Abstract
Staphylococcus aureus is a premier human pathogen and the most common cause of osteoarticular, wound, and implanted device infections. We recently demonstrated S. aureus efficiently binds the classical complement regulator C4b-binding protein (C4BP) inhibiting antibody-initiated complement-mediated opsonization. Here we identify S. aureus surface protein SdrE as a C4BP-binding protein. Recombinant SdrE and recombinant bone sialoprotein-binding protein (Bbp), an allelic variant of SdrE, both efficiently bound to C4BP in heat-inactivated human serum. We previously described SdrE as binding alternative pathway regulator factor H. Recombinant SdrE and Bbp efficiently bound C4BP and factor H in serum without apparent interference. Gain of function studies utilizing Lactococcus lactis clones expressing SdrE or Bbp increased serum C4BP and factor H binding, compared with empty-vector control (WT) approximately 2-fold. Correspondingly, classical pathway-mediated C3-fragment opsonization and bacterial killing by human neutrophils decreased by half for L. lactis clones expressing SdrE or Bbp compared with WT. In summary, we identify SdrE and allelic variant Bbp as S. aureus surface proteins that bind the complement regulator C4BP inhibiting classical pathway-mediated bacterial opsonization and killing.
Keywords: Staphylococcus aureus, Complement, Opsonophagocytosis, C4BP, SdrE
1. Introduction
Staphylococcus aureus is the preeminent human pathogen causing skin and skin-structure infections [9] as well as post-operative infections [12], in addition to being the major cause of many types of invasive bacterial infections [3,39]. Hospital- and community-associated MRSA infections continue to rise [16] making treatment of this pathogen ever more challenging. Additionally, evidence suggests that wild-type infection does not yield protective immunity against repeated infection with the same strain [29] and all attempts to develop a successful vaccine against S. aureus have failed [7].
The lack of protective immunity from infection or immunization suggests that antibody-mediated immunity against S. aureus is inadequate. This is despite the fact that a wide variety of antibodies are generated by the human host during S. aureus infection [37] and many different immunogens have been tried as vaccines in a variety of combinations [33]. Antibody interactions with Fc receptors are altered by S. aureus expression of staphylococcal protein A (SpA) and Sbi [23], but antibody binding to the bacterial surface also initiates potent classical complement pathway-mediated host defenses including opsonization and anaphylatoxin production [24]. Inhibition of classical complement pathway activation in the host is primarily regulated by C4b-binding protein (C4BP) by displacing C2a from the classical C3 convertase and facilitating factor I-mediated cleavage of C4b to inactive forms [6,13,14]. Surface recruitment of C4BP as an immune evasion strategy has been demonstrated for several pathogenic bacteria including Streptococcus pyogenes [1,21], Streptococcus pneumoniae [8], Borrelia burgdorferi [30], and Neisseria meningitidis [20].
We have recently shown that the host complement regulator of classical pathway activation C4BP is recruited to the surface of S. aureus increasing degradation of the classical C3 convertase and contributing to C4b cleavage resulting in decreased opsonization by C3b [18]. These results suggested that S. aureus recruitment of C4BP to its surface likely contributes to the inhibition of antibody-initiated classical complement pathway activation, a mechanism that may result in lack of protective adaptive immunity after infection or immunization.
Here we identify S. aureus surface proteins that bind to C4BP and show that bacterial expression of these proteins alters classical complement pathway-mediated opsonization and bacterial killing. Identification of C4BP-binding surface proteins that inhibit antibody-initiated complement activation and immune effectors provides an opportunity to develop novel strategies to increase vaccine effectiveness against S. aureus.
2. Material and methods
2.1. Ethics Statement
Human blood was obtained from four healthy volunteers for generating serum and neutrophils used as reagents in these studies. Eastern Virginia Medical School IRB approved this study protocol: 02–06-EX-0216. Written informed consent was provided by study participants.
2.2. Bacteria and growth conditions
S. aureus Reynolds strain was grown to mid-logarithmic phase in Columbia 2% NaCl media at 37 °C, as described elsewhere [5]. Lactococcus lactis strains that constitutively express the staphylococcal surface proteins SdrE, Bbp, or that contain the empty vector pKS80 are described elsewhere [26,36]. L. lactis were grown in M17 broth containing 0.5% glucose and 5 μg/ml of erythromycin at 30 °C without shaking, as previously described [32]. MRSA strains from the Nebraska Transposon Mutant Library, described elsewhere [11], were obtained from NARSA.
2.3. Buffers
Binding experiments were performed with GVBS++ buffer (VBS with 0.1% gelatin, 0.15 mM CaCl2, and 1.0 mM MgCl2), or EDTA-GVBS− − buffer (VBS with 0.1% gelatin and 0.01 M EDTA).
2.4. Human serum and purified complement proteins
Normal human serum (NHS) was prepared as previously described [4] from the blood of four healthy donors and pooled. Blood was obtained with consent under an IRB-approved protocol (Eastern Virginia Medical School IRB 02–06-EX-0216). Heat-inactivated sera were generated by incubating NHS at 56 °C for 30 min. Cobra Venom Factor (CVF) treated sera was generated by incubating NHS with CVF (Comp Tech) at 20 μg/ml for 1 h at 37 °C. Heat-inactivated and CVF-treated sera were confirmed to have no complement activity in a CH50 assay. Factor B depleted sera and purified C4b Binding Protein (C4BP) were obtained commercially (Comp Tech). Total IgG in our pooled serum was measured at 16.5 mg/ml by ELISA, as previously described [10]. IgG binding to S. aureus was estimated by incubating 1010 CFU of a Spa-deficient strain (Nebraska Transposon Mutant Library) with 1 ml of heat-inactivated pooled human serum, stripping bound antibody, and measurement by ELISA. We measured 0.0257 mg of IgG bound 1010 CFU, or about 0.15% of total IgG in the serum. Relative amount of anti-SdrE antibody present in the serum was performed by serial dilution comparing antibody binding to rSdrE or a total cell wall preparation (Spa-deficient strain) in a modified ELISA-type assay. Approximately 0.019% of IgG in the serum bound to rSdrE compared to a total cell wall preparation.
