Abstract
Objective:
The goal of the Response Evaluation in Neurofibromatosis and Schwannomatosis Visual Outcomes Committee is to define the best functional outcome measures for future neurofibromatosis type 1 (NF1)-associated optic pathway glioma (OPG) clinical trials.
Methods:
The committee considered the components of vision, other ophthalmologic parameters affected by OPG, potential biomarkers of visual function, and quality of life measures to arrive at consensus-based, evidence-driven recommendations for objective and measurable functional endpoints for OPG trials.
Results:
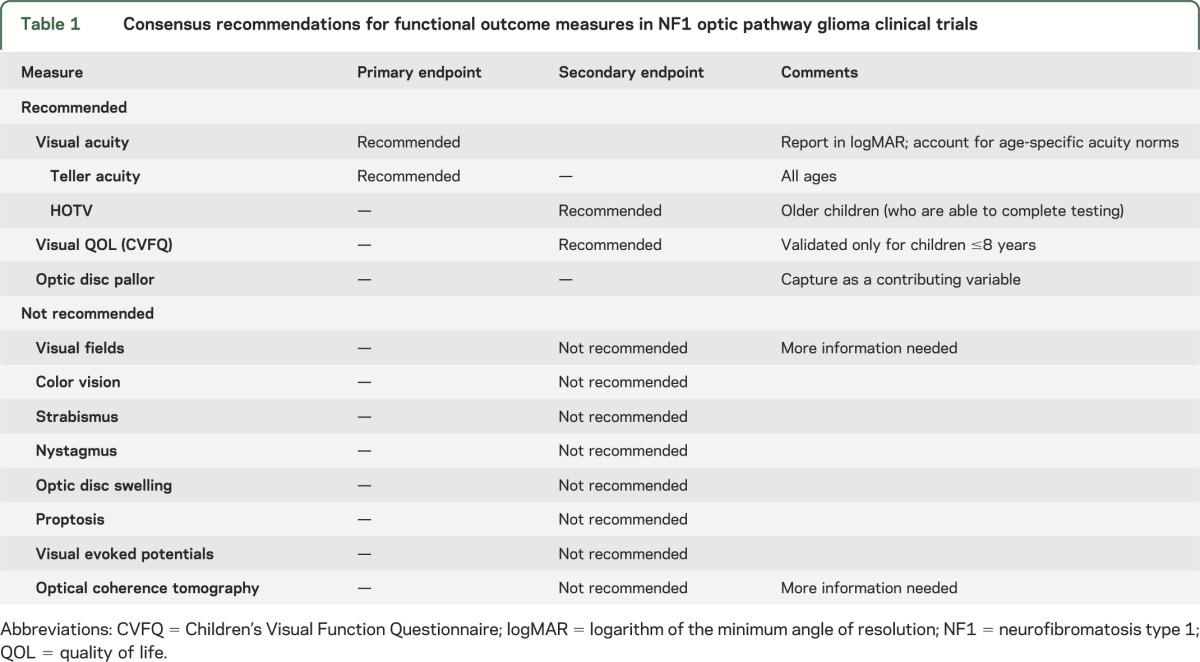
Visual acuity (VA) assessments using consistent quantitative testing methods are recommended as the main functional outcome measure for NF1-OPG clinical trials. Teller acuity cards are recommended for use as the primary VA endpoint, and HOTV as a secondary endpoint once subjects are old enough to complete it. The optic disc should be assessed for pallor, as this appears to be a contributory variable that may affect the interpretation of VA change over time. Given the importance of capturing patient-reported outcomes in clinical trials, evaluating visual quality of life using the Children's Visual Function Questionnaire as a secondary endpoint is also proposed.
Conclusions:
The use of these key functional endpoints will be essential for evaluating the efficacy of future OPG clinical trials.
Optic pathway gliomas (OPG) arise in 15%–20% of children with neurofibromatosis type 1 (NF1), occur preferentially in young children compared with adolescents or adults, and cause vision loss in as many as half of those affected.1 In this regard, the main objective in clinical management of these tumors is preservation of visual function. Although prognostic factors have been identified, there are currently no reliable indicators of future visual loss. This absence of prognostic signs has led clinicians to avoid initiating treatment until visual function has declined. When treatment is indicated, NF1-OPG are typically managed with a combination of carboplatin and vincristine—an approach that has not changed in 15 years.2
To date, OPG clinical trials have focused on imaging outcomes, with tumor response and/or progression-free survival used as measures of treatment success.2–4 However, increasing evidence from case reports, case series, and larger studies indicates that imaging outcomes do not correlate with visual outcomes following treatment.5–7 In fact, in a large multi-institutional retrospective review, only one-third of subjects had concordant visual and imaging outcomes.8 Since the primary goal of treatment is preservation of visual function, therapeutic success should be based on visual rather than imaging endpoints. In order to better understand treatment outcomes for this tumor, future OPG clinical trials will need to mandate functional endpoints as primary outcome measures.
Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) is an international collaborative initiative designed to develop standardized criteria for determining treatment response in patients with NF1, NF2, and schwannomatosis. The overall objective is to identify robust endpoints that can be incorporated into future clinical trials and used to most effectively define and compare treatment efficacy. The goal of the REiNS Visual Outcomes Committee is to define the best functional outcome measures for future clinical trials of NF1-OPG. The committee considered the major psychophysical components of vision (acuity, fields, and color vision), other ophthalmologic elements affected by OPG (optic disc appearance, strabismus, nystagmus, and proptosis), potential biomarkers of visual function (visual evoked potential and optical coherence tomography), and quality of life measures to arrive at consensus-based, evidence-driven recommendations for functional endpoints for OPG trials (table 1).
Table 1.
Consensus recommendations for functional outcome measures in NF1 optic pathway glioma clinical trials
THE BEST FUNCTIONAL ENDPOINT
Visual acuity.
Visual acuity (VA) reflects visual pathway integrity, making it an ideal candidate to objectively measure the visual impact of an OPG. It is likely the most important functional ophthalmologic feature, as modest amounts of VA loss can affect activities of daily living.9,10 There is good test-retest reliability, assessments can be standardized, and the intervals of change (lines on an eye chart) are universally understood and quantifiable. Furthermore, ophthalmologists and ophthalmology technicians universally understand how to perform acuity testing.
