Abstract
A higher chronic expansion of effector cytotoxic CD8+DR+ T-lymphocytes has been reported in common variable immunodeficiency (CVID) patients with complications such as splenomegaly, autoimmune disease and/or granulomatous disease. In order to document the features associated with this T cell activation involving the CD8+ T-compartment, we examined the diversity of the alpha/beta TCR repertoire of the patient's CD8+ T-lymphocytes using the qualitative analysis of the CDR3 lengths (Immunoscope).
Ten CIVD patients were enrolled in this study, four without complications (Group 1), six with complications (Group 2). All patients exhibited non-gaussian altered CDR3 length distributions, albeit to different extent within the different Vβ families. CVID patients with activated CD8+ T-cells show a reduction of their TCR repertoire diversity which is more severe in patients with complications. Viral reactivations such as CMV are suspected to be part of the mechanisms underlying immunosenescence.
Keywords: Common variable immunodeficiency, HLA-DR, Immunoscope, CD8+ T-cell expansion
Highlights
-
•
Some CVID patients with complications have an increase in peripheral blood CD8+DR+ T cells.
-
•
These CD8+ T-cells have an activated cytotoxic phenotype.
-
•
These CD8+ T-cells show a reduction of their TCR repertoire diversity.
-
•
Viral reactivations such as CMV are suspected leading to accelerated immunosenescence.
1. Introduction
Common variable immunodeficiency (CVID) is a highly heterogeneous group of B cell-deficiency syndromes all characterized by defective antibody production and recurrent sino-pulmonary bacterial infections [1,2]. Other important clinical features are also observed in about 25% of patients, including lymphadenopathy, splenomegaly, granulomatosis and/or autoimmune diseases [1–3]. The recently proposed classifications of CVID patients based on flow cytometric quantification of class-switched memory and immature blood B-cells [4], although useful for patients caring, is insufficient as many patients also have circulating immunophenotypic T-cell abnormalities, particularly significantly elevated CD8+ T-cells expressing the activation marker HLA-DR [5]. We reported on an expansion of CD8+HLA-DR+ T-lymphocytes with an effector phenotype in a subset of CVID patients with splenomegaly, lymphoid hyperplasia and/or granulomatosis [5]. To gain further insight into the nature of this expansion of CD8+ T-lymphocytes in this group of CVID patients, we examined their αβTCR repertoire assessing the CDR3 length polymorphism (CDR3-LP) of their CD8+ T-lymphocytes providing a highly sensitive detection of overrepresented CD8+ T-cells.
2. Materials and methods
2.1. Patients
Among the 10 CVID patients enrolled in this study, six were men and four were females. All patients had been diagnosed as having CVID based on recurrent bacterial infections associated with hypogammaglobulinemia (serum IgG and IgA and/or IgM ≥ 2 standard deviations (SD) below the normal mean), confirmed on two occasions 12 weeks apart, while all secondary causes have been excluded [6]. At the time of the evaluation, none of the patients had any sign of acute infection. A single CDR3 length polymorphism evaluation was carried out per patient between 6 years and 30 years following diagnosis, and clinical data available at this time point were collected. All participants gave their written informed consent. All patients, but one, were receiving substitution therapy with intravenous immunoglobulins (IVIgs) preparations. Splenomegaly was diagnosed when the spleen was >13 cm long on computed tomography scan. Granulomatous disease and lymphoid hyperplasia were diagnosed from lymph-node biopsies and/or splenectomy. Immune thrombocytopenia (ITP) was defined according to the published standardized international criteria [7].
2.2. Flow cytometric analysis
Whole blood was collected in tubes containing sodium ethylenediaminetetraacetate (Becton-Dickinson, Le Pont de Claix, France). PBL from CVID pts were analyzed by 4-color flow cytometry (FacsCalibur, Becton-Dickinson, Moutain View, CA). For patients given replacement therapy, blood samples were collected just before the IVIgs infusion. Flow cytometric analyses for patients and controls were performed within the next 24 h following blood withdrawal. We used specific antibodies, conjugated to fluorescein isothiocyanate, phycoerythrin (PE), peridinin chlorophyll protein or allophycocyanin, directed against the following surface markers: CD3, CD8, CD4, CD45, CD57, HLA-DR, CD19, CD27 and sIgD (all from Beckman-Coulter, Marseille, France). IgG1 or IgG2a isotypes were used as isotypic controls. For intracellular labelling of granzyme B and perforin, PBL were first incubated with anti-CD3, -CD8 and HLA-DR for 30 min in order to tag cell surface molecules. Cells were then resuspended in 1× final Permeafix (BDBiosciences, Pont de Claix, France) for 30 min at room temperature before labelling with either anti-granzyme B-PE, anti-perforin-PE or a PE-conjugated isotypic control. Lymphocytes were gated according to their forward and side scatter (only for CD3/CD8/HLA-DR/perforin or granzyme B panels) and highest expression of CD45 characteristics otherwise. For the B-cell phenotyping, patients were classified according the recent EURO-class system which is based on the absence or the presence of circulating B lymphocytes (B− group: CD19+ ≤ 1%; B+ group: CD19+ > 1% of total lymphocytes) [4]. Within the B+ group, patients are classified according to the proportion of switched memory B (smB) cells (smB− group: IgD−CD27+/ CD19+ ≤ 2%; smB+ group: IgD−CD27+/CD19+ > 2%). For all patients, flow cytometric analyses have been carried out repeatedly every 6 months for at least a period of time of 3 years (maximum 6 years) before the repertoire assessment.
