post-burn period. Accordingly, upon verification of deep anesthesia with sodium pentobarbital (40–50 mg/kg intra-peritoneal, i.p.), as determined by absence of any signs of awareness including response to hind limb pinch, rats were subjected to the following steps in quick succession: (i) the back fur was shaved off ∼30% Total Body Surface Area (TBSA), (ii) rats were securely placed in an appropriately sized bottomless plastic mold that allows the shaved area of the skin on the back to be immersed in water while the rest of the body is protected, (iii) the back was immersed in a hot water bath (95–97 °C) for 10 s, (iv) excess hot water was immediately wiped off to avoid an additional injury, and (v) 10 ml of normal saline was injected intra-peritoneal for resuscitation. The resuscitation procedure was sufficient to restore control level of urine output in the experimental group of rats over a period of ∼24 h. Therefore, we did not find any need to compensate for the loss of fluids. Moreover, animals start feeding and drinking normally; hence do not require artificial feeding. The experimental animals were divided into two groups and each group had at least six rats; [1] Burn, [2] Sham control, which go through clipping of back hair, anesthesia, immersion in water bath at room temperature, resuscitation with 10 ml normal saline. All the data reported and analyzed in this study was obtained from first two groups. Each groups had minimum of six animals, three separate samples were collected from each animal for analysis. In the subsequent experiments “n” represents the number of either samples or animals in the study as detailed in the legends of the respective figures. This injury protocol is well established, repeatable and extensively used by the peers in the field of acute burn-injury studies.
2.2. Collection of tissue samples and separation of CD4+ T cells
Burn and sham control animals were anesthetized as detailed in earlier methods, abdomen was opened, MLN were located and chain of upper and lower mesenteric lymph nodes were aseptically removed. For isolation of Peyer's patches, ileum was located and aggregation of lymphoid tissue bulging out of outermost layer of intestine (serosa) of small intestine were aseptically removed with scissors and placed in warm culture media. Peyer's patches were removed across the whole length of small intestine. Both MLN and PP tissues were then crushed under a metallic cell dissociation sieve-Tissue Grinder Kit (Sigma-Aldrich) in separate petri dishes. Single-cell separations were prepared by addition of Collagenase-D, repeated spinning, and reconstitution of culture media. The magnetic microbead cell separation method called (MACS) was standardized for bench use as per protocol of Miltenyi Biotech Inc. Cells obtained from GALT, i.e., mesenteric lymph nodes and Peyer's patches were incubated with microbeads and then ran over a column within magnetic field. CD4+ T cells labeled with magnetic beads stuck to the columns and all other cells passed through as negative selection. The columns were removed from magnetic field and the cells were eluted by elution buffer as positive selection. With this method of separation we were able to obtain 99% purified CD4+ T cells. This method to elute CD4+ T cells was found superior to the nylon wool method which yields total T cells, and cell sorting by FACS which is more time consuming and less cost effective.
2.3. Purification of CD4+CD25+ T cells by magnetic cell sorting (MACS)
FITC Multisort kit using a magnetic bead isolation method was used to isolate CD4+CD25+ T cells. This method enabled us to enrich a double positive cell population in two steps, both using elution of positive selection. Total cells obtained from MLN or PP was stained with FITC-CD4+ and PE-CD25+ labeled antibody. In the first step cells were incubated with CD4+ beads and then passed over columns that retained CD4+ labeled cells in a magnetic field. Negative selection was discarded and positive selection was then preserved. The FITC label on positive selection was then cleaved and the reaction stopped enzymatically. The cells were then incubated with PE microbeads and again passed through second set of columns. Positive selection was eluted as CD4+CD25+ T cells. The second step in elution rendered negative selection, i.e., CD4+CD25− T cells. We were able to enrich this rare population to about 70% purity by this method, whereas 60% purity was achieved for CD4+CD25− T cells. The cell surface expression marker was confirmed by flow cytometry by labeling with antibodies for CD4+ and CD25+ cell surface markers.
2.4. Comparison of CD4+CD25+ T cell sorting by MACS and FACS
CD4+CD25+ T cells were sorted out by using two different techniques. Firstly, MACS cell sorting through magnetic beads of used, there was 2% of T cell population among Burn MLN T cells and 3% in Burn PP. When this result was compared with cell sorting through FACS technique, similar results were obtained, i.e., ∼3% CD4+CD25+ T cell population in both burn MLN and PP. Hence both technique yielded similar results. This experiment was conducted to confirm the validity of microbead method in terms of elution of double positive cells. Both methods could efficiently quantify CD4+CD25+ T cells but FACS cell sorting has a drawback that it is time consuming and gives a poor yield of cells.
2.5. IL-2 cytokine measurement (ELISA)
The supernatants were harvested from various CD4+ T cell cultures as mentioned in results and cytokine content of (IL-2) was determined by using enzyme-linked immunosorbent assay (ELISA) kits (Biosource Inc., CA). The values were depicted as pg/ml.
2.6. Mitochondrial morphology assessment
MitoTracker GreenFM (Molecular Probes, Eugene, OR) was used to assess mitochondrial morphology. GALT (MLN and PP) CD4+ T cells were isolated from day 3 post-burn and sham control animals and were incubated in 500 μl modified RPMI1640 for 30 min at 37 °C/5% CO2 with 200 nmol/L MitoTraker GreenFM. Cytospins were prepared on microscopy glass slides (Fisher Scientific, Pittsburgh, PA) in the dark. The slides were air dried and analyzed by a confocal laser scanning microscope (Bio-Rad Laboratories, Hercules, CA).