VA testing has been used reliably and is sensitive to change in clinical trials for other visual diseases (e.g., amblyopia, diabetic retinopathy).11,12 To date, VA is the only visual outcome measure that has been assessed to any major extent in OPG, as well as the only measure that has been shown to be sensitive to change with treatment. In a large retrospective study, 32% of subjects with NF1-OPG experienced an improvement in VA following treatment with chemotherapy.8 In addition, a decline in VA is the most common reason to initiate therapy for OPG, and other potential markers of functional decline, such as visual field (VF) or color vision loss, typically occur concurrently with acuity decline.8 As such, VA is the best studied and most reliable functional measure of vision for OPG and other visual diseases.
How should VA be tested?
For the purpose of clinical trials, it is crucial that the selection of testing method is quantifiable so that the magnitude of change can be accurately measured over time. Qualitative measures, such as “fix and follow” will not always detect VA changes.13 For example, a 2-year-old whose VA changes from 20/40 to 20/100 will still fix and follow. There are multiple testing methods available to assess VA quantitatively. The choice of method depends on a child's age, developmental/cognitive level, and ability to cooperate. A detailed discussion of VA testing methods and challenges is beyond the scope of this report, but the topic has been previously reviewed.13
Quantitative testing methods (Teller acuity cards [TAC]) exist for children as young as 6 months of age and are reliable measures of VA. TAC (figure) is a preferential looking test that relies on an infant's propensity to redirect his or her gaze toward a visually interesting stimulus (alternating high-contrast black and white lines). VA is quantified by knowing the distance to the stimulus and the width of the smallest lines the child is able to appreciate. Other methods for testing the youngest age group include Cardiff acuity cards, which use pictures of varying contrast; however, these have not been studied as widely as an outcome measure for clinical trials. In older children, testing methods measure the ability to recognize (“recognition acuity”) a figure (e.g., Lea symbols) or letters (e.g., HOTV or Snellen) (figure). The complexity of the test increases with age, such that a higher level of cognition and cooperation is required to complete HOTV compared with Lea, and Snellen testing compared with HOTV. This makes testing in children with NF1 particularly challenging, as a large proportion have baseline deficits in attention and/or learning.
Figure. Visual acuity testing methods.
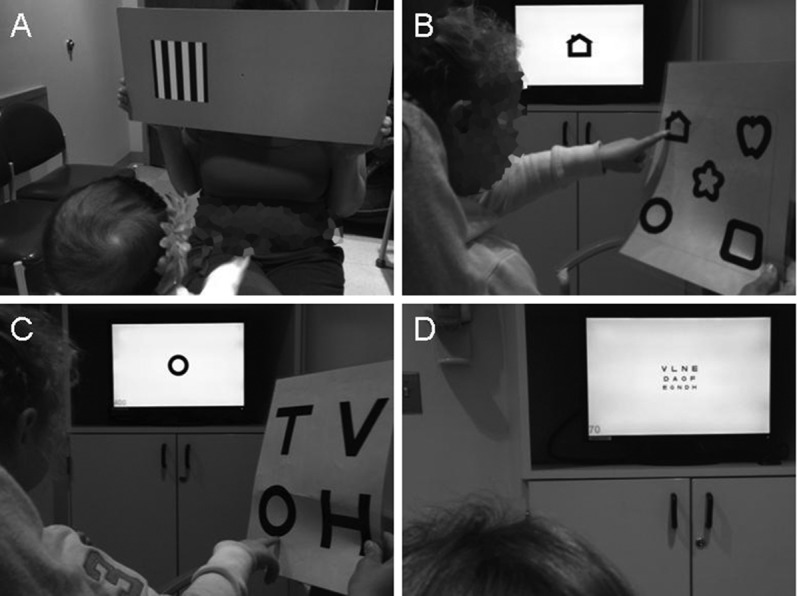
(A) Teller acuity cards, (B) Lea, (C) HOTV, (D) Snellen.
One of the challenges in monitoring VA over time in children enrolled in a clinical trial is that the preferred clinical VA testing method may change as the child gets older. Although there is reasonable correlation of VA results between testing formats, they are not identical.13 For example, when VA is near normal, TAC may underestimate VA relative to recognition acuity.14 In contrast, TAC may overestimate VA compared with recognition acuity when VA is moderately abnormal or worse.15 Hence, transitioning between testing formats may confound the interpretation of acuity changes over time. Therefore, we recommend not switching testing formats for subjects during the treatment and follow-up phases of the study. In addition, we would limit testing methods to TAC and HOTV, as these are relatively easy to perform, standardized testing methods exist, and both methods have been validated in clinical trials for other pediatric ophthalmologic diseases (e.g., retinopathy of prematurity, amblyopia, cataracts).11,12,16 Of note, it is important to use a standardized TAC and HOTV testing method at all study sites.
To provide consistency from study enrollment through study completion, we suggest the use of TAC as the primary VA endpoint for all subjects regardless of age, as this is the only visual testing method that all subjects who are old enough for quantitative VA testing can perform. Of note, in the retrospective study of visual outcomes following chemotherapy, 37.5% of subjects were unable to complete HOTV testing at the start of treatment.8 In addition, in a study of 127 subjects 10 years and younger with OPG (NF1 and non-NF1), 30.7% could not complete HOTV testing, and the number rose to 67.3% in children younger than 5 years of age.17 Although the committee considered allowing HOTV as a primary outcome measure for those capable of performing it at study entry, we rejected this for several reasons. First, in a retrospective study8 a small percentage (3%) of children who were able to perform HOTV at the start of therapy were unable to perform it successfully at study end and required Lea or TAC testing (data not published). Second, although TAC and HOTV acuities are similar, they are not identical, thus making comparisons between subjects challenging.17 Last, the interval between “lines” on the chart for TAC and HOTV is not equivalent.
However, once a subject is old enough to perform testing, we recommend adding HOTV testing as a secondary endpoint. Although Teller acuity can be converted to a recognition acuity equivalent, the latter is a more accurate reflection of how we understand VA. Therefore, it is important to capture a recognition acuity measure that can place the Teller acuity in context. In addition, these data will facilitate a better evaluation of long-term changes in VA as a subject enters adulthood. HOTV testing is preferred because it can be started at a younger age and is more feasible in children with NF1-related cognitive or behavioral problems than other recognition acuity testing methods. Importantly, this recommendation does not preclude testing using an alternate method (e.g., Snellen) for clinical purposes.
How should VA be reported?