2.3. Blood samples, RNA extraction and analysis of CDR3 length
Thirty milliliters of blood were collected by venopuncture and anti-coagulated with sodium EDTA. Peripheral blood mononuclear cells (PBMC) were recovered after a Ficoll-Hypaque gradient (Eurobio, Les Ulis, France) and CD8+ T-cells sorted using MACS CD8 microbeads (Miltenyi, Bergisch Gladbach, Germany). After washing, 2 × 107 CD8+ T-cells were added with Trizol® reagent (Invitrogen™, Life Technologies, CA, USA) for RNA extraction according to manufacturer's instructions. The RNA concentration for each sample was determined by optical density measurement, and RNA quality was checked by running samples on a 1% agarose gel. Two 2 μg of RNA were reverse transcribed using an Invitrogen cDNA synthesis kit (Boeringher Mannheim, Indianapolis, IN) and diluted to a final volume of 100 μL. Complementary DNAs were amplified by PCR using a Cβ primer and one of the 26 specific Vβprimers. The amplifications were performed in a 9600 Perkin-Elmer thermocycler (Applied Biosystems, Foster City, CA, USA) as previously described [8]. Briefly, each amplification product was used for an elongation reaction using a dye-labeled Cβ primer, then heat-denatured, loaded onto a 6% acrylamide-8 M urea gel and electrophoresed for 5 h using an Applied Biosystems 373A DNA sequencer (Perkin-Elmer®).
2.4. TCR repertoire analysis and statistical analysis
Diversity and T-cell selection in the CD8+ compartment were assessed by analysis of TCR (β chain) usage biases. Analysis of CDR3-LD was performed using Immunoscope® software [9]. The percentage of CDR3-LD alteration for each Vβ family and a global percentage of CDR3-LD alteration for each individual or group were calculated as described [10]. The percentage of alteration was defined as the difference between the frequency of each CDR3 length in the distribution profile and the control distribution, calculated from the 13 age- and gender-matched healthy individuals. The global CDR3-LD alteration is represented as a topview TcLandscape® enabling an easy appraisal of the ‘qualitative’ measurement of the CDR3-LD bias (see Figs. 1 and 2). Only CDR3 lengths with an alteration above 30% were taken into account. The level of Vβ RNA was measured by real-time quantitative PCR and expressed as a ratio of a nonregulated or minimally regulated gene, HPRT. The primers used were especially designed for quantitative PCR as previously described [8]. The data were displayed as a three-dimensional TcLandscape® [11–13]. Percentages of CDR3-LD alterations are represented as a color code, from deep blue (−50%) to dark red (+50%). The X-axis displays the 26 human Vβ families, the Y-axis gives the Vβ/HPRT ratios and the Z-axis gives the CDR3 lengths. The color code for the tridimensional TcLandscape® is the same as that used for the corresponding topview. All T cell-subpopulation percentages and qualitative (percentage of alteration) values for each Vβ family were compared between groups using the non-parametric Mann Whitney U-test, with significance set at p = 0.05, and Statistica Inc. software (Statsoft, Tucson, AZ).
Fig. 1.
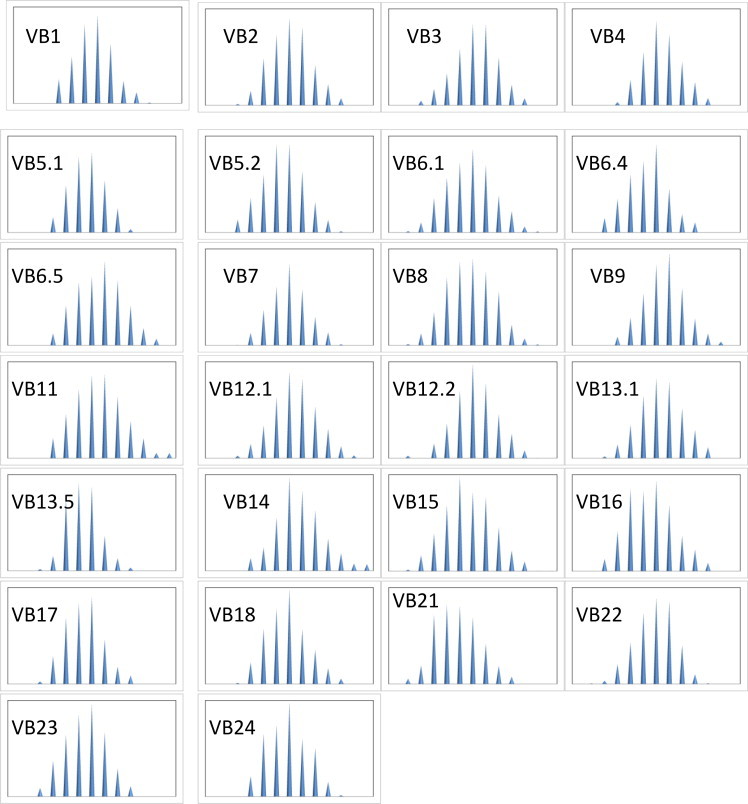
Average Immunoscope profile obtained from the T cell repertoire analysis of 13 healthy individuals. For technical details, see Section 2. The distribution of TCR β CDR3 lengths (peak profile representation) for each Vβ family is a typical Gaussian-like profile which characterizes the polyclonal response usually observed in healthy volunteers.