2.7. Flow cytometry
Flow cytometry available as core facility at Department of Biological Sciences at Chicago State University was used for the determination of T cell type, activation markers, and apoptosis by Annexin-V (Promega). CD4+ and CD4+CD25+ T cells were labeled with respective FITC/PE/APC or CyChrome-tagged rat-specific antibodies at 1–5 μg/ml/106 cells. Isotype control antibodies and unstained cells were used as controls. Cells were fixed in 0.7 ml of 1% paraformaldehyde solution and acquired on a FACScan flow cytometer (Becton Dickinson). The machine is equipped with an argon ion air-cooled laser, which emits a peak line of fluorescence of 488 nm at 15 mW. At least 10,000 events were acquired by using LYSYS II software (Becton Dickinson). Both forward-angle and right-angle light scatter signals were adjusted for optimum results for the detection of T cells. The forward-scatter threshold signal was adjusted to exclude debris and un-lysed erythrocytes. All parameters were optimized on un-stimulated purified T cells that have been loaded with respective antibody.
2.8. CD4+ T cell proliferation assays
CD4+ and/or CD4+CD25+ T cells at different ratios were cultured in 96-well flat-bottomed microtiter plates (Falcon, Lincoln Park, NJ) for 72h/37 °C/5% CO2. Cells were incubated with various activators, i.e., anti-CD3 (1 μg/ml), anti-CD3 (1 μg/ml) plus rIL-2 (1 μg/ml). Tritiated thymidine 1 μCi (37Bq) was then added to each well in the last 16–18 h of culture. Cells were harvested at the end of culture period onto filtermats (Skatron, Sterling, VA) with a semi-automatic PHD cell harvester (Cambridge Technology Inc.). The counts per minute of the filter membrane were measured in scintillation liquid on a Beckman LS 6500 liquid scintillation counter (Fullerton, CA). When T cells were stimulated with anti-CD3, the antibodies were immobilized on the treated tissue culture 96-well plates which were coated with the antibodies (∼10 µg/ml) and incubated at 37 °C for 90 min, prior to cell cultures.
2.9. Adoptive transfer assays
CD4+CD25+ and CD4+CD5− T cells were purified by FACS cell sorter and/or the magnetic bead isolation method from day 3 post-burn Lewis rats and were injected intravenously into sham Lewis rats. The rats were sacrificed day 1 and day 3 post-adoptive transfer. GALT (MLN and PP) were then isolated from these rats, CD4+ T cells were purified as previously described, and CD4+ T cell proliferation by Thymidine incorporation and IL-2 levels in the supernatant by ELISA were determined. The experiments were conducted in Lewis rats that are inbred rats and allow for transfer of cells between MHC matched animals. The recipient rats that were adoptively transferred with CD4+CD25+ T cells from day 3 post-burn rats were also studied for their effect on CD4+ T cell effector functions and their effects on mitochondrial responses of CD4+ T cells.
2.10. Intestinal CD4+ T cells co-culture
T cells from burn and sham rats were co-cultured to study if the effect of burn T cells could modulate sham T cell responses. CD4+CD25+ and CD4+ T cells were isolated by the magnetic bead method from day 3 post-burn and sham MLN and PP of rats and then co-incubated with CD4+ T cells at different effector:responder cell ratios. T cells were stimulated with anti-CD3 (1 μg/ml) and allowed to proliferate 72 h/37°C/5% CO2. Samples were then taken for IL-2 analysis from supernatants of T cells co-cultures, and T cell proliferation assessed by Thymidine incorporation. The effect of CD4+CD25+ T cell on CD4+ T cell was determined in terms of inhibition of proliferation, IL-2 production and/or T cell apoptosis.
3. Results
3.1. Suppression of CD4+ T cell proliferation of MLN and PP following burn injury
CD4+ T cells were isolated from MLN and PP of day 3 post-burn and sham rats by magnetic cell sorting (MACS). T cells were stimulated with anti-CD3 (10 μg/ml) for 72 h and thymidine incorporation (dpm) assessed. These experiments were performed to assess the potential differences in CD4+ T cells proliferation obtained from GALT (Peyer's patches-PP and draining lymph nodes such as Mesenteric lymph nodes-MLN). The results in Fig. 1 showed that CD4+ T cells both obtained from MLN and PP proliferate well with anti-CD3 stimulation in culture. CD4+ T cells obtained from MLN of sham and/or day 3 burn shows higher proliferative indices as compared to PP from MLN and/or PP of sham animals. CD4+ T cell proliferation of MLN and PP obtained from day-3 burn was significantly lower (p < 0.05) as compared to sham MLN and PP. Moreover, CD4+ T cells obtained from Burn PP also showed a significant (p < 0.05) depression in growth as compared to Sham PP. This differential effect was more pronounced in PP CD4+ T cells from burn rats as compared to PP of sham animals.
Fig. 1.
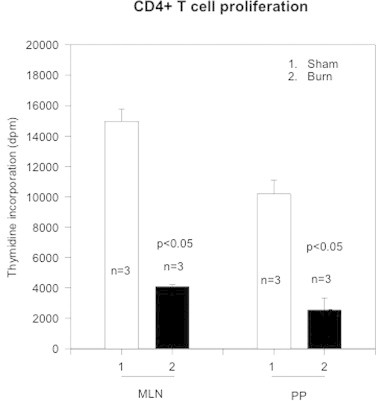
The figure shows CD4+ T cell proliferation as assessed by Thymidine incorporation (dpm). CD4+ T cells were obtained from gut-associated lymphoid tissue (GALT), i.e., mesenteric lymph nodes (MLN) and Peyer's patches (PP) from sham (open bars) and burn (closed bars). The data represents Mean ± SD Thymidine incorporation (dpm) values obtained from sham and day-3 burn rats (n, number of animals = 3). p < 0.05 values show statistical significance.
3.2. Alteration of expression of cell surface markers on GALT-derived CD4+ T cells (Table 1)
Following phenotypic characterization of CD4+ T cells, expression of activation markers was performed in these studies. Enriched CD4+ T cells were obtained from day 3 post-burn and sham rats through MACS separation and T cell activation markers were analyzed by flow cytometry. All phenotype expression studies were performed on un-stimulated CD4+ T cells showing basal or constitutive levels of activation receptor expression.