2.5. Recombinant proteins
Recombinant SdrE (rSdrE), recombinant bone sialoprotein-binding protein (rBbp), and recombinant ClfA (rClfA) were expressed as His-tagged proteins containing their respective unique A regions in an Escherichia coli expression system, as described elsewhere [26,27,36]. Briefly, recombinant proteins were purified from cell lysates by nickel column chromatography and verified by anti-His tag dot blots and SYPRO Ruby (Invitrogen) stained SDS-PAGE.
2.6. Isolation of cell wall proteins binding C4BP
Cell wall extracts were prepared and separated by size exclusion chromatography as described previously [19]. Briefly, cell wall preparations were generated from mid-logarithmic phase S. aureus Reynolds using lysostaphin in a protoplast stabilizing raffinose buffer with DNAse and protease inhibitors. Separation by size-exclusion column chromatography was performed using a HiPrep 16/60 Sephacryl column equilibrated with PBS. Fractions were dot blotted and blocked with 3% BSA / TBS and washed with TBS-Tween. Blot overlay was performed with 10% CVF-NHS for 2 h and then probed with a mouse anti-human C4BP antibody (Quidel) followed by a goat anti-mouse HRP antibody (Sigma-Aldrich). Fractions that bound serum C4BP were then identified by enhanced chemiluminescence (ECL). These fractions of interest were further analyzed by SDS-PAGE with SYPRO Ruby (Invitrogen) total protein stain, or 10% CVF-NHS overlay Western blot. Overlay blot controls were performed without serum or without primary antibody, yielding negative results for each.
2.7. Mass spectrometry identification
Protein bands were excised from SYPRO-Ruby stained SDS-PAGE gels and processed for liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) on a linear trap quadrupole ion trap (LTQ) mass spectrometer (Thermo Fisher) as previously described [17,19,38]. The acquired data was processed by Xcalibur (Thermo Fisher) and the proteins were identified using Mascot Daemon version 2.2.03 (Matrix Science, UK) software using the latest version (2012_10) of the combined indexed Homo sapiens and S. aureus sequences of the non-redundant Swiss-Prot protein database.
2.8. Alignment analysis
Amino acid sequences of SdrE (GenBank accession # YP_001331559.) and Bbp (GenBank Accession # Q14U76.1) as well as human C4BP (GenBank Accession # AAA36507.1) and factor H (GenBank Accession # P08603.4) were aligned using Clustal Omega [15].
2.9. Binding assays
Recombinant Bbp or SdrE was bound to 96-well Immulon II plates at 10 μg/ml in a carbonate coating buffer pH 9.6, and incubated overnight at 4 °C. The plates were then washed with 2 × PBS / 1% Tween-20 three times and blocked with 0.5% gelatin in PBS for 2 h at room temperature. The plates were washed again, and NHS, heat-inactivated sera, or pure C4BP were diluted in blocking buffer, titrated, and incubated for the time indicated at room temperature. After washing, the plates were probed with chicken anti-human factor H antibody (Accurate Chemical), or the mouse anti-human C4BP antibody; antibodies were diluted to 1:1000 in blocking buffer and incubated for 1 h. Secondary probe was performed with goat anti-chicken HRP antibody (Genway) or goat anti-mouse HRP antibody; antibodies were diluted to 1:1000 in blocking buffer and incubated for 1 h. Plates were washed and developed with TMB substrate (Thermo Scientific) and stopped with 2.5 N H2SO4. Absorbance values were read at 450 nm. Values from control wells performed without primary antibodies yielded low background signals, which were subtracted out.
2.10. L. lactis expression of SdrE and Bbp
Log phase L. lactis in coating buffer were used to coat an Immulon II plate starting at 1.3 × 108 cells/ml then serial dilutions. After overnight at 4 °C, wells were washed and blocked with 0.5% gelatin in PBS for 2 h. After washing, wells were incubated with a chicken anti-SdrE/Bbp antibody diluted in block buffer, at 1:1000 for 1 h. The wells were then washed and incubated with anti-chicken HRP antibody (Genway) diluted in block buffer at 1: 1000 for 1 h . Wells were washed and developed with TMB, as described above.
2.11. Factor H and C4BP binding L. lactis strains
L. lactis strains were suspended at OD600 = 4.0, of which 0.25 ml was diluted with heat-inactivated sera and GVBS++ buffer to a total volume of 0.5 ml and incubated for 30 min at 37 °C [32]. Bacteria were washed with GVBS−− buffer, and surface proteins were stripped with 50 μl of 2% SDS and boiled for 5 min. Samples were sedimented and supernatants recovered.
2.12. Factor H and C4BP quantitative dot blot
C4BP dot blot has been previously described [18]. Briefly, after adding samples and washing, the membrane was blocked with 3% BSA, probed with a monoclonal mouse anti-C4BP primary antibody, and then a HRP-labeled goat anti-mouse secondary antibody. The factor H dot blot was probed with a goat anti-human factor H antibody primarily and a rabbit anti-goat HRP antibody secondarily. Purified factor H was used to generate a standard curve and detection was via ECL. Quantity One software (Bio-Rad) was used to assign grey scale values to the standard curve titrations and the samples so that a linear regression could be used to quantify the amount of C4BP or factor H in each sample.
2.13. C3 binding to L. lactis
L. lactis strains were incubated with 10% factor B-depleted sera in GVBS++ buffer for 10 min at 37 °C. These cells were washed thoroughly with GVBS−− buffer, and the cell pellets were resuspended in 50 μL of 25 mM methylamine to strip off covalently bound C3-fragments. Quantity of total C3-fragments was measured via C3 ELISA as previously described [17]. C3-fragments were qualitatively analyzed by Western blot analysis, as previously described [17].