2.5. Statistical analysis
All T cell-subpopulation percentages and qualitative (percentage of alteration) values for each Vβ family were compared between groups using the non-parametric Mann Whitney U-test, with significance set at p = 0.05, and Statistica Inc. software (Statsoft, Tucson, AZ).
3. Results
3.1. Patient population
Among our cohort of 71 CVID pts followed in our Department, we studied the 10 patients for whom we had an extended follow-up time and repeated lymphocytes phenotyping in the history of their disease. Their median ages were 46 years (range: 30–75 years) and their median follow-up were 9 years (minimum 6 years, maximum 30 years). Their individual clinical characteristics are summarized in Table 1. Recurrent bacterial respiratory tract infections were common to all CVID patients but among them, six (pts 5–10) had other clinical manifestations including autoimmune diseases (2 ITP, patients 8 and 10), chronic granulomatous disease (patients 5 and 7) and/or organomegaly caused by lymphoid hyperplasia (patients 5, 6, 8, 9 and 10). Patients were then divided into two groups: the Group 1 including those for which the only clinical features noted have been sino-pulmonary bacterial infections before IVIg substitution (n = 4, patients 1–4) and the Group 2 gathering the patients with other additional clinical complications (n = 6, patients 5–10).
Table 1.
Summary of the demographic and clinical characteristics of CVID patients.
| Patients no. | Age/sex | Age at diagnosis | Infectious manifestationsa | Splenomegaly | Autoimmune diseasesb | Lymphoid proliferation | Granulomatous disease |
|---|---|---|---|---|---|---|---|
| Group 1 | |||||||
| 1 | 57/F | 38 | S, B, An | − | − | − | |
| 2 | 49/M | 26 | S, B, P, G | − | − | − | − |
| 3 | 32/F | 21 | S, An, B | − | − | − | − |
| 4 | 30/F | 27 | G, P, digestive infections (Salmonella), Herpes | − | − | − | − |
| Group 2 | |||||||
| 5 | 61/F | 52 | S, P, B, urinary infections | + | − | + | + |
| 6 | 37/M | 31 | S, G, B, P, Sep | + | − | + | − |
| 7 | 75/M | 68 | S, B, P | + | − | − | + |
| 8 | 43/M | 27 | An, S, B, P | + | + (ITP) | + | − |
| 9 | 54/M | 24 | S, B, P | − | − | + | − |
| 10 | 39/M | 30 | S, B, P | + | + (ITP) | + | − |
Patients in Group 2 were diagnosed at a later age than Group 1 (median values: 30.5 years versus 26.5 years, respectively, not statistically significant). Complications were present in all patients in Group 2 at diagnosis and, in four of them, they revealed CVID. At the time of the study, all patients, except patients 7, received IVIgs treatment every 3 or 4 weeks, and all had a residual serum IgG level >8 g/l.
An: angina; B: bronchitis; G: giardiasis infection; P: pneumonia; S: sinusitis; and Sep: septicemia.
ITP: idiopathic thrombocytopenic purpura.
Patients in Group 2 were diagnosed at a later age than Group 1 (median values: 30.5 years versus 26.5 years, respectively, not statistically significant). Complications were present in all patients in Group 2 at diagnosis and, in four of them, they revealed CVID. At the time of the study, all patients, except patient 7, received IVIgs treatment every 3 or 4 weeks, and all had a residual serum IgG level >8 g/l.
3.2. Activation of a high proportion of CD8+ T lymphocytes in CVID patients
Phenotypic characteristics of peripheral circulating lymphocytes in the 10 CVID patients are reported in Table 2. All had a significant percentage of detectable circulating B cells (CD19+ >1% of total lymphocytes) and were then classified in the B+ group according to the EURO-class system. Among them, 4 CVID patients belonged to the smB− group (% of CD19+ expressing CD27 but sIgD− ≤2% of B cells), and 6 belonged to the smB+ group (% of CD19+ expressing CD27 but sIgD− >2%). We did not observe any correlation between the EURO-Class and the two groups of patients based on the occurrence of clinical complications probably due to the small sample of our population. Three patients had a T-cell lymphocytosis (patients 5–7), which could be explained primarily by an expansion of the CD8+ T-compartment. Three others (patients 4, 9 and 10) had a CD4+ T-cell lymphopenia between 300 and 500/μl. Patient 4 showed a CD8+ T-cell lymphopenia of unknown origin. All Group 2 patients displayed a diminished CD4+/CD8+ ratio, either because of CD4+ lymphopenia or/and CD8+ lymphocytosis, while all patients of the Group 1 had a normal ratio CD4+/CD8+.