3.2.1. Cell surface expression of regulatory marker (CD25)
CD25 is the alpha chain of the IL-2 receptor. It is a type I transmembrane protein present on activated T cells. Our results (Table 1) indicate that CD4+ T cells co-express CD25 regulatory marker; sham rats MLN (10%), PP (5.5%) and day 3 post-burn MLN (16%), PP (10%) respectively. These data exhibit that burn injury promotes an upregulation of CD25 regulatory markers, both of MLN and PP origin.
Table 1.
Percentage expression of T cell receptor in Lewis rats.
| T cell receptor | Sham |
Burn |
||
|---|---|---|---|---|
| MLN | PP | MLN | PP | |
| αβ | 88 | 67 | ND | ND |
| γδ | 2 | 4 | 4 | 6 |
| CD25 | 10 | 5.5 | 16 | 10 |
| CD49d | 64 | 32 | 59 | 25 |
| CD11a | 97 | 45 | 96 | 35 |
| CTLA-4 | 3 | 6 | 5 | 9 |
| CD62L | 44 | 17 | 51 | 14 |
3.2.2. Cell surface expression of co-stimulatory molecule (CTLA-4)
CTLA-4 also known as CD152 is a negative regulator of cell-mediated immune responses. Our results (Table 1) showed an up-regulation of surface marker expression of CTLA-4 on CD4+ T cells in sham PP and day 3 post-burn PP. However CTLA-4 expression was very weak in un-stimulated CD4+ T cells.
3.2.3. Cell surface expression of adhesion molecules (CD62L, CD11a)
Also known as L-selectin (LECAM-1) is expressed on lymphocytes that bind to sialylated oligosaccharides determinants on high endothelial venules (HEV) in peripheral lymph nodes. l-selectin is known to be rapidly shed from T cells upon activation. The level of CD62L expression distinguishes naïve T cell from effector/memory T helper cells. The results (Table 1) showed that CD62L expression was markedly down regulated in CD4+ T cells obtained from both MLN and PP of day 3 post-burn and sham rats. Similarly, the results (Table 1) demonstrated that CD11a receptor expression was down regulated both in day 3 post-burn and sham MLN and PP of CD4+ T cells indicating that peripheral lymph nodes mostly host activated cell types. CD11a (LFA), also known as CD11a or Integrin αl chain mediates adhesion through interaction with ICAM 1 and 2. It has also been reported to inhibit T cells infiltration in several in vivo models of inflammation.
3.2.4. Cell surface expression of homing receptor (CD49d)
Also known as Integrin α-4 chain expressed as a heterodimer with either of two β chains, β1 or β7. The α4β1 (VLA-4) integrin is expressed on peripheral T and B cells and monocytes, while α4β7 is expressed on peripheral T cells. This integrin binds to VCAM-1. The interaction between the CD49d antigen and VCAM-1 is known to play an important role in stabilizing the adhesion of lymphocytes to endothelial cells. Results in Table 1 showed a down-regulation of CD49d receptor expression in CD4+ T cells of both MLN and PP obtained from day 3 post-burn and sham rats.
3.3. Burn injury suppresses GALT CD4+CD25+ T cell proliferation and IL-2 release
It is a known fact that the level of expression of adhesion as well as homing receptors is generally considered as an index of activity of a T cell. The naïve T cells possess these markers at high levels, which are later, shed off or down regulated, or inverted after the T cell activation and, when it reaches peripheral lymph node or passes from circulation to tissue. In these experiments CD4+CD25+ T cells were obtained from day 3 post-burn and sham Lewis rats through MACS separation and T cell activation marker receptor was analyzed by flow cytometry. All phenotype expression studies were performed on un-stimulated CD4+CD25+ T cells showing basal or constitutive levels of receptor expression. CD25+ marker is considered to be an index of activated T cell. Based upon our previous experiments where we showed presence of CD25+ marker on T cells of GALT, we decided to explore this subset of T cells. These experiments were performed to find the proliferation of CD4+CD25+ T cells from day-3 burn and sham rats and determine if there were any differential growth or suppressive patterns between MLN and PP. MLN and PP from day 3 post-burn and sham control Lewis rats were enriched for CD4+CD25+ and CD4+CD25− T cells by magnetic cell separation (MACS). Both groups were then stimulated with anti-CD3 (10 μg/ml) for 72 h and thymidine incorporation (dpm) was assessed. The results in Fig. 2 showed that CD4+CD25+ T cell obtained from MLN and/or PP of burn animals showed lower proliferative indices (∼50%) as compared to MLN and/or PP of sham CD4+ T cells (Fig. 2). This significant (p < 0.05) depressive effect was more pronounced in CD4+CD25+ T cells obtained from PP.
Fig. 2.
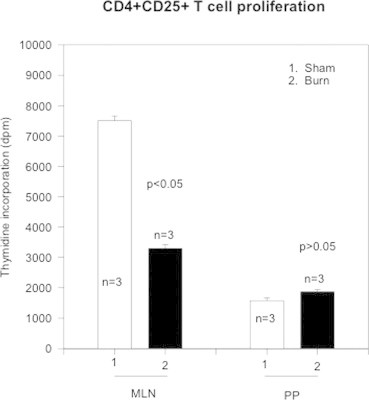
The figure shows CD4+CD25+ T cell proliferation as assessed by Thymidine incorporation (dpm). CD4+CD25+ T cells were obtained from mesenteric lymph nodes (MLN) and Peyer's patches (PP) from sham (open bars) and burn (closed bars). The data represents Mean ± SD values of sham and day-3 burn rats (n, number of animals = 3). Statistical analysis of p < 0.05 shows significance and p > 0.05 shows no significance.