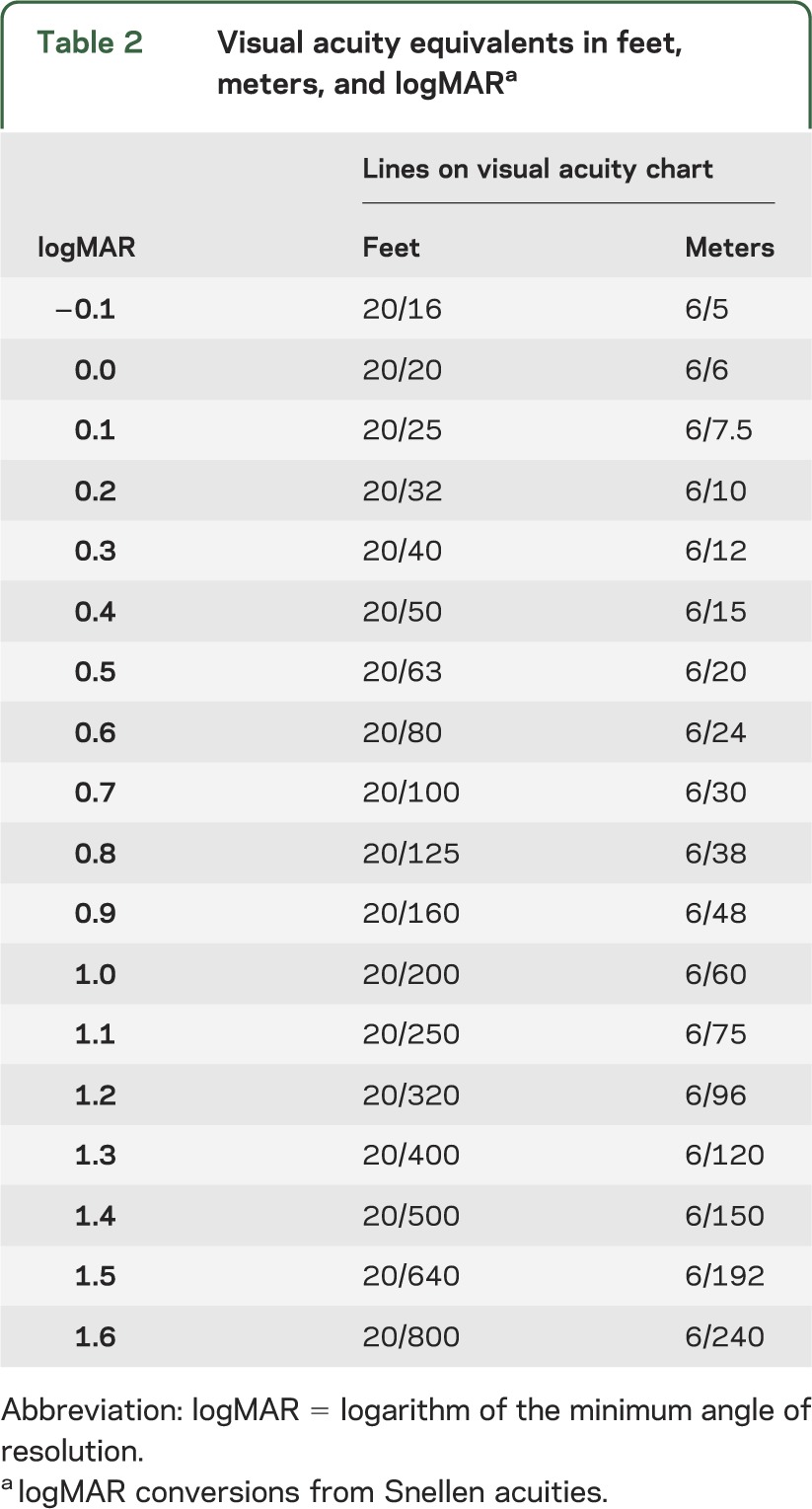
Clinically, VA is typically reported as a fraction (e.g., 20/20 in feet, 6/6 in meters), and change in acuity is usually described by the difference in the number of lines on the eye chart between testing sessions. Unfortunately, the difference between lines can vary not only between testing methods but also within the same method.13 Therefore, in order to standardize the quantification of magnitude of change, we recommend that VA be reported using the logarithm of the minimum angle of resolution (logMAR). For recognition acuity measures, this linear scale is created by calculating the base 10 logarithm of 1/(VA decimal notation [e.g., the decimal equivalent for 20/40 = 0.5]), a practice widely used in clinical ophthalmologic research. Intervals of change between lines on logMAR charts are therefore of equal magnitude (table 2). TAC can also be directly converted to logMAR (logMAR = −1 × log[spatial frequency/30]). It is worth recognizing that classifying VA into categories (e.g., good, fair, poor) is problematic as small nonfunctional changes in VA can result in a change in VA category. Reporting VA as a continuous measure will allow for a more detailed evaluation of visual change over time.
Table 2.
Visual acuity equivalents in feet, meters, and logMARa
It is equally important to account for the normal development of VA during early childhood. Normal VA improves with age in young children, thereby necessitating different age-based norms (e.g., 20/40 is normal at age 3 years, 20/20 is normal at age 6 years).13 Thus, we recommend calculating an age-based VA by comparing all values to normal for age VA (normal VA for age − current VA) and reporting the difference in logMAR from normal.
When reporting study results, we suggest reporting both per-subject and per-eye outcomes. The latter is standard in the ophthalmology literature and is important as vision may be affected in only one eye. However, in a subject with a unilateral optic nerve glioma, the unaffected eye should be excluded, as it will bias the results. Per-subject reporting is also informative as illustrated by the following potential scenario: the VA improves in one eye but worsens in the other during therapy; this subject should be coded as a treatment failure and come off study. In addition, although reporting vision by the better or worse eye may be useful when reporting long-term outcomes, its relevance for assessing the effectiveness of a chemotherapeutic agent is unclear. Reporting intervals should match those of radiologic reporting. For consistency, we recommend reporting results at one or more of the following times: end of therapy and 1-, 2-, 3-, and/or 5-year follow-up.
What constitutes visual progressive disease or response?
At present, there is no validated definition of clinically significant VA change. Several previous OPG studies have used a 2-line change (approximately 0.2 logMAR) from baseline, but this has not been validated. However, given that the ophthalmologic literature reveals a roughly 1-line variation between observers and testing sessions,18,19 using a 2-line change is reasonable. We recognize the potential risk of decreased specificity by using such a narrow definition; however, we feel this is offset by increased sensitivity to early decline in VA. We therefore recommend defining a significant VA change as a 0.2 or greater change in logMAR. When visual response is detected, it should be confirmed at a subsequent study visit to be considered “durable.”