2.14. L. lactis killing assay
The complement-mediated killing assay was performed as described previously [32]. Briefly, in 1.0 ml total volume, 6 × 106 CFU L. lactis, 5 × 106 neutrophils and 10% factor B-depleted sera diluted in HBSS with Ca++ were tumbled at 37 °C. At 15 and 30 min, samples were diluted in sterile water and plated on GM17 agar supplemented with erythromycin. After overnight growth at 30 °C, colonies were counted. The number of bacteria killed was calculated by subtracting the bacterial count of the sample with neutrophils from the bacterial count of a matched base-line sample without neutrophils. Additional controls performed without serum yielded zero killing.
2.15. Statistical analysis
Means and standard error of the means (SEM) were calculated from ‘n’ independent experiments performed on separate days. Data were analyzed by student's t test; calculated P values of ≤ 0.05 were considered statistically significant.
3. Results
3.1. Identification of S. aureus cell wall protein SdrE as a C4BP binding protein.
In order to identify S. aureus cell wall proteins that bind C4BP we utilized methodology we have previously reported [19,32]. Briefly, S. aureus cell wall preparations were generated, fractionated by column chromatography and analyzed by overlay C4BP dot blot (Fig. 1A). Cell wall fractions demonstrating the highest C4BP binding were concentrated and assayed by serum C4BP overlay Western blot identifying three bands of interest (Fig. 1B). Control Western blots performed without serum overlay or without primary antibody were negative. Corresponding bands were excised from total protein stained SDS-PAGE gels and subjected to in gel digestion and mass spectrometry analysis (LC-ESI-MS/MS) in order to identify peptide sequences. All three bands of interest yielded multiple significant identifications of peptides from the S. aureus cell wall protein SdrE (Table 1). Peptide scores > 30 and Expect scores < 0.05 were considered significant. These identified peptides yielded a peptide map for SdrE with 58% coverage of the non-SD-repeat region (Fig. 1C).
Fig. 1.
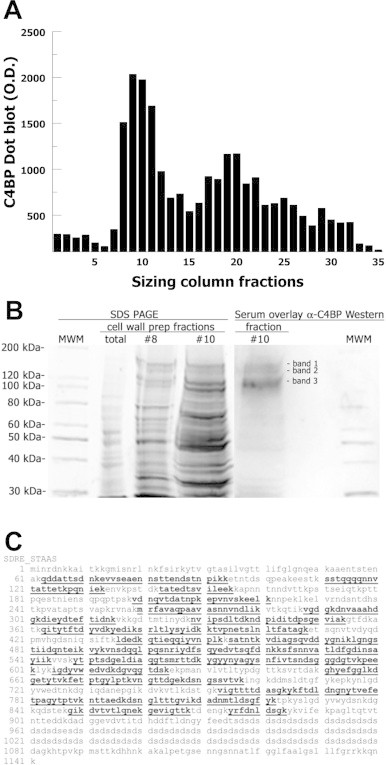
Identification of SdrE as a binding partner for C4BP. (A) S. aureus cell wall preparation fractions separated by size-exclusion chromatography then assayed for C4BP binding by dot blot. (B) Cell wall proteins from concentrated fractions were separated by SDS-PAGE and visualized by total protein stain (left). Blotted proteins were overlayed with serum (CVF-treated) and then probed for bound C4BP (right). Three bands were excised and analyzed by mass spectrometry. (C) Peptide coverage map for SdrE peptides identified by mass spectrometry in bold and underlined.
Table 1.
Mass spectrometry analysis of S. aureus cell wall protein binding C4BP.
| Mr (expt) | Mr (calc) | Peptide score | Expect score | Peptide sequence |
|---|---|---|---|---|
| 1213.3464 | 1213.5727 | 49 | 0.014 | K.YRFDNLDSGK.Y |
| 1242.8066 | 1242.6860 | 48 | 0.018 | R.LTLYSYIDKK.T |
| 1252.6867 | 1252.6340 | 51 | 0.0089 | K.FETPTGYLPTK.V |
| 1385.2488 | 1384.6695 | 72 | 9.1e-05 | K.VNSDQQLPQSNR.I |
| 1435.3694 | 1434.7090 | 78 | 2e-05 | K.TATEDTSVILEEK.K |
| 1440.9608 | 1440.7460 | 78 | 2.3e-05 | K.VIGTTTTDASGKYK.F |
| 1443.6196 | 1443.7933 | 79 | 1.8e-05 | K.GIKDVTVTLQNEK.G |
| 1492.2922 | 1492.6028 | 69 | 0.00018 | K.DADNMTLDSGFYK.T |
| 1594.4430 | 1593.7522 | 73 | 8.4e-05 | K.VDIAGSQVDDYGNIK.L |
| 1603.4596 | 1602.8101 | 62 | 0.0011 | K.LGNGSTIIDQNTEIK.V |
| 1764.2652 | 1763.8941 | 48 | 0.029 | K.TVPNETSLNLTFATAGK.E |
| 1775.5260 | 1774.8962 | 69 | 0.00023 | K.VYKVNSDQQLPQSNR.I |
| 1895.0746 | 1894.8756 | 62 | 0.0012 | K.VNGTTDGEKDSNGSSVTVK.I |
| 1900.6288 | 1899.9003 | 78 | 3.4e-05 | K.GHYEFGGLKDGETYTVK.F |
| 1931.1484 | 1931.0211 | 87 | 3.6e-06 | K.DVTVTLQNEKGEVIGTTK.T |
| 1943.5350 | 1943.0000 | 96 | 5e-07 | R.FAVAQPAAVASNNVNDLIK.V |
| 1957.3312 | 1956.8735 | 78 | 3.5e-05 | K.YTPTSDGELDIAQGTSMR.T |
| 1964.6628 | 1963.9698 | 106 | 4.7e-08 | K.NTTAEDKDSNGLTTTGVIK.D |
| 1992.8768 | 1992.0528 | 77 | 3.8e-05 | K.LGNGSTIIDQNTEIKVYK.V |
| 2098.5814 | 2097.9167 | 82 | 1.6e-05 | R.IYDFSQYEDVTSQFDNK.K |
| 2141.7474 | 2141.0205 | 69 | 0.0003 | K.QITYTFTDYVDKYEDIK.S |