CD4+CD25+ T cells were purified by MACS and cultured with anti-CD3 (10 μg/ml) for 72 h. ELISA determined IL-2 levels produced by CD4+CD25+ T cells. Fig. 3 shows the data obtained from three animals. The representative data of mean ± SD values is shown. The results showed elevated levels of IL-2 (>1100 pg/ml) in MLN CD4+CD25− T cells obtained from sham rats. There was a statistical reduction (p < 0.05) in IL-2 production from CD4+CD25+ T cells obtained from MLN and/or of PP from burn animals. However, no such difference in IL-2 production was noticed in PP of either sham or day 3 post-burn rats. Although, there was a down regulation of IL-2 in CD4+CD25+ expressing T cells whether obtained from sham or burn-injured animals, no obvious difference in IL-2 production was noted in CD4+CD25− T cells, both derived from sham or burn animals. Hundred percent enriched cell population of CD4+CD25+ T cells were obtained through Cell sorting by FACS from both sham and day 3 post-burn rats and their dependency for IL-2 assessed. The results showed that CD4+CD25+ T cells were IL-2 dependent and needed IL-2 in the media for their ex-vivo expansion. There was retardation of growth of CD4+ T cells when there were enriched for CD4+CD25+ T cells in culture. However this effect could be abrogated by addition of IL-2. We used recombinant IL-2 (5 ng/ml) for 3 days to grow enriched CD4+CD25+ T cell population. The difference between this experiment and IL-2 production by CD4+CD25+ T cells was that IL-2 production was determined in cells obtained through MACS and this IL-2 dependency experiment was done on 100% enriched CD4+CD25+ T cells obtained by cell sorting by FACS.
Fig. 3.

Figure shows IL-2 production by CD4+CD25− and CD4+CD25+ T cells following 72 h of incubation. Supernatants were harvested from CD4+ T cell cultures and assessed for IL-2 by ELISA kit. IL-2 (pg/ml) values are depicted on the x-axis. The data represents Mean ± SD (n, number of samples = 3) obtained from sham and day-3 burn MLN (closed bars) and PP (open bars).
3.4. CD4+CD25+ T cells obtained from burn-injured rats inhibited CD4+ T cell proliferation of sham control rats following adoptive transfer
This experiment was performed to find if CD4+CD25+ T cells from day 3 post-burn animals could affect T cells of the sham control Lewis rats. In these experiments CD4+CD25+ and CD4+CD25− T cells from MLN and PP were obtained from day 3 post-burn rats and injected intravenously into sham rats in separate experiments. Cell sorting by flow cytometry was used to get 100% enriched CD4+CD25+ T cells. 106 MLN and PP CD4+CD25+ and/or CD4+CD25− T cells were obtained from day 3 post-burn rats and given intravenously to sham rats. The rats were then sacrificed day 1 and day 3 post adoptive transfer and CD4+T cell proliferation responses of the recipient animals were assessed by Thymidine incorporation. The results (Fig. 4A and B) indicated that when burn CD4+CD25+ T cells were adoptively transferred to sham rats, T cell proliferation of all groups (CD4+, CD4+CD25+ and CD4+CD25− T cells) of sham rat were found to be significantly depressed. T cell proliferation was significantly inhibited in the sham rats that were adoptively transferred with CD+CD25+ T cells from burn rats as compared to those who were infused CD4+CD25− T cells. This effect was repeatedly seen in MLN and PP of sham rats on both day 1 (Fig. 4A) and day 3 (Fig. 4B) post adoptive transfer. However, maximum inhibition of proliferation was found in CD4+CD25+ T cells obtained from PP of sham rats (p < 0.05). Expression of activation markers on recipient CD4+ T cells was also determined. CD4+CD25+ T cells were obtained through MACS separation and the following T cell activation marker receptor expression analyzed and shown in Table 2. The results showed that adoptive transfer of CD4+CD25+ T cells from burn rats did not influence the phenotypic expression of CD4+ T cells of sham rats. The percentage expressions of CD25, LFA, and CD62L remained same before and after adoptive transfer. Moreover, this lack of significant change in surface expression of activation markers did not change over time, i.e., day 1 or day 3 post adoptive transfers.
Fig. 4.
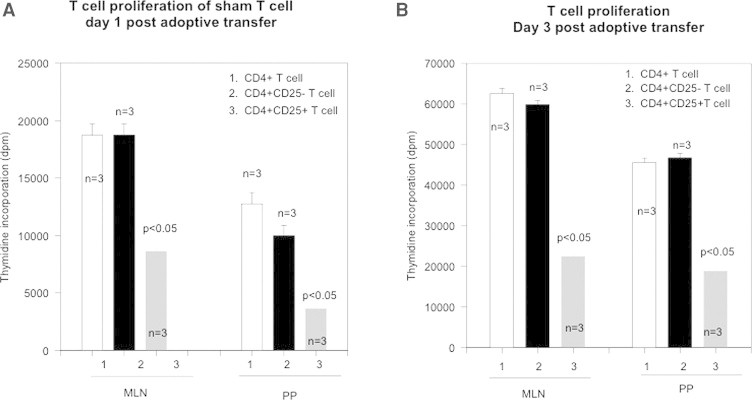
(A) CD4+CD25+ T cells were obtained from day-3 burn Lewis rats and were adoptively transferred into sham Lewis rats. After day-1 post transfer MLN and PP were obtained from sham recipients, T cell sub-types isolated and thymidine incorporation assesses their proliferative responses. Figure shows T cell proliferation of different subsets of T cells, i.e., CD4+, CD4+CD25+ and CD4+CD25− T cells obtained from sham rats. Data represents mean ± SD (n, is the number of animals = 3) and p < 0.05 shows statistically significant values. (B) CD4+CD25+ T cells were obtained from day-3 burn Lewis rats and were adoptively transferred into sham Lewis rats. After day-3 post transfer MLN and PP were obtained from sham recipients, T cell sub-types isolated and thymidine incorporation (dpm) assesses their proliferative responses. Figure shows T cell proliferation of different subsets of T cells, i.e., CD4+, CD4+CD25+ and CD4+CD25− T cells obtained from sham rats. Data represents mean ± SD (n, is the number of animals = 3) and p < 0.05 shows statistically significant value.