Table 2.
Summary of the immunologic characteristics of CVID patients.
| Pt-1 | Pt-2 | Pt-3 | Pt-4 | Pt-5 | Pt-6 | Pt-7 | Pt-8 | Pt-9 | Pt-10 | Healthy controls (n = 12) | Group 1 (n = 4) | Group 2 (n = 6) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % CD19+-B cells | 14.92 | 14.87 | 6.8 | 11.9 | 5.12 | 7.68 | 5.5 | 8 | 5 | 4.5 | 11.89 (8.32–13.25) | 13.39 (9.36–14.9) | 5.31 (5–7.68)a,b |
| CD19+-B cells/μl | 299 | 132 | 152 | 99 | 305 | 261 | 268 | 191 | 135 | 82 | 269 (211–338.5) | 142 (115.5–225.5) | 226 (135–268) |
| % CD19+ IgD− CD27+- B cells | 20.81 | 3 | 4.12 | 5.53 | 1.8 | 6.23 | 1.34 | 3 | 0.12 | 1.87 | 16.04 (11.88–20) | 4.83 (3.56–13.17) | 1.83 (1.34–3)a |
| EURO-CLASS | smB+ | smB+ | smB+ | smB+ | smB− | smB+ | smB− | smB+ | SmB− | SmB− | |||
| CD3+-T cells (/μl) | 1234 | 1651 | 1368 | 622 | 5112 | 2871 | 3572 | 1166 | 1203 | 1324 | 1530.5 (1308–1734) | 1301 (928–1509.5) | 2097 (1203–3572) |
| CD4+-T cells (/μl) | 673 | 1053 | 726 | 450 | 1811 | 778 | 701 | 536 | 389 | 396 | 875 (753–1120) | 699.5 (561.5–889.5) | 618.5 (396–778) |
| CD8+-T cells (/μl) | 494 | 527 | 530 | 141 | 3072 | 1967 | 2773 | 543 | 750 | 906 | 484 (413–595) | 510.5 (317.5–528.5) | 1436 (750–2773)a,c |
| CD4+/CD8+ ratio | 1.36 | 1.99 | 1.36 | 3.19 | 0.59 | 0.40 | 0.25 | 0.98 | 0.52 | 0.43 | 1.85 (1.60–2.31) | 1.68 (1.36–2.59) | 0.475 (0.4–0.59)a,c |
| % CD3+–DR+-T cells | 16.71 | 2.64 | 9.76 | 4.6 | 59 | 53.88 | 46.27 | 51.96 | 32.15 | 26.48 | 6.90 (5.40–8.65) | 7.18 (3.62–13.23) | 49.11 (32.15–53.88)a,c |
| % CD4+–DR+-T cells | 7.5 | 1.6 | 6.2 | 4.4 | 59 | 28.78 | 32.1 | 41.38 | 1.8 | 20.72 | 2.77 (2.25–3.78) | 5.30 (3–6.85) | 30.44 (20.72–41.38)a |
| % CD8+–DR+-T cells | 21.03 | 5.51 | 10.81 | 6.1 | 77.32 | 65.15 | 50.95 | 62.98 | 40.45 | 36.46 | 3.31 (2.57–4.94) | 10.68 (8.32–15.92)d | 56.96 (40.45–65.15)a,c |
| % CD8+–DR+-Perforin+ T cells | 2.69 | 15 | 1.64 | 8 | 48.91 | 51.05 | 56 | 37.33 | 19.83 | 18.67 | 5.43 (2.11–6.75) | 5.34 (2.16–11.5) | 43.12 (19.83–51.05)a,c |
| % CD8+–DR+-Granzyme+ T cells | 5.20 | 11 | 2.52 | 7 | 58.95 | 60.39 | 66 | 50.21 | 24.72 | 29.87 | 5.31 (3.48–7.62) | 6.1 (3.86–9) | 54.58 (29.87–60.39)a,c |
For Groups 1 and 2, results are expressed as medians and (25th; 75th percentiles). Comparisons were made with the non-parametric Mann-Whitney U-test. Other between-group comparisons were not statistically significant.
p ≤ 0.001 versus healthy controls.
p = 0.03 versus Group I.
p ≤ 0.009 versus Group 1.
p = 0.0002 versus healthy controls.