| 2146.6474 | 2146.0470 | 76 | 6.4e-05 | K.DGETYTVKFETPTGYLPTK.V |
| 2173.6574 | 2173.1266 | 80 | 2.4e-05 | K.LDEDKQTIEQQIYVNPLK.K |
| 2195.7294 | 2195.0706 | 98 | 3.9e-07 | K.SATNTKVDIAGSQVDDYGNIK.L |
| 2226.5414 | 2225.9965 | 70 | 0.00028 | K.IGDYVWEDVDKDGVQGTDSK.E |
| 2226.6934 | 2226.9957 | 84 | 1.1e-05 | R.IYDFSQYEDVTSQFDNKK.S |
| 2246.0554 | 2246.1365 | 71 | 0.00022 | K.MRFAVAQPAAVASNNVNDLIK.V |
| 2290.5614 | 2290.1005 | 93 | 1.3e-06 | K.SFSNNVATLDFGDINSAYIIK.V |
| 2302.7994 | 2302.2056 | 59 | 0.0031 | K.LDEDKQTIEQQIYVNPLKK.S |
| 2384.7054 | 2384.1536 | 69 | 0.00034 | K.QITYTFTDYVDKYEDIKSR.L |
| 2554.9747 | 2554.2762 | 75 | 0.0001 | K.VDNQVTDATNPKEPVNVSKEELK.N |
| 2576.0263 | 2576.2202 | 66 | 0.00078 | K.SSTQQQQNNVTATTETKPQNIEK.E |
| 2679.6994 | 2679.2228 | 75 | 9.4e-05 | K.FTDLDNGNYTVEFETPAGYTPTVK.N |
| 2766.0661 | 2766.3811 | 56 | 0.0086 | K.NVIPSDLTDKNDPIDITDPSGEVIAK.G |
| 2971.6754 | 2971.3651 | 65 | 0.0011 | K.YKFTDLDNGNYTVEFETPAGYTPTVK.N |
| 3038.1532 | 3038.3629 | 75 | 0.00011 | K.VGDGKDNVAAAHDGKDIEYDTEFTIDNK.V |
| 3130.4722 | 3130.4830 | 64 | 0.0014 | K.FETPTGYLPTKVNGTTDGEKDSNGSSVTVK.I |
| 3178.8622 | 3178.5517 | 85 | 1e-05 | K.VDIAGSQVDDYGNIKLGNGSTIIDQNTEIK.V |
| 3439.2682 | 3439.5461 | 60 | 0.0038 | K.NTTAEDKDSNGLTTTGVIKDADNMTLDSGFYK.T |
| 3482.7922 | 3482.5292 | 94 | 1.4e-06 | K.QDDATTSDNKEVVSEAENNSTTENDSTNPIKK.E |
| 3571.1422 | 3570.7464 | 70 | 0.00038 | K.VDIAGSQVDDYGNIKLGNGSTIIDQNTEIKVYK.V |
| 3593.4952 | 3593.6546 | 65 | 0.0012 | K.VNSDQQLPQSNRIYDFSQYEDVTSQFDNKK.S |
| 3780.7162 | 3780.8541 | 57 | 0.0085 | K.SATNTKVDIAGSQVDDYGNIKLGNGSTIIDQNTEIK.V |
| 3799.4212 | 3798.6697 | 116 | 1e-08 | R.TTDKYGYYNYAGYSNFIVTSNDSGGGDGTVKPEEK.L |
We have previously reported identification of SdrE as a S. aureus cell wall receptor for the host complement regulator factor H that decreases complement alternative pathway-mediated opsonization and bacterial killing [32]. The most common configuration of C4BP is seven alpha chains and one beta chain [2]. The 1231 amino acid factor H and the 597 amino acid alpha-chain of C4BP are comprised of complement control protein (CCP) domains [22] with regions of limited homology (Supplemental Fig. S1A).
3.2. Binding of C4BP or factor H with SdrE or Bbp.
S. aureus bone sialoprotein-binding protein (Bbp) is an allelic variant of SdrE [35] with 75% sequence identity between the two molecules (Supplemental Fig. S1B). Thus, we analyzed the binding of C4BP and factor H to both SdrE and Bbp, as well as a control LPXTG-linked S. aureus surface protein, ClfA. C4BP in heat-inactivated serum was allowed to interact with surface-bound recombinant proteins for 60 min in an ELISA-type assay. Heat-inactivated serum was used to prevent C4BP or factor H from binding with deposited C4b or C3b, respectively. C4BP in heat-inactivated serum bound recombinant SdrE and Bbp in a dose-dependent manner compared with minimal binding for rClfA (Fig. 2A). Time-course experiments were performed with 25% heat-inactivated serum allowing C4BP to bind to recombinant proteins in ELISA-type assays. C4BP in heat-inactivated serum demonstrated time-dependent binding to rSdrE and rBbp (Fig. 2B). Testing C4BP binding to rSdrE using either normal human serum (NHS) or heat-inactivated serum yielded similar binding (Fig. 2C). In previously published experiments testing C4BP binding to the S. aureus surface, heat-inactivated serum demonstrated decreased C4BP binding compared with NHS [18]. Purified C4BP demonstrated dose-dependent binding to rSdrE as well as rBbp (Fig. 2D). Half-maximal binding for pure C4BP was observed at 15 nmol/l for rSdrE and 30 nmol/l for rBbp, where the recombinant proteins were coated at 10 μg/ml. These findings suggest that C4BP may have a have a higher affinity for SdrE than Bbp. Absorbance values for 50% heat-inactivated serum showed a 3-fold increase (p = 0.02) for factor H binding to rBbp compared with fH binding to rSdrE (Fig. 2E); however half-maximal binding values were similar. Time-course experiments also suggested increased absorbance values for factor H in heat-inactivated serum binding to rBbp compared with rSdrE (Fig. 2F). These findings suggest that Bbp, in addition to SdrE, may also be a factor H-binding protein.