Table 2.
Percentage expression of MLN T cell receptor in Lewis rats.
| T cell activation marker receptor | Day postburn |
Adoptive transfer CD25+ |
Adoptive transfer CD25− |
|||
|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | |
| CD25 | ND | 6.61 | 5.50 | 6.23 | 6.38 | 6.53 |
| CD11a | ND | 76 | 75 | 71 | 73 | 73 |
| CD62L | ND | 48 | 62 | 63 | 58 | 64 |
3.5. CD4+CD25+ T cells from burn-injured animals induce apoptosis of naïve CD4+ T cells
In these experiments CD4+CD25+ T cells were enriched by magnetic bead method from day 3 post-burn and sham Lewis rats. The results showed that there was a significant decrease (p < 0.05) in apoptosis of CD4+CD25+ T cells (Fig. 5) when freshly obtained from burn MLN and PP cells and induced with camptothecin as compared to CD4+CD25+ T cells obtained from MLN and PP of sham rats. There was no change in percentage apoptotic cells in freshly isolated CD4+CD25− T cell population (Fig. 6). However when CD4+CD25+ T cells were grown in culture with IL-2 and anti-CD3 (10 μg/ml) for 72 h there was a significant increase in apoptosis of burn MLN and PP as compared to sham T cells (Fig. 7). This effect was also seen irrespective of camptothecin induction. Camptothecin is a known inducer of apoptosis in normal and tumor cell lines. It arrests cell at the G2/M phase by binding irreversibly to the DNA-topoisomerase I complex, inhibiting the re-association of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. Furthermore, experiments were also designed to determine the impact of CD4+CD25+ T cells on apoptosis of CD4+CD25− T cells in co-culture assays. CD4+CD25− T cells were obtained from both day 3 post-burn and sham rats through MACS separation and were co-cultured with CD4+CD25+ T cells for 16 h and then assessed for mitochondrial swelling. Confocal microscopy was performed to assess mitochondrial swelling by the addition of fluorescent probe MitoTracker Green. The results indicated (Fig. 8) that CD4+ T cell form day-3 post-burn exhibited characteristics swelling of mitochondria as compared to sham rat CD4+ T cells. Further studies also showed that CD4+CD25+ T cells obtained from burn rats induced mitochondrial swelling of CD4+CD25− T cells of sham rats when grown in co-culture.
Fig. 5.
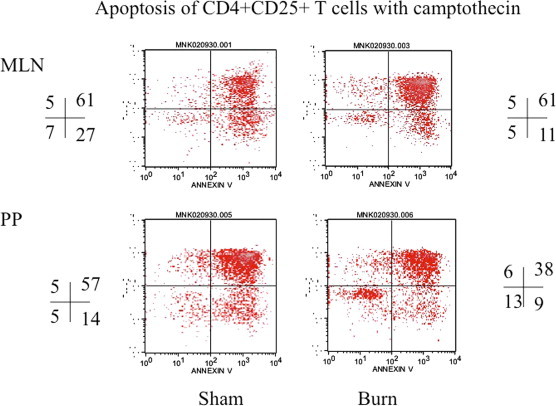
This is representative flow cytograph showing apoptosis of CD4+CD25+ T cells following induction of apoptosis by camptothecin. Camptothecin was used to induce apoptosis in these cultured CD4+ T cells. CD4+CD25+ T cells were obtained from MLN and PP of sham and day-3 post-burn rats. The quadrants represent percentage of apoptotic cells in different gated populations of CD4+CD25+ T cells of sham and day-3 post-burn animals.
Fig. 6.
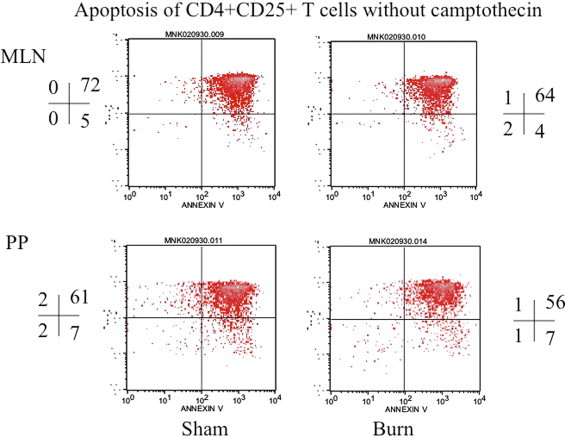
This is representative flow cytograph showing apoptosis of CD4+CD25+ T cells without camptothecin induction. CD4+CD25+ T cells were obtained from MLN and PP of sham and day-3 post-burn rats. The quadrants represent percentage of apoptotic cells in different gated populations of CD4+CD25+ T cells of sham and day-3 post-burn animals.
Fig. 7.
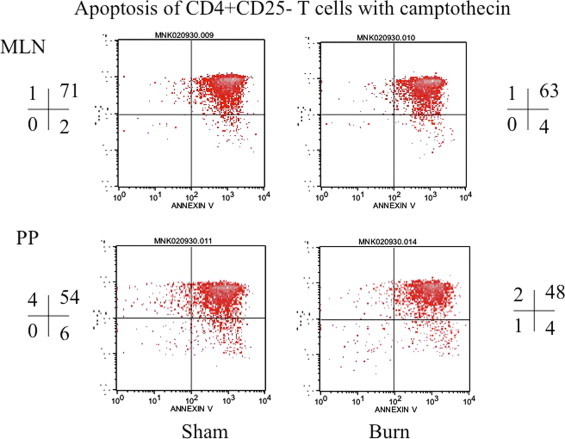
This is representative flow cytograph showing apoptosis of CD4+CD25− T cells following induction of apoptosis by camptothecin. CD4+CD25−T cells were obtained from MLN and PP of sham and day-3 post-burn rats. The quadrants represent percentage of apoptotic cells in different gated populations of CD4+CD25− T cells of sham and day-3 post-burn animals.