As shown in Table 2, Group 2 patients were characterized by a statistically significant increase in their percentages of HLA-DR-expressing CD3+ T-cells when compared to Group 1 patients (p = 0.009). This activated phenotype was principally contributed by the CD3+ CD8+ T-lymphocytes (p = 0.009) and marginally by the CD3+ CD4+ T-cells (p = 0.06). This difference could be partially due to a significantly higher number and percentages of circulating CD3+ CD8+ T-cells in the Group 2 compared with the Group 1 (p = 0.009), whereas we did not notice any difference regarding the number and the percentages of circulating CD3+ or CD3+ CD4+ T-cells between the 2 groups. We observed a strict correlation between the number of circulating CD3+ CD8+ T-cells and the percentage of circulating HLA-DR-expressing CD8+ T-cells (Spearman test: r = 0.85, p = 0.001). The ratio CD4+/CD8+ was very different between the two groups (median values: 1.68 in the Group 1 versus 0.47 in the Group 2, p = 0.009) which always reflected the CD8+ T-cell expansion and not a CD4+ lymphopenia. Moreover, when we considered the activation status within each circulating lymphocytes subsets, the CD8+ T-cells were still significantly more activated in the Group 2 than in the Group 1 (p = 0.009), whereas no difference was observed in the CD4+ T-cells between the 2 groups (p = 0.06).
We also confirmed our previous results that median percentages of intracellular expression of perforin or granzyme B in peripheral blood CD8+DR+ T-cells were significantly more elevated in the Group 2 linking the presence of complications in these patients to an activated CD8+ T-lymphocytes effector (CCR7− and CD45RA+) cytotoxic phenotype (data not shown) [5].
This phenotyping profile has been stable for at least the 3 last years of patients' follow-up with a maximum follow-up of 6 years.
3.3. Repertoire analysis of blood CD8+ T cell subsets
To see whether the activated CD3+CD8+ T-cells were the result of a clonal expansion, we undertook their TCR repertoire analysis. Using the immunoscope method, the distribution of TCR β CDR3 lengths for each Vβ is visualized as a series of peaks separated by a distance of three nucleotides corresponding to in-frame transcripts [9]. A single CDR3-LD evaluation was carried out per patient, and clinical data available at this time point were collected. A physiologically diverse repertoire yields a Gaussian-like profile (see Fig. 1), whereas the presence of T-cell clonal expansion manifests as larger and fewer peaks [14,15]. All patients exhibited altered T-cell repertoires as shown in Fig. 2. One or more peaks accumulated within different Vβ families for each patient. From one patient to another, these expansions did not concern the same Vβ and no recurrence of a particular peak was found. However, these T cell expansions were more marked for the Group 2 patients in which they appeared to be oligoclonal with single or double dominant peaks in several Vβ families, particularly for the two patients with granulomatous disease (see Fig. 2).
Fig. 2.
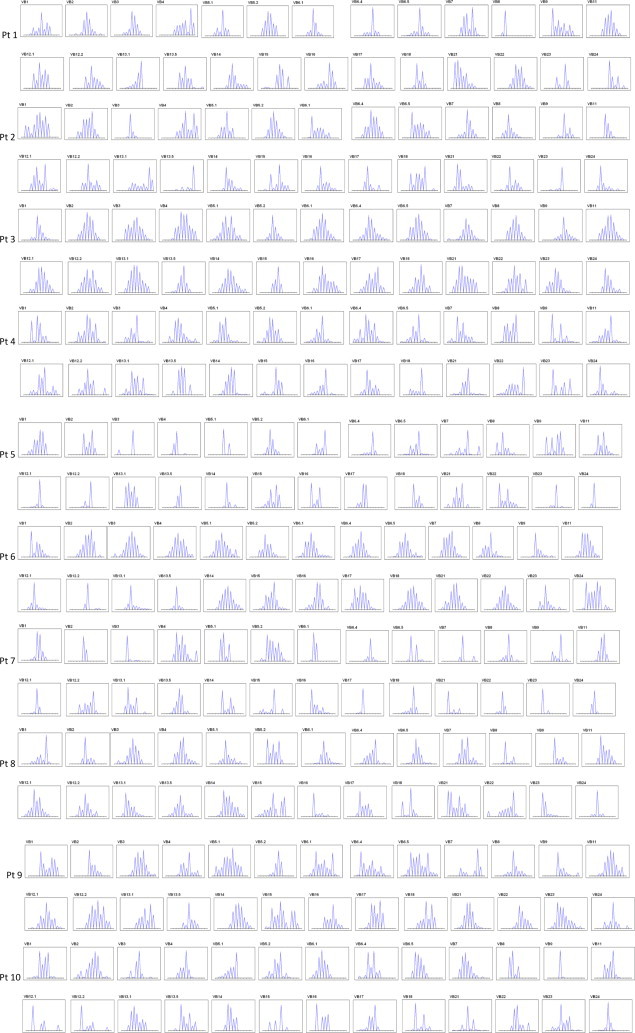
Immunoscope analysis of the T cell repertoire in ten CVID patients. For technical details, see section Methods. Pt = Patient. T-cell clonal expansions manifest as a distribution skewed by the presence of larger peaks that accumulate above the Gaussian-like background of polyclonal T cells, particularly in CVID patients with granulomatosis (patients 5 and 7).
A percentage of CDR3-LD alteration for each Vβ family was calculated as described (see Table 3) [11]. More was the repertoire biased greater was the score for one particular Vβ family. A biclonal picture such as pt 1 Vβ8 was granted a score of 90%, whereas the Gaussian distribution obtained from pt 3 Vβ11 yielded a score of 7% of alteration. When compared to the Group 1, the median percentage of CDR3-LD was significantly higher in the group of CVID pts with complications for the V2 (31.7% versus 17.16%, p = 0.01) and the V5.1 beta families (30.44% versus 17.97%, p = 0.03). However, recurrence of a particular peak was not found among patients .There were no Vβ family untouched, nor Vβ families consistently concerned except the two aforementioned.