Fig. 2.
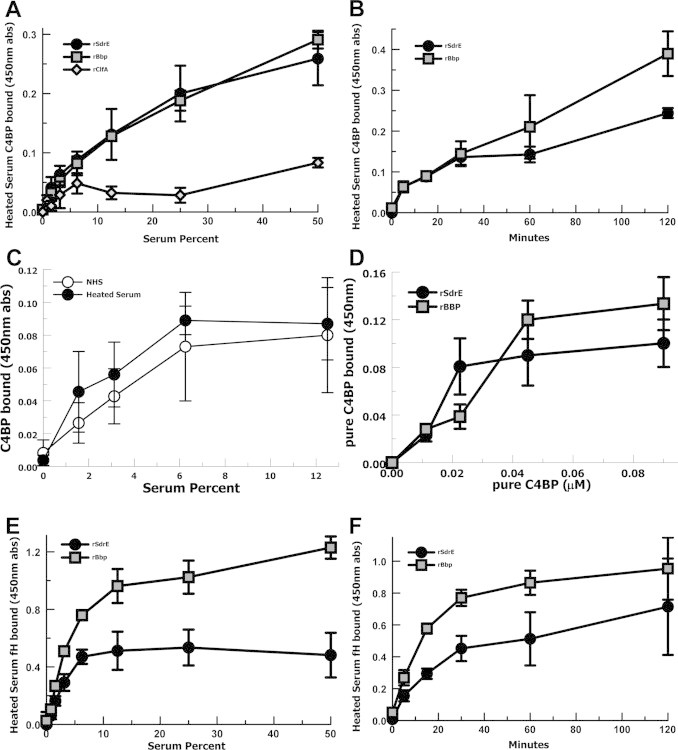
ELISA-type binding assays for C4BP and factor H with recombinant SdrE and recombinant Bbp. (A) C4BP in heat-inactivated serum binding rSdrE, rBbp, or rClfA (n = 5). (B) Time-course of C4BP in heat-inactivated serum binding rSdrE or rBbp (n = 4). (C) C4BP in normal human serum (NHS) or heat-inactivated serum binding rSdrE. (D) Purified C4BP binding rSdrE or rBbp (n = 4). (E) Factor H in heat-inactivated serum binding rSdrE or rBbp (n = 5). (F) Time-course of Factor H in heat-inactivated serum binding rSdrE or rBbp (n = 3). Data are the means of results of independent experiments. Error bars denote SEM.
3.3. Binding of C4BP and factor H to L. lactis expressing SdrE or Bbp.
In order to evaluate the binding of C4BP to SdrE or Bbp expressed on a bacterial surface we used gain-of-function L. lactis clones expressing SdrE, or Bbp, or containing an empty vector. L. lactis clones were measured for SdrE and Bbp expression using whole cells in a modified ELISA-type assay (Fig. 3A) demonstrating significant expression compared with empty vector. L. lactis clones were incubated in heated-sera and then stripped of surface-bound proteins which were measured by dot blot assays. Control dot blots were performed for L. lactis without serum or without primary antibody demonstrating minimal background. L. lactis clones in 10% heated-sera over 30 min bound more C4BP when expressing SdrE (p = 0.04) or Bbp (p = 0.03) compared with the empty vector strain (Fig. 3B). Similarly, L. lactis clones bound more factor H when expressing SdrE (p = 0.01) or Bbp (p < 0.01) compared with the empty vector strain (Fig. 3C). When the L. lactis clones expressing SdrE were incubated in heated-sera and evaluated at 10 and 30 min, the amount of fH and C4BP bound did not statistically differ at either 10 min (p = 0.7) or 30 min (p = 0.09) (Fig. 3D). These results suggest that in serum both factor H and C4BP are able to bind SdrE expressed on a bacterial surface without either one preventing the binding of the other. We then evaluated C4BP binding to an SdrE-deficient MRSA and control strains from the Nebraska Transposon Mutant Library (Fig. 3E). These strains were incubated in 10% heat-inactivated serum for 30 min, washed and stripped of surface-bound proteins were assayed for C4BP by dot blot. The SdrE-deficient strain showed a 30% (p = 0.05) decrease in C4BP binding compared with the wild-type. Similar C4BP binding to wild-type was found for the Sbi-deficient, CHIPS-deficient, and capsule-deficient strains.
Fig. 3.
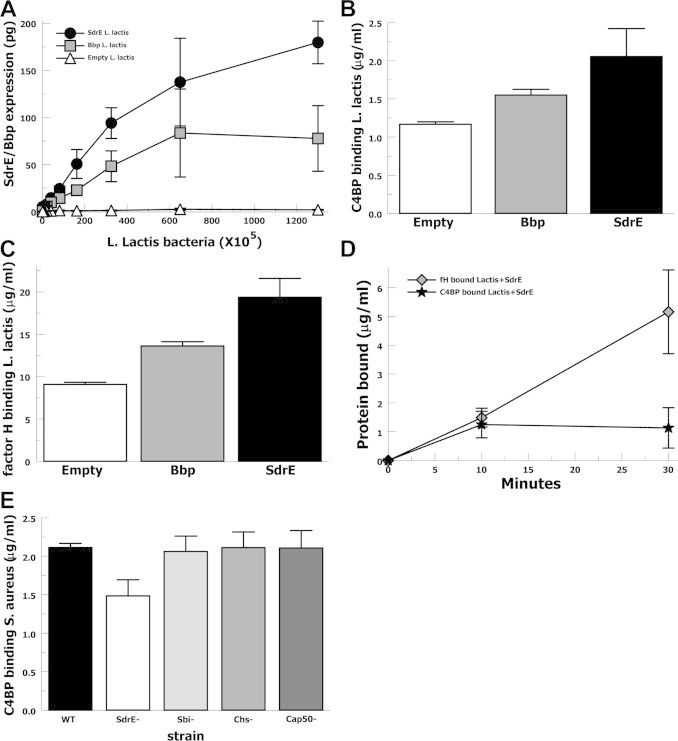
Serum C4BP binding to L. lactis expressing SdrE, or Bbp, or empty vector control. (A) SdrE and Bbp expression by L. lactis strains assayed by modified ELISA. (B) C4BP in 10% heat-inactivated serum binding to L. lactis strains at 30 min assayed by dot blot of stripped surface proteins (n = 4). (C) Factor H in 10% heat-inactivated serum binding L. lactis strains at 30 min assayed by dot blot of stripped surface proteins (n = 4). (D) C4BP and factor H in 20% heat-inactivated serum binding to SdrE-expressing L. lactis over 30 min assayed by dot blot of stripped surface proteins (n = 4). (E) C4BP in 10% heat-inactivated serum binding to S. aureus strains including wild type (WT), SdrE-deficient (SdrE−), Sbi-deficient (Sbi−), CHIPS-deficient (Chs−), and capsule-deficient (Cap50−) assayed by dot blot of stripped surface proteins (n = 4). Data are the means of results of independent experiments. Error bars denote SEM.
