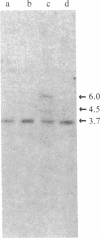
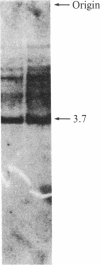
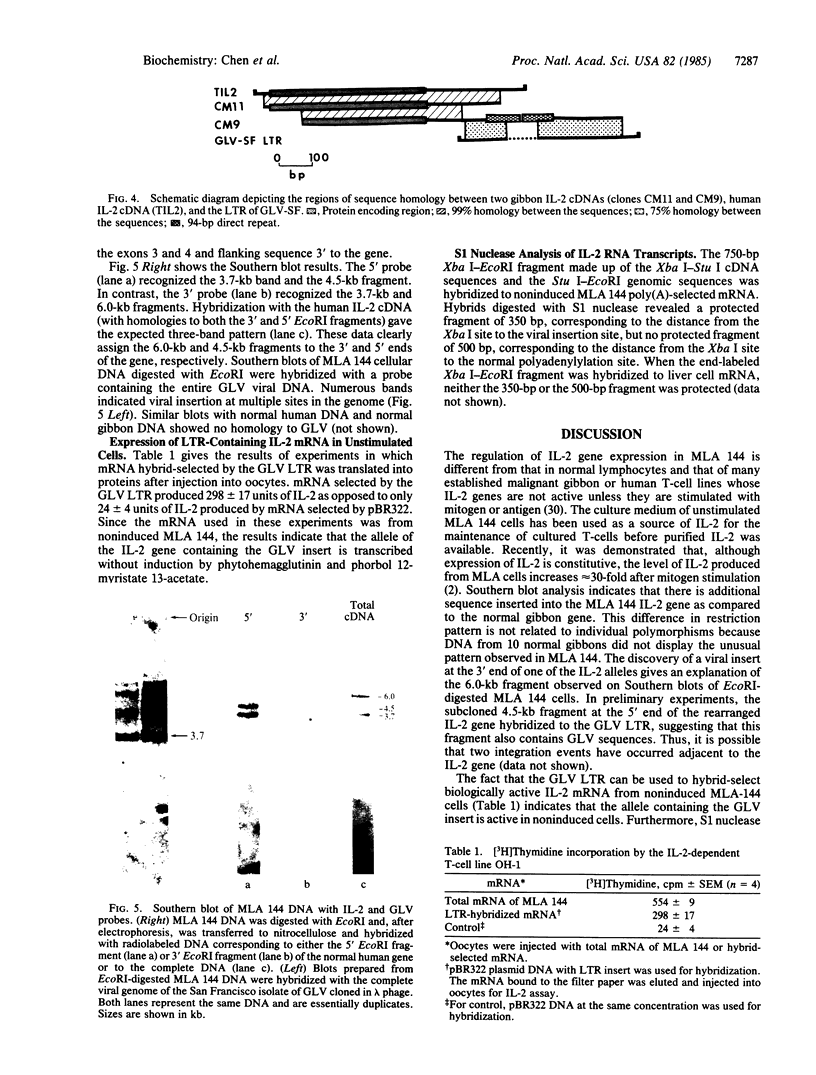
Abstract
The gibbon leukemia cell line MLA 144 differs from every other T-lymphocyte line in that it constitutively makes interleukin 2 (IL-2) (also called T-cell growth factor) without stimulation by antigen, lectin, or tumor promoters. Previous work in which glucocorticoids were used to inhibit IL-2 production has indicated that proliferation of this cell line is dependent upon endogenously produced IL-2. We have found that the MLA 144 cell line has a copy of the gibbon leukemia virus inserted into the 3' nontranslated region of the IL-2 gene. This integration event produces a composite mRNA made up of the protein coding sequences of the IL-2 gene transcript but incorporating the viral long terminal repeat (LTR) in the 3' nontranslated region of the mRNA. This composite mRNA transcript uses the polyadenylylation signal in the viral 5' LTR and incorporates the viral transcriptional control regions. The integration event must involve only one allele of the IL-2 gene, since transcripts essentially identical to normal human IL-2 mRNA are also produced in cloned sublines of MLA 144. That the viral LTR contains a 94-base-pair repeat reminiscent of enhancer sequences in several viruses suggests that the integration of the viral LTR at the 3' end of the IL-2 gene is responsible for the constitutive production of IL-2 in the MLA 144 cell line.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Organization of the rat gamma-fibrinogen gene: alternative mRNA splice patterns produce the gamma A and gamma B (gamma ') chains of fibrinogen. Cell. 1982 Nov;31(1):159–166. doi: 10.1016/0092-8674(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Takaoka C., Matsui H., Taniguchi T. Structure of the human interleukin 2 gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7437–7441. doi: 10.1073/pnas.80.24.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980 Dec 1;152(6):1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Hewetson J. F., Hopkins R. F., 3rd, Sowder R. C., Neubauer R. H., Rabin H. A rapid, large scale purification procedure for gibbon interleukin 2. J Immunol. 1983 Aug;131(2):810–815. [PubMed] [Google Scholar]
- Holbrook N. J., Lieber M., Crabtree G. R. DNA sequence of the 5' flanking region of the human interleukin 2 gene: homologies with adult T-cell leukemia virus. Nucleic Acids Res. 1984 Jun 25;12(12):5005–5013. doi: 10.1093/nar/12.12.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Smith K. A., Fornace A. J., Jr, Comeau C. M., Wiskocil R. L., Crabtree G. R. T-cell growth factor: complete nucleotide sequence and organization of the gene in normal and malignant cells. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1634–1638. doi: 10.1073/pnas.81.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Skalka A. M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Robb R. J. Induction of interleukin 2 production in the gibbon ape T cell line MLA 144. Lymphokine Res. 1985 Winter;4(1):1–4. [PubMed] [Google Scholar]
- Lin Y., Stadler B. M., Rabin H. Synthesis of biologically active interleukin 2 by Xenopus oocytes in response to poly(A)-RNA from a gibbon T-cell line. J Biol Chem. 1982 Feb 25;257(4):1587–1590. [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. L., McKereghan K., Kaplan H. S., Fry K. E. Molecular cloning and partial characterization of unintegrated linear DNA from gibbon ape leukemia virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4213–4217. doi: 10.1073/pnas.78.7.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor and glucocorticoids: opposing regulatory hormones in neoplastic T-cell growth. Immunobiology. 1982 Mar;161(1-2):157–173. doi: 10.1016/S0171-2985(82)80023-5. [DOI] [PubMed] [Google Scholar]
- Snyder S. P., Dungworth D. L., Kawakami T. G., Callaway E., Lau D. T. Lymphosarcomas in two gibbons (Hylobates lar) with associated C-type virus. J Natl Cancer Inst. 1973 Jul;51(1):89–94. doi: 10.1093/jnci/51.1.89. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Function of the retrovirus long terminal repeat. Cell. 1982 Jan;28(1):3–5. doi: 10.1016/0092-8674(82)90367-1. [DOI] [PubMed] [Google Scholar]
- Trainor C. D., Scott M. L., Josephs S. F., Fry K. E., Reitz M. S., Jr Nucleotide sequence of the large terminal repeat of two different strains of gibbon ape leukemia virus. Virology. 1984 Aug;137(1):201–205. doi: 10.1016/0042-6822(84)90025-4. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]