Fig. 8.
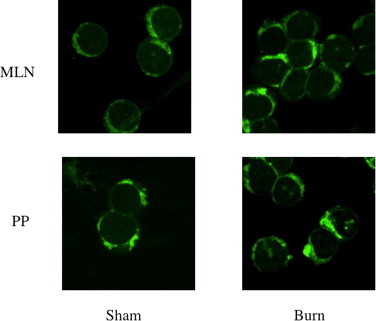
The figure shows MitoTracker GreenFM dye staining of mitochondria of CD4+ and CD4+CD25+ T cells of sham (A) and Day-3 post-burn (B) as determined by confocal microscopy. The cells depicted as having swollen mitochondria (increased uptake of green dye) and have taken the dye are considered as apoptotic cells.
3.6. Statistical analysis
All statistical analyses were carried out using the Statistical Package, Social Sciences Software Program (SPSS, SigmaStat version 2.0, Chicago, IL). To determine inter- and intragroup differences between variables, one-way repeated-measures ANOVA, followed by a pairwise multiple comparisons procedure (Tukey's post hoc test) was performed. The statistical analysis of the different experimental groups included the comparison of sham, and burn injury. p < 0.05 was considered as statistically significant.
4. Discussion
It is well established that burn-injury initiates an early pro-inflammatory innate immune response followed by an adaptive counter-inflammatory response [16–19]. The first 48 h are very critical in the care of burn patients, especially when they are in intensive care unit and a pathophysiology is developing which eventual will determine the success or failure of immune response [20]. Several mechanisms have been postulated to explain the immunosuppression following burn and/or burn-sepsis injuries. These include decreased production of the Th1-inducing cytokine IL-12, release of immunosuppressive prostaglandins, increased levels of corticosteroids [21]. T-cell dysfunction is known to contribute to immune paralysis observed in burned patients. T-cell unresponsiveness to infection has been attributed to under- or over-activation of effector cells, and imbalance of pro- and/or anti-inflammatory cytokines [1,6]. This dysfunction has been attributed to dendritic cell and T cell interactions [7]. Others have documented alterations of activated protein 1 (AP-1) and nuclear factor-kB in T-cells, which regulate genes for IL-2 production and activation of T cell [10]. This immunological deficit has also known to contribute to anergy or apoptotic death of activated T cells [22]. TNF-alpha and other stress mediator like glucocorticoids are involved in death of T cell by apoptosis [23]. This ongoing apoptotic T cell death accompanied with an unresponsive state (anergy) can lead to immunosuppression following burn injury [24]. Our current studies look at an early immune response (day-3 post-burn) and demonstrate the role of CD4+ T cells at a mucosal battlefront than traditional draining lymph nodes which drain injury site such as skin in scald burn. Our studies focus on Peyer's patches (PP) of intestine and mesenteric lymph nodes (MLN) that are challenged by gut immune response to a major burn injury. We previously have shown that early burn injury (1–3 days post-burn) modulates the intestinal mucosal barrier, allowing intestinal normal flora to invade and become a source of infection for burn victims [15]. Therefore we focused on CD4+ T cells obtained from Peyer's patches and compared them to mesenteric lymph nodes (MLN) where the lymph flows as a part of gut-associated lymphoid tissue (GALT). Peyer's patches (PP) are the sites in the gut wall where effector T cells are at work mounting antigen specific defense against the pathogenic/nonpathogenic antigens that are crossing the intestinal mucosal barrier. The effector T cells found in PP are derived from MLN as well as those that initially recognized the antigen in PP and had also undergone activation in PP. Thus, a loss of responsiveness of CD4+ T cells to antigen and their deletion due to apoptosis in both MLN and gut associated lymphoid tissue-GALT (PP) CD4+ cells can be harmful to the burn-injured hosts. There is support for the concept that burn-injury may exacerbate the inherent mucosal tolerance phenomenon in the PP T cells, and that such exacerbation leads to mucosal invasion of gut commensal bacteria and/or their breakdown products. However, to date studies have not evaluated the possibility that burn-injury alters interactions between PP's CD4+ T cells which play a role in the generation losses of CD4+ T cell responsiveness and may contribute to their inappropriate apoptosis. In this series of experiments, we hypothesized that burn-injury related immunosuppression is caused by PP CD4+ T cells deficits arising from their interactions with PP CD4+ T cells. Our studies have indicated that proportion of CD25+ cells among the CD4+ T cells in burn rat Peyer's Patches and (PP) mesenteric lymph nodes (MLN) is higher than that found in sham rat PP and MLN CD4+ T cells. This is indicative of activated CD4+ T cells as CD25+ receptor represents activity like up-regulation of IL-2 receptor. These findings suggest that the increase in the proportion of CD25+ T cells with burn injury represented induced CD4+CD25+ T cells, and that there might have been a shift of CD4+CD25− T cells to CD4+CD25+ T cells during the course of burn injury. IL-2 not only controls the survival and proliferation of T cells but also is implicated in the homeostasis and differentiation of Th1, Th2 and Th17 T cells [25].