3.4. Classical complement pathway opsonization and bacterial killing.
In order to evaluate the effect of bacterial expression of Bbp or SdrE on classical pathway mediated opsonization, we measured C3-fragment binding to L. lactis strains incubated in 10% factor B-depleted sera (Fig. 4A). Factor B depleted serum was used to isolate activation to the classical and lectin pathways in the absence of the alternative pathway. At 10 min, L. lactis expressing Bbp bound 47% fewer C3-fragments compared with empty vector (p = 0.05) and L. lactis expressing SdrE bound 33% fewer C3-fragments compared with empty vector (p = 0.03). C3-fragment binding comparing SdrE and Bbp clones were not statistically different. Anti-C3 Western bot analysis of stripped C3-fragments demonstrated that the bound C3-fragments were opsonic forms C3b and iC3b (Fig. 4B). Anti-C3 Western blot analysis also suggested that the C3-fragments 75 kDa (β), 68 kDa (α′1), and 42 kDa (α′2) appeared more dense for the empty vector compared with the SdrE/Bbp-expressing clones, consistent with quantitative ELISA results. These results suggest that SdrE or Bbp mediated recruitment of C4BP to the bacterial surface significantly decreased classical complement pathway-mediated opsonization by C3-fragments.
Fig. 4.
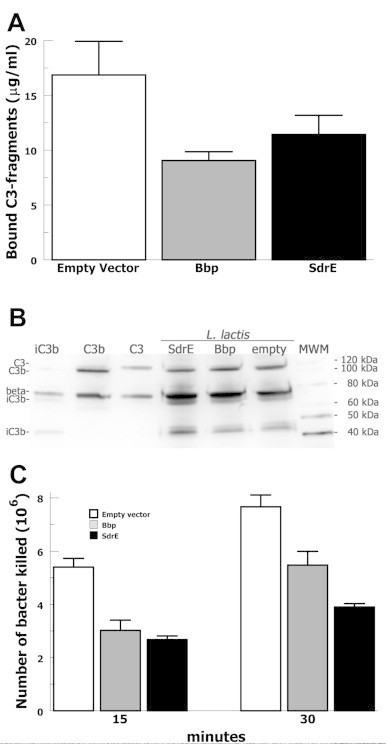
Functional testing of complement-mediated opsonization and opsonophagocytic killing of L. lactis expressing SdrE, or Bbp, or empty vector control. (A) C3-fragment opsonization of L. lactis strains incubated in 10% factor B-depleted sera for 30 min assayed by ELISA of stripped surface proteins (n = 4). (B) Anti-C3 Western blot of stripped surface proteins from L. lactis clones incubated with 10% factor B-depleted serum. (C) Neutrophil killing of L. lactis strains incubated in 10% factor B-depleted sera and purified human neutrophils for the times indicated and then plated for colony counting (n = 4). Data are the means of results of independent experiments. Error bars denote SEM.
In order to evaluate the effect of bacterial expression of Bbp of SdrE on classical complement pathway mediated bacterial killing by neutrophils, we assayed survival or L. lactis strains incubated in 10% factor B-depleted serum incubated with human neutrophils (Fig. 4C). Baseline controls were performed by incubating L. lactis in 10% factor B-depleted serum without neutrophils. At 15 min, L. lactis expressing Bbp showed a 44% decrease in bacterial killing compared with empty vector (p < 0.01) and L. lactis expressing SdrE demonstrated a 50% decrease in killing compared with empty vector (p < 0.01). At 30 min, L. lactis expressing Bbp showed a 29% decrease in bacterial killing compared with empty vector (p = 0.04) and L. lactis expressing SdrE demonstrated a 49% decrease in killing compared with empty vector (p = 0.01). These data suggest that bacterial expression of Bbp or SdrE significantly increase bacterial survival of classical complement pathway-mediated neutrophil killing.
4. Discussion
These experiments identify SdrE and Bbp as S. aureus cell wall proteins that bind the host classical complement pathway regulator C4BP. SdrE and Bbp belong to the structurally related Sdr family of S. aureus proteins named for a repeating region of serine and aspartic acid (SD) dipeptides proximal to their LPXTG cell wall linkage [25]. SdrE has been implicated in platelet activation [26] and recently identified as a ligand for factor H [32]. Bbp, an allelic variant of SdrE [28], has previously been noted to bind fibrinogen and bone sialoprotein, for which it was named [35].
The distribution of sdrE has previously been reported to be as high as 90%, including nasal carriage, invasive strains, and MRSA strains [31]. Another study evaluating sdrE and bbp among invasive isolates found 56% and 38% prevalence, respectively, for a combined 94% [28]. These studies demonstrate that SdrE and Bbp are highly prevalent among clinical isolates and suggest they likely play an important role in infection.