How should ceiling and floor effects be handled?
It is critical to consider ceiling and floor effects for VA when establishing clinical trial enrollment criteria and analyzing study data. For example, eyes with normal VA (20/20 = 0.0 logMAR or better) at baseline cannot improve and should be eliminated from a study targeting visual response. In contrast, blind eyes cannot worsen and should be excluded from studies of visual progression-free survival (PFS). Based on our definition of visual progression or response (±0.2 logMAR or greater change), we recommend a ceiling of 0.2 logMAR below normal for age or 0.2 logMAR below a previous reliably documented VA. We set the floor at 1.36 logMAR (20/470), which is the VA equivalent of the lowest Teller acuity card from which a 0.2 logMAR decline can be measured with good reliability (using a standard testing distance of 55 cm). Based on these considerations, we recommend that one eye must be evaluable (0.2 logMAR below normal for age or below a previous reliably documented VA − 1.36 logMAR) in order to enroll in a therapeutic OPG trial. When analyzing results, if the study endpoint is best visual response, then eyes with VA at the ceiling should be excluded. If the study endpoint is visual PFS, then eyes with VA at the floor should be excluded in the analysis.
How often should VA be monitored while on study?
At present, no evidence exists to recommend an ideal monitoring interval to determine visual progression or response in a clinical trial. Thus, we recommend monitoring on the same schedule as imaging outcomes, which is typically every 3 months while on treatment. Following completion of treatment, we recommend monitoring at 3, 6, 12, 18, 24, 30, 36, 48, and 60 months.
What if the examination is unreliable due to poor cooperation or a change in VA is detected or suspected?
If the results of a VA evaluation are in doubt because of poor effort or cooperation, testing should be repeated in 1 to 2 weeks. If the result is still unreliable, then no data should be entered for that visit. If the clinician is absolutely certain the VA change is not related to effort/cooperation, then a repeat examination is not required. In most instances, however, the exam will need to be repeated. If VA loss is observed, it is crucial to exclude refractive error, amblyopia, or other non-OPG-related causes.
OTHER POSSIBLE FUNCTIONAL ENDPOINTS
Visual fields.
VF deficits may occur in association with OPG; therefore, recommendations for testing visual function in children with NF1 have included VF testing during selected ophthalmology evaluations.1,20 There is a range of methods for VF testing, including simple confrontation (testing the ability to see fingers in all 4 quadrants) and computerized techniques (Goldmann or Humphrey perimetry). Confrontation testing is not as sensitive as perimetry but can be useful for detecting large deficits (e.g., quadrantanopia or hemianopia).21 In contrast, the clinical significance of the smaller changes noted with perimetry for patients with OPG is unclear. Although all VF testing methods require the patient to be alert and cooperative and to maintain fixation reliably, the testing time for perimetry (5–7 minutes per eye) is longer than for other measures of visual function (e.g., acuity). Given the high incidence of learning difficulties and attention deficit disorder in children with NF1, there are concerns about the reliability of testing as well as the elevated false-positive and false-negative rates in children younger than 10 years of age. In an audit of children with NF1 up to 7 years of age, none of the children was mature enough to reliably complete VF testing.22
In the OPG literature, most studies do not report VF, do so only in a small subset of subjects, or do not provide within-subjects comparisons. In addition, details regarding VF testing method or consistency with quantification are often lacking. Several studies reveal that VF deficits are usually (89%–100%) associated with concurrent VA deficits,8,23 VF change in the absence of VA change is rarely a treatment indication,8 and VF outcome mostly mirrors VA outcome following treatment.8
In summary, given the concerns about the reliability of VF testing in this patient population, the lack of adequate data from prior studies (especially compared with VA), and the fact that VF deficits are usually associated with concurrent VA deficits, the committee does not endorse VF as a primary outcome measure for OPG clinical trials. A more thorough evaluation of VF in NF1-OPG is required before routine inclusion as a secondary outcome measure can be recommended.
Color vision.
Standard color vision testing (e.g., Ishihara) requires cooperation and the ability to read numbers or identify shapes; thus, most very young children with NF1-OPG are unable to complete testing.22 Color vision data are rarely collected,24 and changes in color vision over time are rarely reported in OPG studies. Testing color vision may be helpful in differentiating the cause of VA loss. In this respect, color vision is usually spared in amblyopia or refractive error, whereas color vision loss typically accompanies the VA loss due to OPG.1 Therefore, color vision loss is unlikely to provide additional information about disease progression relative to VA. In summary, while color vision testing may be a good adjunct measure in clinical trials, further study is needed before it can be recommended for routine inclusion.
Strabismus.
Strabismus (ocular misalignment) is measured in prism diopters, but the variability in measurements may be as much as 6 diopters, and it may be difficult to quantify reliably in uncooperative children.25 Although eye deviation in OPG may be due to optic nerve enlargement with downward displacement of the globe, it is usually secondary to VA loss in the affected eye (i.e., sensory strabismus).1 No correlation has been reported between the amount of VA loss and the occurrence or severity of strabismus. In addition, strabismus is rarely commented on or followed longitudinally in large OPG series, and its incidence is much lower than that of VA loss.8,26–28 Therefore, at present strabismus is not recommended as an outcome measure to follow OPGs.
Nystagmus.
Nystagmus (rhythmic oscillation of the eyes) can be a presenting sign of an OPG, typically one located in the optic chiasm/hypothalamus. As with strabismus, nystagmus in OPG is usually secondary to VA loss in the affected eye(s).1 Few studies report on nystagmus, but in those that do the incidence is low (2%–19%).8,27,28 It is difficult to quantify nystagmus without sophisticated eye movement recordings, and there does not appear to be a correlation between continued VA decline and the severity of the nystagmus. Given these factors, the committee does not recommend following nystagmus as an outcome measure for OPG.
Optic disc swelling.
Optic disc swelling is the visible elevation of the optic disc with blurring of the disc margin seen on fundus exam. Its incidence in OPG varies widely (up to 21%) in the few studies that report on optic disc swelling.8,26,28 It appears to be more commonly associated with tumor involvement of the optic nerve.28 Disc swelling from chiasmal lesions more frequently reflects elevated intracranial pressure from obstruction of CSF flow. Although disc swelling may be associated with a change in the size of the OPG on MRI scan, it is not always associated with demonstrable VA loss. In addition, optic disc swelling is not predictive of VA outcome, as it improves in almost all subjects (91%) following chemotherapy.8 For these reasons, it is not recommended as an outcome measure to follow OPGs.