A major outcome of our studies is the finding that GALT-derived CD4+CD25+ T cells obtained from PP and MLN of burn-injured rats have the capability of functionally down-regulating naïve CD4+CD25− T cells of sham control rats. This observation was made through ex vivo experiments in which burn rat CD4+CD25+ T cells were co-cultured with CD4+CD25− T cells from naïve rats, and in experiments in which burn rat (of the inbred Lewis strain) CD4+CD25+ T cells were adoptively transferred into naïve Lewis strain rats. In the latter experiment, CD4+CD25− T cells were isolated from the recipient rats and were shown to have attenuated IL-2 production and proliferative response. Our experiments initially determined the extent of CD4+CD25− T cells’ functional down-regulation with burn injury. There might be differences in the effects of this burn-injury condition that may be consequential in the determination of morbidity and mortality of injured hosts in these injury conditions. We also assessed some of the known markers of CD4+ helper T cells’ functional down-regulation in the course of burn. The general, approach in this series of experiments was to study purified activated CD4+ T cells and their of potential alterations in CD4+ T cell associated IL-2, proliferative activity, apoptosis, and an assessment of association between these T cells. Secondly, we adoptively transferred both CD4+CD25+ T cells from PP and LP of burn-injured rats’ and evaluated CD4+ T cell IL-2 production, proliferation and apoptosis in co-culture of CD4+T cells with CD25+ T cells. The over all objective of these experiments was to correlate PP and LP T cell functional alterations in burn-injured animals with intestinal permeability alterations and bacterial invasion in gut tissue as well as gut bacterial translocation to extra-intestinal site such as mesenteric lymph nodes (MLN). The latter approach was to ascertain if CD4+ T cell deficits, decreased responsiveness to antigen and increased apoptosis, contribute to an increased bacterial invasion in the gut, such that bacteria or bacterial products in turn or the innate immune components, macrophages cause damage to intestinal epithelial barrier integrity. T lymphocytes are found in the GI tract in the mucosal epithelial layer (intraepithelial T cells), PP, and lamina propria. While the intraepithelial T cells are primarily CD8+ cells, the majority of T cells in the lamina propria are CD4+ cells. PP contains predominantly B cells and a relatively small population of CD4+ T cells; their lymphoid follicles resemble those in spleens and other lymph nodes. In the current studies, we determined the proportion of CD4+CD25+ T cells, as well as of αβ and γδ TCR distribution, among the T cells in PP, and in the MLN of rat intestine. The CD4+T cells probably recognize and are activated by the antigens, upon its presentation by the accessory cells, in the MLN, as antigens pass through the gut lymph into MLN. T cells activated in MLN circulate to the lamina propria. Previous studies in rats have also shown that activated CD4+ T cells originating from MLN preferentially recirculate to the intestinal sites, where the antigen was initially recognized, and on returning to these sites (lamina propria, PP, MLN), if re-stimulated, their proliferation is highest in MLN. The proliferation of CD4+ CD25+ T cells in the MLN was also higher than that of PP T cells. Whereas MLN contained both naïve T cells (CD45RC high) and activated/memory cells (CD45RC low), lamina propria and PP have been shown to contain mostly activated T cells [12,13]. Assessments of modulations in T cell activation, proliferation, and apoptosis in MLN in some respect allow for evaluations of CD4+CD25+ T cells that eventually populate the intestinal mucosal sites. Since the microenvironment of T cells varies it would be important to make assessments of T cell functions/responses in samples from each of the two intestinal sites, i.e., MLN and PP.
We have previously shown the deficits in CD4+ T cell: APC interactions and the contribution of CTLA-4 exhibited on effector T cells following burn injury [12,13]. The current data suggest that GALT CD4+ T cells in these studies are CTLA-4-positive and express the activation and maturation surface markers that constitute an effector CD4+ T cell immune response. We studied surface expression of activation markers on CD4+ T cells derived from MLN and PP of day 3 post-burn and sham rats in detail and found, of all effector CD4+T cells 6–16% (Table 1) were CD25+. The fact that CD4+CD25+CTLA+ T cells may be considered as regulatory T cells but we did not check for the Foxp3 transcription factor to make certain that we were dealing with Tregs in the current study. However, we did separate our study of effector immune responses based upon; presence (CD4+CD25+) and absence (CD4+CD25−) activation marker. There is emerging and existing data depicting a protective and/or a detrimental role of regulatory T cells (Tregs) [26–31]. Other T cell markers CD49D appeared to be expressed on both MLN CD4+ T cells of sham and burn but expression was reduced on PP as compare to MLN. Similar was the case for expression of CD11a, which was expressed on all GALT CD4+ T cells regardless of their origin albeit less on PP. Adhesion marker (CD62L) had similar pattern of expression on GALT CD4+ T cells with significantly less expression on PP of sham or burn animals.
To surmise our data shows that day 3 post burn-injury does modulate surface expression of effector CD4+ T cells of GALT origin and their sensitivity to apoptosis which may regulate immune homeostasis in gut. More specifically, up-regulation of CD25+ regulatory marker, down regulation of adhesion (CD62L, CD11a) and homing receptor (CD49d) expression, and up-regulation of negative co-stimulatory (CTLA-4) molecule. Furthermore, a big pool of GALT-derived effector T cells being vulnerable to acute burn injury may also impact immune suppression that we normally observe in this type of injury. Our future studies are addressing a cross-talk between CD4+ T cells and dendritic cells in the microenvironemnt of the gut.