Immunization studies were previously conducted in mice by Stranger-Jones et al. using 19 different recombinantly expressed S. aureus surface proteins [34]. These studies showed that rSdrE was one of the most effective immunogens in reducing S. aureus colony counts (> 4 logs) in mouse kidneys. Of the effective surface protein immunogens, rSdrE immunization yielded serum with the highest level of complement-mediated opsonophagocytic killing of S. aureus. These studies suggest that in mice SdrE is an excellent immunogen against S. aureus and this effect is likely mediated, at least in part, by increasing complement-mediated opsonophagocytosis. Our findings show that bacterial surface expression of SdrE/Bbp increases recruitment of C4BP and decreases classical complement pathway-mediated opsonization and bacterial killing. Thus, our results are congruent with those of Stranger-Jones et al. and suggest that the effectiveness of SdrE as a protective immunogen may have been mediated, in part, via altering its interaction with C4BP.
We have previously identified SdrE as a factor H-binding protein and shown that bacterial expression of SdrE decreases alternative complement pathway-mediated opsonization, anaphylatoxin production, and bacterial killing [32]. Here we show that bacteria expressing SdrE are able to recruit factor H and C4BP simultaneously from human serum without evidence of interference. This suggests that SdrE may play a vital role in the modulation of classical and alternative pathway activation on the S. aureus surface via C4BP and factor H, respectively. Thus, SdrE/Bbp appears to be attractive targets to inhibit these mechanisms of immune evasion and optimize complement-mediated opsonophagocytosis and killing of S. aureus.
Our findings show that SdrE/Bbp-mediated binding of C4BP inhibits classical complement pathway activation and subsequent opsonization and bacterial killing. Inhibition of antibody-initiated complement activation likely contributes, in part, to lack of effective protective immunity after S. aureus infection or immunization. Disruption of SdrE/Bbp-mediated recruitment of C4BP will likely improve antibody-initiated complement effectors and could prove an effective strategy to improve anti-S. aureus vaccine development.
Acknowledgements
Access to the mass spectrometers of the Leroy T. Canoles Jr. Cancer Research Center was kindly provided by Dr. O. John Semmes
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix. Supplementary Materials
Amino acid alignments. (A) Alignment of C4BP alpha-chain with factor H and (B) SdrE with Bbp as determined by Clustal Omega analysis. “*” indicates identical residues, “:” indicates conserved residues, “.” indicates semi-conserved residues between the sequences. The last 553 amino acids of fH are not shown as there was no alignment between the two proteins in this region.
References
- 1.Andre I., Persson J., Blom A.M., Nilsson H., Drakenberg T., Lindahl G., Linse S. Streptococcal M protein: structural studies of the hypervariable region, free and bound to human C4BP. Biochemistry. 2006;45:4559–4568. doi: 10.1021/bi052455c. [DOI] [PubMed] [Google Scholar]
- 2.Blom A.M., Villoutreix B.O., Dahlback B. Functions of human complement inhibitor C4b-binding protein in relation to its structure. Arch immunol ther exp. 2004;52:83–95. [PubMed] [Google Scholar]
- 3.Calhoun J.H., Manring M.M., Shirtliff M. Osteomyelitis of the long bones. Semin Plast Surg. 2009;23:59–72. doi: 10.1055/s-0029-1214158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunnion K.M., Hair P.S., Buescher E.S. Cleavage of complement C3b to iC3b on the surface of Staphylococcus aureus is mediated by serum complement factor I. Infect Immun. 2004;72:2858–2863. doi: 10.1128/IAI.72.5.2858-2863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunnion K.M., Lee J.C., Frank M.M. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immun. 2001;69:6796–6803. doi: 10.1128/IAI.69.11.6796-6803.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daha M.R., van Es L.A. Relative resistance of the F-42-stabilized classical pathway C3 convertase to inactivation by C4-binding protein. J Immunol. 1980;125:2051–2054. [PubMed] [Google Scholar]
- 7.Deresinski S., Herrera V. Immunotherapies for Staphylococcus aureus: current challenges and future prospects. Infect Control Hosp Epidemiol. 2010;31(Suppl. 1):S45–S47. doi: 10.1086/655992. [DOI] [PubMed] [Google Scholar]
- 8.Dieudonne-Vatran A., Krentz S., Blom A.M., Meri S., Henriques-Normark B., Riesbeck K., Albiger B. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor C4b-binding protein in a PspC allele-dependent fashion. J Immunol. 2009;182:7865–7877. doi: 10.4049/jimmunol.0802376. [DOI] [PubMed] [Google Scholar]
- 9.Dryden M.S. Skin and soft tissue infection: microbiology and epidemiology. Int J Antimicrob Agents. 2009;34(Suppl. 1):S2–S7. doi: 10.1016/S0924-8579(09)70541-2. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez Falcon M.F., Echague C.G., Hair P.S., Nyalwidhe J.O., Cunnion K.M. Protease inhibitors decrease IgG shedding from Staphylococcus aureus, increasing complement activation and phagocytosis efficiency. J Med Microbiol. 2011;60:1415–1422. doi: 10.1099/jmm.0.027557-0. [DOI] [PubMed] [Google Scholar]
- 11.Fey P.D., Endres J.L., Yajjala V.K., Widhelm T.J., Boissy R.J., Bose J.L., Bayles K.W. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio. 2013;4:e00537–12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry D.E., Barie P.S. The changing face of Staphylococcus aureus: a continuing surgical challenge. Surg Infect. 2011;12:191–203. doi: 10.1089/sur.2011.068. [DOI] [PubMed] [Google Scholar]
- 13.Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978;148:1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gigli I., Fujita T., Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci USA. 1979;76:6596–6600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goujon M., McWilliam H., Li W., Valentin F., Squizzato S., Paern J., Lopez R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:W695–9. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould I.M., Reilly J., Bunyan D., Walker A. Costs of healthcare-associated methicillin-resistant Staphylococcus aureus and its control. Clin Microbiol Infect. 2010;16:1721–1728. doi: 10.1111/j.1469-0691.2010.03365.x. [DOI] [PubMed] [Google Scholar]