Optic disc pallor.
Optic disc pallor corresponds to atrophy of the optic nerve fibers and can reflect damage anywhere along the optic pathway from the retina to the lateral geniculate nucleus. It is easy to visualize on fundus exam, is usually commented on in OPG studies, and is present in almost half of OPG cases (combined data of 18 studies). Unfortunately, it is not clear whether disc pallor is associated with vision loss, given that few studies have attempted to evaluate this relationship, often with small sample sizes and mixtures of NF1/sporadic OPG and treated/untreated OPG. Optic disc pallor often occurs in patients with OPG without a VA or VF deficit and may be absent in those with vision loss. In addition, the development of disc pallor can lag behind the appearance of a VA deficit and vice versa. One study suggests that optic disc pallor at the start of chemotherapy may be associated with worse VA outcomes; however, change in disc pallor over time is not a useful marker, as it almost never improves.8
In external compressive tumors of the optic chiasm (e.g., pituitary adenoma or craniopharyngioma), disc pallor correlates with the presence of decreased VA but not the degree of vision loss.29–31 However, the predictive value of pallor for VA recovery following tumor decompression is variable, although it appears that those with “mild” pallor may be more likely to have some recovery of vision.32,33 This observation is also supported by optical coherence tomography studies of the retinal nerve fiber layer in this population.34 The degree of disc pallor has also been suggested to be an important factor in VA outcome for patients with OPG5 and those with atrophy from any cause35; however, in these studies, the degree of pallor was determined subjectively (which is affected by the degree of pigmentation of the fundus) or by using photographic slides (in which the color of the optic disc depends on the length of exposure).
In summary, monitoring changes in disc pallor over time during a clinical trial does not appear useful, as it rarely improves. Disc pallor is likely associated with VA loss, but not universally. Although the degree of disc pallor may be the more relevant feature, at present there is no accepted scale to grade pallor or data indicating that it can be measured reliably, even using photographs. However, because of the correlation between baseline disc pallor and VA outcomes in treated subjects as well as the possible implication that pallor may be an indicator of preexisting damage that heralds subsequent vision loss,5,8 we recommend capturing disc pallor (present or not) to define its role as a contributing variable or correlative marker in future OPG trials.
Proptosis.
There are scant objective data on measuring proptosis in orbital tumors. Measurements can be performed by exophthalmometry,36 and normative measurements based on age have been reported in children.37,38 Although there is interobserver reliability of exophthalmometry in healthy adults with and without Graves disease,39 there is no known large study examining exophthalmometry in children with orbital tumors. In contrast, proptosis has been measured successfully and serially using MRI in children with optic nerve gliomas40 and may be a more accurate method of measuring the degree of proptosis; however, a recent study demonstrated that improvement in proptosis did not correlate with tumor shrinkage.41 Most importantly, it is unclear how to quantify the functional impact of a change in proptosis. In addition, patients with proptosis comprise a minority of subjects enrolled in OPG trials. Further study will be required before considering the inclusion of routine proptosis measurements in future therapeutic studies.
Visual evoked potentials.
Visual evoked potentials (VEPs) are an electrophysiologic test believed to provide a functional measure of visual pathway integrity. Although VEP testing may detect OPG with some sensitivity,42–46 some patients with NF1 have abnormal VEP testing despite no evidence of glioma.47 Of greater concern is the poor diagnostic sensitivity of VEP for VA loss48 and the poor correlation of VEP changes over time with VA changes and response to treatment.49,50 Several other issues limit the utility of VEP for OPG clinical trials, including the challenge of testing young children because of the level of cooperation required, the lack of standardization of testing methods, the absence of a validated definition of the amount of change that defines “worsening” in longitudinal studies, the lack of universal availability of the equipment, and the variability in measures using different equipment. Therefore, the committee does not recommend the routine inclusion of VEP testing for OPG clinical trials.
Optical coherence tomography.
The axons of the retinal ganglion cells, termed the retinal nerve fiber layer (RNFL), combine to form the pregeniculate portion of the afferent visual pathway (i.e., optic nerve, chiasm, and tracts). RNFL thickness, a structural marker of visual pathway integrity, can be measured using optical coherence tomography (OCT). Thinning of the RNFL correlates with VA and/or VF deficits in patients with optic neuritis and multiple sclerosis (MS).51,52 Studies also suggest that significant RNFL thinning predicts persistence of visual deficits over time for both MS and compressive tumors of the optic chiasm.34,53 In addition, decreased RNFL thickness correlated with visual loss (VA and/or VF) in a cross-sectional study of children (6–21 years of age) with OPG.54 This study is currently being replicated in children with OPG younger than 6 years using portable OCT equipment (necessary for evaluating young children).55 Ultimately, longitudinal studies will be required to determine whether a decrease in RNFL thickness is predictive of future vision loss and whether changes in RNFL thickness over time correlate with changes in visual function. In addition, portable OCT equipment is not yet widely available, RNFL measurements vary between different OCT machines, and the reliability of RNFL measures between centers using the same equipment is unknown. Hence, routine inclusion in clinical trials for OPG cannot be recommended at this time.
Visual quality of life.
Patient-reported outcomes, specifically those assessing quality of life (QOL), have emerged as important measures for use in clinical treatment trials. VA loss has been reported to affect markedly an individual's employment and overall QOL.9,10 To evaluate the direct impact of vision loss on particular QOL domains, vision-specific QOL instruments have been developed for adults and were found to correlate with the degree of visual impairment.56–58 Visual ability and QOL measures that assess the impact of vision loss in children have been examined in a variety of pediatric eye diseases, with most designed to evaluate children between 8 and 18 years of age.59–63 Using the review process developed by the REiNS Patient-Reported Outcomes Subcommittee (see Wolters et al., this supplement), we reviewed the available pediatric questionnaires. Of these, 2 examined visual ability rather than vision-specific QOL.62,63 Two vision-specific QOL measures have been developed for children,59,60 although the Children's Visual Function Questionnaire (CVFQ)59 is the only instrument designed to evaluate children 8 years of age and younger, the time during which most patients with NF1-OPG become symptomatic. To date, no studies have been published that evaluate the impact of OPG-related vision loss on vision-specific QOL in children. The inclusion of a vision-specific QOL measure in NF1-OPG clinical trials is complicated by a number of factors, including the potential differential impact of VA vs VF loss, the use of parent proxy reporting, the wide age range of subjects enrolled, and the relevance of the vision loss based on age. For example, moderate vision loss may have only a modest impact on QOL for a 3-year-old, whereas in an adolescent the same degree of vision loss may result in the inability to drive a motor vehicle, which might result in a more profound effect on QOL. Additionally, it is unclear whether and how the known developmental and behavioral complications of NF1 might influence the accuracy and relevance of QOL measures. Therefore, the committee does not recommend a vision-specific QOL measure as a primary outcome at this time, although the CVFQ could be considered as a secondary outcome measure in children 8 years of age and younger. Ultimately, development of a NF1-OPG-specific QOL measure or adapting the CVFQ to include domains relevant to all age groups may be helpful and is currently being considered.