Acknowledgements
The author wishes to acknowledge generous support from College of Pharmacy, and CTRE Chicago State University grant to the author. Special thanks to Dr. Ashraf Ali, Director, FACS Facility of Biological Sciences Department of Chicago State University for helping in flow cytometric analyses.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Barlow Y. T lymphocytes and immunosuppression in the burned patient: a review. Burns. 1994;20(6):487–490. doi: 10.1016/0305-4179(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 2.Fukuzuka K, Edwards IIIrd CK, Clare-Salzler M, Copeland IIIrd EM, Moldawer LL, Mozingo DW. Glucocorticoid-induced, caspase-dependent organ apoptosis early after burn injury. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2000;278(4):R1005–R1018. doi: 10.1152/ajpregu.2000.278.4.R1005. [DOI] [PubMed] [Google Scholar]
- 3.Duan X, Yarmush D, Lederer A, Yarmush ML, Mitchell RN. Burn-induced immunosuppression: attenuated T cell signaling independent of IFN-gamma- and nitric oxide-mediated pathways. Journal of Leukocyte Biology. 2008;83(2):305–313. doi: 10.1189/jlb.0407228. [Epub 2007 Nov 16] [DOI] [PubMed] [Google Scholar]
- 4.Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. Journal of Leukocyte Biology. 2012;92(3):529–538. doi: 10.1189/jlb.0212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnerty CC, Przkora R, Herndon DN, Jeschke MG. Cytokine expression profile over time in burned mice. Cytokine. 2009;45(1):20–25. doi: 10.1016/j.cyto.2008.10.005. [Epub 2008 Nov 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patenaude J, D’Elia M, Hamelin C, Garrel D, Bernier J. Burn injury induces a change in T cell homeostasis affecting preferentially CD4+ T cells. Journal of Leukocyte Biology. 2005;77(2):141–150. doi: 10.1189/jlb.0703314. [DOI] [PubMed] [Google Scholar]
- 7.Fazal N. OX62+OX6+OX35+ rat dendritic cells are unable to prime CD4+ T cell for an effective immune response following acute burn injury. Results in Immunology. 2013;(3):64–72. doi: 10.1016/j.rinim.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. doi: 10.1016/s0305-4179(02)00187-0. [Review] [DOI] [PubMed] [Google Scholar]
- 9.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. Journal of Trauma. 2006;61(2):293–298. doi: 10.1097/01.ta.0000228969.46633.bb. [Discussion 298–9] [DOI] [PubMed] [Google Scholar]
- 10.O’Suilleabhain CB, Kim S, Rodrick MR, Mannick JA, Lederer JA. Injury induces alterations in T-cell NFkappaB and AP-1 activation. Shock. 2001;15(6):432–437. doi: 10.1097/00024382-200115060-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lebedev MJ, Ptitsina JS, Vilkov SA, Korablev SB, Novikov VV. Membrane and soluble forms of Fas (CD95) in peripheral blood lymphocytes and in serum from burns patients. Burns. 2001;27(7):669–673. doi: 10.1016/s0305-4179(01)00036-5. [DOI] [PubMed] [Google Scholar]
- 12.Fazal N, Al-Ghoul WM. Thermal injury-plus-sepsis contributes to a substantial deletion of intestinal mesenteric lymph node CD4 T cell via apoptosis. International Journal of Biological Sciences. 2007;3(6):393–401. doi: 10.7150/ijbs.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazal N, Raziuddin S, Khan M, Al-Ghoul WM. Antigen presenting cells (APCs) from thermally injured and/or septic rats modulate CD4+ T cell responses of naive rat. Biochimica et Biophysica Acta. 2006;1762(1):46–53. doi: 10.1016/j.bbadis.2005.07.005. [Epub 2005 Oct 11] [DOI] [PubMed] [Google Scholar]
- 14.Fazal N, Choudhry MA, Sayeed MM. Inhibition of T cell MAPKs (Erk 1/2, p38) with thermal injury is related to down-regulation of Ca2+ signaling. Biochimica et Biophysica Acta. 2005;1741(1–2):113–119. doi: 10.1016/j.bbadis.2004.10.006. [Epub 2004 Oct 30] [DOI] [PubMed] [Google Scholar]
- 15.Fazal N, Shamim M, Khan SS, Gamelli RL, Sayeed MM. Neutrophil depletion in rats reduces burn-injury induced intestinal bacterial translocation. Critical Care Medicine. 2000;28(5):1550–1555. doi: 10.1097/00003246-200005000-00048. [DOI] [PubMed] [Google Scholar]
- 16.Moore FA, Moore EE, Read RA. Post-injury multiple organ failure: role of extrathoracic injury and sepsis in adult respiratory distress syndrome. New Horizons. 1993;1(4):538–549. [PubMed] [Google Scholar]
- 17.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Baue AE. MOF, MODS, and SIRS: what is in a name or an acronym? Shock. 2006;26:438–449. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- 19.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Critical Care Medicine. 1996;24:1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Latenser BA. Critical care of the burn patient: the first 48 hours. Critical Care Medicine. 2009;37(10):2819–2826. doi: 10.1097/CCM.0b013e3181b3a08f. [DOI] [PubMed] [Google Scholar]
- 21.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. Journal of Immunology. 2006;176(1):225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 22.Mountz JD, Zhou T, Wu J, Wang W, Su X, Cheng J. Regulation of apoptosis in immune cells. Journal of Clinical Immunology. 1995;15(1):1–16. doi: 10.1007/BF01489485. [DOI] [PubMed] [Google Scholar]
- 23.Ayala A, Chung CS, Xu YX, Evans TA, Redmond KM, Chaudry IH. Increased inducible apoptosis in CD4+ T lymphocytes during polymicrobial sepsis is mediated by Fas ligand and not endotoxin. Immunology. 1999;97(1):45–55. doi: 10.1046/j.1365-2567.1999.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargreaves RG, Borthwick NJ, Gilardini Montani MS, Piccolella E, Carmichael P, Lechler RI, Akbar AN, Lombardi G. Induction of apoptosis following antigen presentation by T cells: anergy and apoptosis are two separate phenomena. Transplantation Proceedings. 1997;29(1–2):1102–1104. doi: 10.1016/s0041-1345(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 25.Létourneau S, Krieg C, Pantaleo G, Boyman O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. Journal of Allergy and Clinical Immunology. 2009;123(4):758–762. doi: 10.1016/j.jaci.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Banz A, Pontoux C, Papiernik M. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells. Journal of Immunology. 2002;169(2):750–757. doi: 10.4049/jimmunol.169.2.750. [DOI] [PubMed] [Google Scholar]
- 27.Hein F, Massin F, Cravoisy-Popovic A, Barraud D, Levy B, Bollaert PE, Gibot S. The relationship between CD4+CD25+CD127− regulatory T cells and inflammatory response and outcome during shock states. Critical Care. 2010;14(1):R19. doi: 10.1186/cc8876. [Epub 2010 Feb 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Critical Care Medicine. 2003;31(7):2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 29.Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. Journal of Immunology. 2005;174(5):2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 30.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. European Journal of Immunology. 2008;38(4):918–921. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29(3):362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