- 17.Hair P.S., Echague C.G., Rohn R.D., Krishna N.K., Nyalwidhe J.O., Cunnion K.M. Hyperglycemic conditions inhibit C3-mediated immunologic control of Staphylococcus aureus. J Transl Med. 2012;10:35. doi: 10.1186/1479-5876-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hair P.S., Wagner S.M., Friederich P.T., Drake R.R., Nyalwidhe J.O., Cunnion K.M. Complement regulator C4BP binds to Staphylococcus aureus and decreases opsonization. Mol Immunol. 2012;50:253–261. doi: 10.1016/j.molimm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Hair P.S., Ward M.D., Semmes O.J., Foster T.J., Cunnion K.M. Staphylococcus aureus clumping factor A binds to complement regulator factor I and increases factor I cleavage of C3b. J Infect Dis. 2008;198:125–133. doi: 10.1086/588825. [DOI] [PubMed] [Google Scholar]
- 20.Jarva H., Ram S., Vogel U., Blom A.M., Meri S. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J Immunol. 2005;174:6299–6307. doi: 10.4049/jimmunol.174.10.6299. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins H.T., Mark L., Ball G., Persson J., Lindahl G., Uhrin D., Blom A.M., Barlow P.N. Human C4b-binding protein, structural basis for interaction with streptococcal M protein, a major bacterial virulence factor. J Biol Chem. 2006;281:3690–3697. doi: 10.1074/jbc.M511563200. [DOI] [PubMed] [Google Scholar]
- 22.Kask L., Villoutreix B.O., Steen M., Ramesh B., Dahlback B., Blom A.M. Structural stability and heat-induced conformational change of two complement inhibitors: C4b-binding protein and factor H. Protein Sci: Publ Protein Soc. 2004;13:1356–1364. doi: 10.1110/ps.03516504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H.K., Thammavongsa V., Schneewind O., Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol. 2012;15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambris J.D., Sahu A., Wetsel R.A. Marcel Dekker; New York: 1998. The chemistry and biology of C3, C4, and C5; pp. 83–118. [Google Scholar]
- 25.McCrea K.W., Hartford O., Davis S., Eidhin D.N., Lina G., Speziale P., Foster T.J., Hook M. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology (Read, Engl) 2000;146(Pt 7):1535–1546. doi: 10.1099/00221287-146-7-1535. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien L., Kerrigan G., Kaw S.W., Hogan M., Penades J., Litt D., Fitzgerald D.J., Foster T.J., Cox D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol. 2002;44:1033–1044. doi: 10.1046/j.1365-2958.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Connell D.P., Nanavaty T., McDevitt D., Gurusiddappa S., Hook M., Foster T.J. The fibrinogen-binding MSCRAMM (clumping factor) of Staphylococcus aureus has a Ca2+-dependent inhibitory site. J Biol Chem. 1998;273:6821–6829. doi: 10.1074/jbc.273.12.6821. [DOI] [PubMed] [Google Scholar]
- 28.Peacock S.J., Moore C.E., Justice A., Kantzanou M., Story L., Mackie K., O’Neill G., Day N.P. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun. 2002;70:4987–4996. doi: 10.1128/IAI.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Roth E., Alcoba-Florez J., Lopez-Aguilar C., Gutierrez-Gonzalez I., Rivero-Perez B., Mendez-Alvarez S. Familial furunculosis associated with community-acquired leukocidin-positive methicillin-susceptible Staphylococcus aureus ST152. J Clin Microbiol. 2010;48:329–332. doi: 10.1128/JCM.00622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietikainen J., Meri T., Blom A.M., Meri S. Binding of the complement inhibitor C4b-binding protein to Lyme disease Borreliae. Mol Immunol. 2010;47:1299–1305. doi: 10.1016/j.molimm.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Sabat A., Melles D.C., Martirosian G., Grundmann H., van Belkum A., Hryniewicz W. Distribution of the serine-aspartate repeat protein-encoding sdr genes among nasal-carriage and invasive Staphylococcus aureus strains. J Clin Microbiol. 2006;44:1135–1138. doi: 10.1128/JCM.44.3.1135-1138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharp J.A., Echague C.G., Hair P.S., Ward M.D., Nyalwidhe J.O., Geoghegan J.A., Foster T.J., Cunnion K.M. Staphylococcus aureus surface protein SdrE binds complement regulator factor H as an immune evasion tactic. PLoS ONE. 2012;7:e38407. doi: 10.1371/journal.pone.0038407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spellberg B., Daum R. A new view on development of a Staphylococcus aureus vaccine: insights from mice and men. Hum Vaccines. 2010;6 doi: 10.4161/hv.6.10.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stranger-Jones Y.K., Bae T., Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad SciUSA. 2006;103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung H., Guss B., Hellman U., Persson L., Rubin K., Ryden C. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. Biochem J. 2000;345(Pt 3):611–619. [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez V., Liang X., Horndahl J.K., Ganesh V.K., Smeds E., Foster T.J., Hook M. Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp) J Biol Chem. 2012;286:29797–29805. doi: 10.1074/jbc.M110.214981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vytvytska O., Nagy E., Bluggel M., Meyer H.E., Kurzbauer R., Huber L.A., Klade C.S. Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics. 2002;2:580–590. doi: 10.1002/1615-9861(200205)2:5<580::AID-PROT580>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 38.Yang L., Nyalwidhe J.O., Guo S., Drake R.R., Semmes O.J. Targeted identification of metastasis-associated cell-surface sialoglycoproteins in prostate cancer. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.007294. M110 007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young T.P., Maas L., Thorp A.W., Brown L. Etiology of septic arthritis in children: an update for the new millennium. Am J Emerg Med. 2011;29:899–902. doi: 10.1016/j.ajem.2010.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid alignments. (A) Alignment of C4BP alpha-chain with factor H and (B) SdrE with Bbp as determined by Clustal Omega analysis. “*” indicates identical residues, “:” indicates conserved residues, “.” indicates semi-conserved residues between the sequences. The last 553 amino acids of fH are not shown as there was no alignment between the two proteins in this region.