Table 3.
Percentages of CDR3-LD alteration for each Vβ family calculated as described in Ref. [11].
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vβ 1 | 32.26 | 31.76 | 19.15 | 34,31 | 15.24 | 30.01 | 23.22 | 44.89 | 48,34 | 24,50 |
| Vβ 2 | 17.64 | 19.28 | 9.19 | 16.69 | 35.9 | 23.58 | 56.76 | 36.66 | 27.5 | 18.85 |
| Vβ 3 | 16.86 | 55.31 | 16.84 | 31.19 | 76.61 | 15.14 | 77.35 | 9.86 | 18.95 | 37.79 |
| Vβ 4 | 50.37 | 48.15 | 19.73 | 33.28 | 71.84 | 21.18 | 17.5 | 12.37 | 28.7 | 33.37 |
| Vβ 5.1 | 21.68 | 16.98 | 18.77 | 17.18 | 57.88 | 18.72 | 37.01 | 26.01 | 27.57 | 33.31 |
| Vβ 5.2 | 24.45 | 15.24 | 20,89 | 13.84 | 22.74 | 14.07 | 22.15 | 25.03 | 44.95 | 20.61 |
| Vβ 6.1 | 31.91 | 25.39 | 9.03 | 25.68 | 43.56 | 30.36 | 69.22 | 22.48 | 29.75 | 26.26 |
| Vβ 6.4 | 37.02 | 15.85 | 1.61 | 20.52 | 58.72 | 7.59 | 58.51 | 23.13 | 35.67 | 30.17 |
| Vβ 6.5 | 34.54 | 29.51 | 22.56 | 26.13 | 31.67 | 20.5 | 52.74 | 35.58 | 31.6 | 26.42 |
| Vβ 7 | 25.3 | 20.99 | 14.79 | 31.40 | 53.19 | 12.11 | 68.15 | 20.86 | 69.94 | 35.02 |
| Vβ 8 | 90.25 | 38.3 | 21.25 | 29.50 | 44.77 | 38.44 | 45.33 | 52.02 | 31.24 | 52.71 |
| Vβ 9 | 34.99 | 46.19 | 29.17 | 49.50 | 30.27 | 38.42 | 53.27 | 40.85 | 41.7 | 79.65 |
| Vβ 11 | 15.05 | 48.72 | 6.98 | 24.51 | 26.31 | 19.93 | 37.26 | 16.68 | 13.22 | 30.68 |
| Vβ 12.1 | 22.55 | 35.36 | 8.31 | 26.64 | 55.12 | 64.6 | 54.89 | 33.53 | 15.39 | 60.69 |
| Vβ 12.2 | 21.31 | 28.01 | 18.17 | 26.88 | 52.34 | 53.14 | 29.15 | 29.3 | 18.16 | 56.12 |
| Vβ 13.1 | 33.19 | 53.14 | 12.06 | 25.06 | 32.58 | 37.31 | 36.32 | 42.26 | 33.14 | 29.75 |
| Vβ 13.5 | 19.17 | 81.68 | 15.37 | 24.55 | 40.31 | 37.98 | 27.58 | 19.25 | 39.89 | 30.10 |
| Vβ 14 | 20.36 | 27.98 | 22.31 | 16.37 | 47.7 | 8.71 | 47.19 | 17.12 | 11.14 | 27.42 |
| Vβ 15 | 38 | 39.19 | 28.89 | 37.40 | 28.74 | 16.75 | 51.28 | 38.74 | 36.55 | 53.22 |
| Vβ 16 | 43.24 | 31.2 | 19.58 | 29.42 | 48.45 | 27.35 | 30.23 | 48.81 | 29.41 | 38.24 |
| Vβ 17 | 23.86 | 37.12 | 33.37 | 21.48 | 33.88 | 22.38 | 62.68 | 24.72 | 20.65 | 25.56 |
| Vβ 18 | 31.75 | 29.64 | 13.6 | 43.65 | 37.75 | 22.21 | 46.74 | 54.12 | 26.65 | 51.13 |
| Vβ 21 | 45.68 | 34.88 | 20.83 | 30.28 | 27.39 | 12.3 | 52.2 | 32.01 | 13.78 | 47.57 |
| Vβ 22 | 26.64 | 27.81 | 18.12 | 31.71 | 33.11 | 20.72 | 39.92 | 47.32 | 28.92 | 31.34 |
| Vβ 23 | 51.24 | 66.22 | 28.2 | 49.10 | 66.53 | 31.66 | 71.74 | 56.38 | 26.06 | 29.23 |
| Vβ 24 | 48.33 | 43.24 | 20.48 | 47.50 | 78.53 | 25.73 | 50.3 | 48.38 | 31.55 | 47.63 |
4. Disscussion
Because we and others have shown that the T-cell activation in CVID concerned mostly the CD8+ T-compartment [5,16,17], we focused our attention on its TCR diversity. We used the immunoscope approach, which allowed for a global estimation of the T-cell repertoire and represented a highly sensitive detection of overrepresented T-cell populations. We showed that CVID patients, notably those with complications, exhibited oligoclonal expansions in their CD8+ T-cells. These observations were not the result of an acute clinical infectious event, but reflected a stable phenotypic T-cell pattern as the CD8+ T-cell activation was consistently observed along six years independently of clinical status. However, we could not establish any relationship between repertoire bias and the clinical manifestations seen in these patients.