REiNS RECOMMENDATIONS
VA should be the main functional outcome measure in clinical trials of children with NF1-OPG. The use of quantitative testing methods is essential, and the testing format should not be changed during the study. To that end, we recommend the use of TAC as the primary VA endpoint and HOTV as a secondary endpoint once subjects are old enough. Results should be reported in logMAR, while taking into account the acuity age-specific norms. The optic disc should be assessed for pallor, as this appears to be a contributory variable that may affect the interpretation of VA change over time. Given the importance of capturing patient-reported outcomes in clinical trials, the committee endorses collecting visual QOL using the CVFQ as a secondary endpoint, as this is currently the best available measure. Collectively, the implementation of these endpoints in future clinical trials will facilitate the evaluation of potential promising agents for the treatment of NF1-OPG.
ACKNOWLEDGMENT
The committee acknowledges Vanessa Merker, BS, Massachusetts General Hospital; Mark Kieran, MD, PhD, Boston Children's Hospital; and Graham Quinn, MD, The Children's Hospital of Philadelphia for their helpful input as well as Scott Plotkin, MD, PhD, Massachusetts General Hospital, Brigitte Widemann MD, National Cancer Institute, and the other members of the REiNS International Collaboration. The committee also acknowledges the helpful input of the representatives of the International Society of Pediatric Oncology Low Grade Glioma committee (Joseph Abbott, Silva Atamian, Catherine Cassiman, Maurizio Clementi, Pablo Hernáiz Driever, Chris Hammond, Darren Hargrave, Kamilla Rothe Nissen, Enrico Opocher, Susan Picton, Arun Reginald, Astrid Sehested, Ian Simmons, Irene Slavc, David Walker).
GLOSSARY
- CVFQ
Children’s Visual Function Questionnaire
- logMAR
logarithm of the minimum angle of resolution
- MS
multiple sclerosis
- NF1
neurofibromatosis type 1
- OCT
optical coherence tomography
- OPG
optic pathway glioma
- PFS
progression-free survival
- QOL
quality of life
- REiNS
Response Evaluation in Neurofibromatosis and Schwannomatosis
- RNFL
retinal nerve fiber layer
- TAC
Teller acuity cards
- VA
visual acuity
- VEP
visual evoked potential
- VF
visual field
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
MJ Fisher: drafting the manuscript, study concept, interpretation of data. RA Avery: revising the manuscript for content, study concept, interpretation of data. JC Allen: revising the manuscript for content, study concept, interpretation of data. SL Ardern-Holmes: revising the manuscript for content, study concept, interpretation of data. LT Bilaniuk: revising the manuscript for content, study concept, interpretation of data. RE Ferner: revising the manuscript for content, study concept, interpretation of data. DH Gutmann: revising the manuscript for content, study concept, interpretation of data. R Listernick: revising the manuscript for content, study concept, interpretation of data. S Martin: revising the manuscript for content, study concept, interpretation of data. NJ Ullrich: revising the manuscript for content, study concept, interpretation of data. GT Liu: revising the manuscript for content, study concept, interpretation of data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
M. Fisher received reimbursement from the Children's Tumor Foundation to attend their annual Neurofibromatosis Conference, is funded by the Department of Defense (W81XWH-12-1-0155, W81XWH-05-1-0615), Thrasher Research Fund, the Children's Tumor Foundation, and Sarcoma Alliance for Research through Collaboration, and received research support from the Pediatric Low Grade Astrocytoma Foundation, Bayer, Children's Discovery Institute, NIH (NR009651-01), and the Department of Defense (W81XWH-08-1-0051). R. Avery is funded by NIH grants K23-EY022673 and UL1RR031988/UL1TR000075, and received research support from the Gilbert Family Neurofibromatosis Institute. J. Allen reports no disclosures. S. Ardern-Holmes served on a scientific advisory board for Novartis Pharmaceuticals Australia, received funding for a trip from Novartis Pharmaceuticals Australia, and receives research support from The Children's Tumor Foundation of Australia. L. Bilaniuk receives research support from the NICHD #UO1HD068541-01. R. Ferner received funding for travel from the Children's Tumor Foundation and the European Neurofibromatosis Association. She receives royalties from Springer for the book Neurofibromatoses in Clinical Practice. D. Gutmann serves on the scientific advisory board of the Brain Tumor Funder's Collaborative and the editorial board of Experimental Neurology. He holds the patent on the neurofibromatosis gene (U.S. Patent No. 5,859,195), for which he receives royalties annually, and a patent for Neurofibromin Pathway Modulators (U.S. Patent No. 8,101,606). He has received honoraria for invited lectureships at UCSD, Johns Hopkins University, Memorial Sloan-Kettering Cancer Center, Cleveland Clinic Foundation, University of Minnesota, Biomarin, MD Anderson Cancer Center, University of Toronto, Dana Farber Cancer Institute, and the University of Chicago. He receives research support from the National Brain Tumor Society, James S. McDonnell Foundation, Department of Defense, National Cancer Institute (grants CA136573, CA141549, and CA160882), Children's Discovery Institute (grant MC-II-2012-212), and the NIH (grants NS065547 and NS072916). R. Listernick serves as an editorial board member of Pediatric Annals. He received honoraria for oral presentations from Children's Hospital of Colorado and American Academy of Pediatrics. S. Martin received funding for a trip from the Children's Tumor Foundation. N. Ullrich receives funding from the Department of Defense (W81XWH-05-1-0615), the NIH (NCT00879034), the National Cancer Foundation Children's Oncology Group, and the Children's Tumor Foundation. She holds patents for a method of diagnosing and treating gliomas (US 5905027, 6028174, 6319891, 6429187, and 6870029), for which she receives royalty payments from the University of Alabama at Birmingham Research Foundation. She also receives royalties from UpToDate for the publication of “The “choking game” and other strangulation activities in children and adolescents.” She has received travel expenses and/or honoraria for lectures or educational activities not funded by industry, and has served as an expert witness for O’Connor, O’Connor, Bresee & First and Bays, Lung, Rose & Holma. G. Liu has consulted for Ipsen and received book royalties from Elsevier. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol 2007;61:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg 1997;86:747–754 [DOI] [PubMed] [Google Scholar]