There is a natural accumulation of clonal populations in CD8+ T-cells in aging [18,19], which could be related to subclinical viral infection [20]. The CD8+ T-cells expansion seems closely associated with cytomegalovirus (CMV) infection which is a potent immunogen and during aging a progressive accumulation of CMV-specific T-cells is observed which can reach until 25% or more of the CD8+ pool [21–23]. CMV DNA is frequently detected in the urine of elderly subjects [24]. It is speculated that this specific T-cell expansion could inhibit the function of other antigen-specific T-cell populations. We cannot exclude the role of the age in the T-cells expansions of our patients, but the age cannot alone explain the overall activation of the T-cell compartment because oligoclonal expansion of CD8+ T cells is also observed in young patients (patients 2, 4 and 9).
However, a chronic viral replication, generated by the immunodepression, and leading to a constriction of the T-cell repertoire could be involved in CVID patients as suggested by recent findings reported in patients with chronic lymphocytic leukemia (CLL), another disease also characterized by hypogammaglobulinemia. CMV-specific CD4+ and CD8+ T-cell populations are both increased in CMV-seropositive CLL patients [25,26]. Recently, Pourgheysari et al. speculated that the immunosuppression associated with CLL triggered a CMV reactivation that in turn activated and expanded CMV-specific T-cells [26]. They showed in CMV-seropositive CLL patients an increment in CD3+ and CD8+ T-cell counts and a marked expansion of CMV-specific CD4+ T-cells which was more pronounced in patients who received chemotherapy. CMV reactivation was not detected by PCR in the blood of CLL patients suggesting that the viral replication was controlled by the immune response. The authors suggest that such expanded T-cell populations could have negative consequences for immunity with a constriction of the T-cell repertoire leading to a loss of certain essential memory T-cell populations directed towards another micro-organisms. This could explain the frequent resurgence of Varicella zoster virus (VZV) infections in CLL patients. In the same way, we could hypothesize an identical model in CVID, the humoral defect favoring a chronic viral replication which in turns could induce T-cells expansions. From our study, the T-cell activation was correlated with the importance of the humoral defect. However, we did not detect CMV in the blood of our patients by PCR, but CMV DNA has been detected in the urine in older CMV-seropositive subjects and then the detection of CMV DNA in the urine of CVID patients should be determined. Moreover, conversely to CLL patients, CVID patients do not exhibit VZV infections. Several CVID patients do not have any T-cell activation whereas they exhibit a profound hypogammaglobulinemia (patient 7 for example). At least, multiple CD8+ T-cell expansions were found in CVID patients, suggesting that these cells might target several antigens. The T cell expansions identified in this study differed from one patient to another but two selected BV families (V2 and V5.1) were more represented in the group with complications. Detection of CMV-specific CD4+ T cells should be determined in CVID patients to precise the role of the virus in the contraction of the T-cell repertoire.
The influence of IVIG substitution on T-cell activation can also be suggested. However, the patient 3 did not receive intravenous immunoglobulins replacement and this patient presented a contracted T-cell repertoire.
Finally, the perturbations of the T-cell repertoire were particularly restricted to the group of CVID patients with auto-immune and/or organomegaly complications which suggested a link between the mechanisms leading to the CD8 TCR repertoire restriction and those leading to the complications in these patients, but viral reactivation might not be the sole explanation. Usually, auto-antibodies are not detected in these patients, perhaps because of the hypogammaglobulinemia but this does not eliminate the participation of T-cells in the auto-immune response in these patients as a cause for their activation.
In summary, CVID patients exhibit a contraction of their CD8+ T-cell repertoire whose causes remain to be deciphered but which are likely multifactorial. In this regard, the exploration of their CMV immune statuses may be of interest for their management on the long term as suggested by Marashi et al. [27].