- 3.Ater J, Holmes E, Zhou T, et al. Abstracts from the thirteenth international symposium on pediatric neuro-oncology: results of COG protocol A9952- a randomized phase 3 study of two chemotherapy regimens for incompletely resected low-grade glioma in young children. Neuro Oncol 2008;10:451 [Google Scholar]
- 4.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol 1997;32:235–241 [DOI] [PubMed] [Google Scholar]
- 5.Campagna M, Opocher E, Viscardi E, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer 2010;55:1083–1088 [DOI] [PubMed] [Google Scholar]
- 6.Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer 2010;46:2253–2259 [DOI] [PubMed] [Google Scholar]
- 7.Shofty B, Ben-Sira L, Freedman S, et al. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer 2011;57:481–485 [DOI] [PubMed] [Google Scholar]
- 8.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol 2012;14:790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahi JS, Cumberland PM, Peckham CS. Visual function in working-age adults: early life influences and associations with health and social outcomes. Ophthalmology 2009;116:1866–1871 [DOI] [PubMed] [Google Scholar]
- 10.Rahi JS, Cumberland PM, Peckham CS. Visual impairment and vision-related quality of life in working-age adults: findings in the 1958 British birth cohort. Ophthalmology 2009;116:270–274 [DOI] [PubMed] [Google Scholar]
- 11.Dobson V, Quinn GE, Biglan AW, Tung B, Flynn JT, Palmer EA. Acuity card assessment of visual function in the cryotherapy for retinopathy of prematurity trial. Invest Ophthalmol Vis Sci 1990;31:1702–1708 [PubMed] [Google Scholar]
- 12.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol 2001;132:903–909 [DOI] [PubMed] [Google Scholar]
- 13.Avery RA, Ferner RE, Listernick R, Fisher MJ, Gutmann DH, Liu GT. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol 2012;110:1–7 [DOI] [PubMed] [Google Scholar]
- 14.Dobson V, Quinn GE, Tung B, Palmer EA, Reynolds JD. Comparison of recognition and grating acuities in very-low-birth-weight children with and without retinal residua of retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Invest Ophthalmol Vis Sci 1995;36:692–702 [PubMed] [Google Scholar]
- 15.Kushner BJ, Lucchese NJ, Morton GV. Grating visual acuity with Teller cards compared with Snellen visual acuity in literate patients. Arch Ophthalmol 1995;113:485–493 [DOI] [PubMed] [Google Scholar]
- 16.Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol 2010;128:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery RA, Bouffet E, Packer RJ, Reginald A. Feasibility and comparison of visual acuity testing methods in children with neurofibromatosis type 1 and/or optic pathway gliomas. Invest Ophthalmol Vis Sci 2013;54:1034–1038 [DOI] [PubMed] [Google Scholar]
- 18.Getz LM, Dobson V, Luna B, Mash C. Interobserver reliability of the Teller Acuity Card procedure in pediatric patients. Invest Ophthalmol Vis Sci 1996;37:180–187 [PubMed] [Google Scholar]
- 19.Harvey EM, Dobson V, Tung B, Quinn GE, Hardy RJ. Interobserver agreement for grating acuity and letter acuity assessment in 1- to 5.5-year-olds with severe retinopathy of prematurity. Invest Ophthalmol Vis Sci 1999;40:1565–1576 [PubMed] [Google Scholar]
- 20.Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet 2007;44:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr NM, Chew SS, Eady EK, Gamble GD, Danesh-Meyer HV. Diagnostic accuracy of confrontation visual field tests. Neurology 2010;74:1184–1190 [DOI] [PubMed] [Google Scholar]
- 22.Pilling RF, Lloyd IC, Huson S. Utility of optic pathway glioma screening in young children with neurofibromatosis type I: questions generated by a clinical audit. Eye (Lond) 2010;24:1603–1605 [DOI] [PubMed] [Google Scholar]
- 23.Sigorini M, Zuccoli G, Ferrozzi F, et al. Magnetic resonance findings and ophthalmologic abnormalities are correlated in patients with neurofibromatosis type 1 (NF1). Am J Med Genet 2000;93:269–272 [DOI] [PubMed] [Google Scholar]
- 24.Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol 2007;9:430–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu GT, Volpe NJ, Galetta SL. Neuro-Ophthalmology: Diagnosis and Management, 2nd ed London: Saunders Elsevier; 2010 [Google Scholar]
- 26.Nicolin G, Parkin P, Mabbott D, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer 2009;53:1231–1237 [DOI] [PubMed] [Google Scholar]
- 27.Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology 2004;111:568–577 [DOI] [PubMed] [Google Scholar]
- 28.Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol 2003;28:262–270 [DOI] [PubMed] [Google Scholar]
- 29.Hollenhorst RW, Younge BR. Ocular manifestations produced by adenomas of the pituitary gland: analysis of 1,000 cases. In: Kohler PO, Ross GT, eds. Diagnosis and Treatment of Pituitary Tumors. Amsterdam: Excerpta Medica-American Elsevier; 1972:53–64 [Google Scholar]
- 30.Kennedy HB, Smith RJ. Eye signs in craniopharyngioma. Br J Ophthalmol 1975;59:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melen O. Neuro-ophthalmologic features of pituitary tumors. Endocrinol Metab Clin North Am 1987;16:585–608 [PubMed] [Google Scholar]
- 32.Cohen AR, Cooper PR, Kupersmith MJ, Flamm ES, Ransohoff J. Visual recovery after transsphenoidal removal of pituitary adenomas. Neurosurgery 1985;17:446–452 [DOI] [PubMed] [Google Scholar]
- 33.Lennerstrand G. Visual recovery after treatment for pituitary adenoma. Acta Ophthalmol (Copenh) 1983;61:1104–1117 [DOI] [PubMed] [Google Scholar]
- 34.Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci 2008;49:1879–1885 [DOI] [PubMed] [Google Scholar]
- 35.DeWitt CA, Johnson LN, Schoenleber DB, Hainsworth DP, Madsen RW. Visual function in patients with optic nerve pallor (optic atrophy). J Natl Med Assoc 2003;95:394–397 [PMC free article] [PubMed] [Google Scholar]
- 36.Hertel E, Simonsz HJ. A simple exophthalmometer. Strabismus 2008;16:89–91 [DOI] [PubMed] [Google Scholar]
- 37.Dijkstal JM, Bothun ED, Harrison AR, Lee MS. Normal exophthalmometry measurements in a United States pediatric population. Ophthal Plast Reconstr Surg 2012;28:54–56 [DOI] [PubMed] [Google Scholar]
- 38.Nucci P, Brancato R, Bandello F, Alfarano R, Bianchi S. Normal exophthalmometric values in children. Am J Ophthalmol 1989;108:582–584 [DOI] [PubMed] [Google Scholar]
- 39.Mourits MP, Lombardo SH, van der Sluijs FA, Fenton S. Reliability of exophthalmos measurement and the exophthalmometry value distribution in a healthy Dutch population and in Graves' patients. An exploratory study. Orbit 2004;23:161–168 [DOI] [PubMed] [Google Scholar]
- 40.Diaz RJ, Laughlin S, Nicolin G, Buncic JR, Bouffet E, Bartels U. Assessment of chemotherapeutic response in children with proptosis due to optic nerve glioma. Childs Nerv Syst 2008;24:707–712 [DOI] [PubMed] [Google Scholar]
- 41.Nguyen-Phuc AY, Khrichenko D, Feygin T, Fisher MJ, Liu GT. Abstracts from the 65th annual meeting of the American Academy of Neurology: proptosis in optic pathway gliomas associated with neurofibromatosis: response to chemotherapy. Neurology 2013. (http://www.abstracts2view.com/aan/view.php?nu=AAN13L_P06.004). [Google Scholar]
- 42.Chang BC, Mirabella G, Yagev R, et al. Screening and diagnosis of optic pathway gliomas in children with neurofibromatosis type 1 by using sweep visual evoked potentials. Invest Ophthalmol Vis Sci 2007;48:2895–2902 [DOI] [PubMed] [Google Scholar]
- 43.Jabbari B, Maitland CG, Morris LM, Morales J, Gunderson CH. The value of visual evoked potential as a screening test in neurofibromatosis. Arch Neurol 1985;42:1072–1074 [DOI] [PubMed] [Google Scholar]
- 44.Lund AM, Skovby F. Optic gliomas in children with neurofibromatosis type 1. Eur J Pediatr 1991;150:835–838 [DOI] [PubMed] [Google Scholar]
- 45.North K, Cochineas C, Tang E, Fagan E. Optic gliomas in neurofibromatosis type 1: role of visual evoked potentials. Pediatr Neurol 1994;10:117–123 [DOI] [PubMed] [Google Scholar]
- 46.Wolsey DH, Larson SA, Creel D, Hoffman R. Can screening for optic nerve gliomas in patients with neurofibromatosis type I be performed with visual-evoked potential testing? J AAPOS 2006;10:307–311 [DOI] [PubMed] [Google Scholar]
- 47.Iannaccone A, McCluney RA, Brewer VR, et al. Visual evoked potentials in children with neurofibromatosis type 1. Doc Ophthalmol 2002;105:63–81 [DOI] [PubMed] [Google Scholar]
- 48.Ng Y, North KN. Visual-evoked potentials in the assessment of optic gliomas. Pediatr Neurol 2001;24:44–48 [DOI] [PubMed] [Google Scholar]
- 49.Falsini B, Ziccardi L, Lazzareschi I, et al. Longitudinal assessment of childhood optic gliomas: relationship between flicker visual evoked potentials and magnetic resonance imaging findings. J Neurooncol 2008;88:87–96 [DOI] [PubMed] [Google Scholar]
- 50.Kelly JP, Leary S, Khanna P, Weiss AH. Longitudinal measures of visual function, tumor volume, and prediction of visual outcomes after treatment of optic pathway gliomas. Ophthalmology 2012;119:1231–1237 [DOI] [PubMed] [Google Scholar]
- 51.Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci 2007;48:5798–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010;67:749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler 2008;14:893–905 [DOI] [PubMed] [Google Scholar]
- 54.Avery RA, Liu GT, Fisher MJ, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol 2011;151:542–549 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avery R, Hwang E, Acosta M, et al. Abstracts from the fifteenth international symposium on pediatric neuro-oncology: Hand-held optical coherence tomography during sedation detects visual acuity and visual field loss in young children with optic pathway gliomas. Neuro Oncol 2012;14:i69 [Google Scholar]
- 56.Cole SR, Beck RW, Moke PS, Gal RL, Long DT. The National Eye Institute Visual Function Questionnaire: experience of the ONTT. Optic neuritis treatment trial. Invest Ophthalmol Vis Sci 2000;41:1017–1021 [PubMed] [Google Scholar]
- 57.Jampel HD, Schwartz A, Pollack I, Abrams D, Weiss H, Miller R. Glaucoma patients' assessment of their visual function and quality of life. J Glaucoma 2002;11:154–163 [DOI] [PubMed] [Google Scholar]
- 58.Mowry EM, Loguidice MJ, Daniels AB, et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry 2009;80:767–772 [DOI] [PubMed] [Google Scholar]
- 59.Birch EE, Cheng CS, Felius J. Validity and reliability of the Children's Visual Function Questionnaire (CVFQ). J AAPOS 2007;11:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cochrane GM, Marella M, Keeffe JE, Lamoureux EL. The Impact of Vision Impairment for Children (IVI_C): validation of a vision-specific pediatric quality-of-life questionnaire using Rasch analysis. Invest Ophthalmol Vis Sci 2011;52:1632–1640 [DOI] [PubMed] [Google Scholar]
- 61.Felius J, Stager DR, Sr, Berry PM, et al. Development of an instrument to assess vision-related quality of life in young children. Am J Ophthalmol 2004;138:362–372 [DOI] [PubMed] [Google Scholar]
- 62.Gothwal VK, Lovie-Kitchin JE, Nutheti R. The development of the LV Prasad-Functional Vision Questionnaire: a measure of functional vision performance of visually impaired children. Invest Ophthalmol Vis Sci 2003;44:4131–4139 [DOI] [PubMed] [Google Scholar]
- 63.Khadka J, Ryan B, Margrain TH, Court H, Woodhouse JM. Development of the 25-item Cardiff Visual Ability Questionnaire for Children (CVAQC). Br J Ophthalmol 2010;94:730–735 [DOI] [PubMed] [Google Scholar]