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Park M.A., Li J.T., Hagan J.B., Maddox D.E., Abraham R.S. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372:489–502. doi: 10.1016/S0140-6736(08)61199-X. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C., Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clinical Immunology. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 3.Oksenhendler E. Infections in 252 patients with common variable immunodeficiency. Clinical Infectious Diseases. 2008;46:1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 4.Wehr C., Kivioja T., Schmitt C., Ferry B., Witte T., Eren E. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 5.Viallard J.F., Blanco P., André M., Etienne G., Liferman F., Neau D., Vidal E., Moreau J.F., Pellegrin J.L. CD8+HLA-DR+ T lymphocytes are increased in common variable immunodeficiency patients with impaired memory B-cell differentiation. Clinical Immunology. 2006;119:51–58. doi: 10.1016/j.clim.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.International Union of Immunological Societies Expert Committee on Primary Immunodeficiencies. Notarangelo L.D., Fischer A., Geha R.S., Casanova J.L., Chapel H., Conley M.E. Primary immunodeficiencies: 2009 update. Journal of Allergy and Clinical Immunology. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D., Arnold D.M. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;114:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 8.Gagne K., Brouard S., Giral M., Sebille F., Moreau A., Guillet M. Highly altered V beta repertoire of T cells infiltrating long-term rejected kidney allografts. Journal of Immunology. 2000;164:1553–1563. doi: 10.4049/jimmunol.164.3.1553. [DOI] [PubMed] [Google Scholar]
- 9.Pannetier C., Even J., Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunology Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 10.Gorochov G., Neumann A.U., Kereveur A., Parizot C., Li T., Katlama C. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nature Medicine. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 11.Sebille F., Gagne K., Guillet M., Degauque N., Pallier A., Brouard S. Direct recognition of foreign MHC determinants by naive T cells mobilizes specific Vbeta families without skewing of the complementarity-determining region 3 length distribution. Journal of Immunology. 2001;167:3082–3088. doi: 10.4049/jimmunol.167.6.3082. [DOI] [PubMed] [Google Scholar]
- 12.Guillet M., Brouard S., Gagne K., Sebille F., Cuturi M.C., Delsuc M.A., Soulillou J.P. Different qualitative and quantitative regulation of V beta TCR transcripts during early acute allograft rejection and tolerance induction. Journal of Immunology. 2002;168:5088–5095. doi: 10.4049/jimmunol.168.10.5088. [DOI] [PubMed] [Google Scholar]
- 13.Miqueu P., Guillet M., Degauque N., Doré J.C., Soulillou J.P., Brouard S. Statistical analysis of CDR3 length distributions for the assessment of T and B cell repertoire biases. Molecular Immunology. 2007;44:1057–1064. doi: 10.1016/j.molimm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Benveniste O., Chérin P., Maisonobe T., Merat R., Chosidow O., Mouthon L., Guillevin L., Flahault A., Burland M.C., Klatzmann D., Herson S., Boyer O. Severe perturbations of the blood T cell repertoire in polymyositis, but not dermatomyositis patients. Journal of Immunology. 2001;167:3521–3529. doi: 10.4049/jimmunol.167.6.3521. [DOI] [PubMed] [Google Scholar]
- 15.Berthelot L., Miqueu P., Pettré S., Guillet M., Moynard J., Wiertlewski S., Lefrère F., Brouard S., Soulillou J.P., Laplaud D.A. Failure of glatiramer acetate to modify the peripheral T cell repertoire of relapsing-remitting multiple sclerosis patients. Clinical Immunology. 2010;135:33–42. doi: 10.1016/j.clim.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Wright J.J., Wagner D.K., Blaese R.M., Hagengruber C., Waldmann T.A., Fleisher T.A. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990;76:2046–2051. [PubMed] [Google Scholar]
- 17.Mouillot G., Carmagnat M., Gérard L., Garnier J.L., Fieschi C., Vince N. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. Journal of Clinical Immunology. 2010;30:746–755. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 18.Posnett D.N., Sinha R., Kabak S., Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. Journal of Experimental Medicine. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hingorani R., Choi I.H., Akolkar P., Gulwani-Akolkar B., Pergolizzi R., Silver J., Gregersen P.K. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. Journal of Immunology. 1993;151:5762–5769. [PubMed] [Google Scholar]
- 20.Silins S.L., Cross S.M., Krauer K.G., Moss D.J., Schmidt C.W., Misko I.S. A functional link for major TCR expansions in healthy adults caused by persistent Epstein-Barr virus infection. Journal of Clinical Investigation. 1998;102:1551–1558. doi: 10.1172/JCI4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan N., Shariff N., Cobbold M., Bruton R., Ainsworth J.A., Sinclair A.J. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. Journal of Immunology. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 22.Wikby A., Johansson B., Olsson J., Löfgren S., Nilsson B.O., Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Experimental Gerontology. 2002;37:445–453. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 23.Solana R., Tarazona R., Aiello A.E., Akbar A.N., Appay V., Beswick M. CMV and immunosenescence: from basics to clinics. Immunityand Ageing. 2012;9:23. doi: 10.1186/1742-4933-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stowe R.P., Kozlova E.V., Yetman D.L., Walling D.M., Goodwin J.S., Glaser R. Chronic herpesvirus reactivation occurs in aging. Experimental Gerontology. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackus W.J., Frakking F.N., Grummels A., Gamadia L.E., De Bree G.J., Hamann D. Expansion of CMV-specific CD8+CD45RA+CD27- T cells in B-cell chronic lymphocytic leukemia. Blood. 2003;102:1057–1063. doi: 10.1182/blood-2003-01-0182. [DOI] [PubMed] [Google Scholar]
- 26.Pourgheysari B., Bruton R., Parry H., Billingham L., Fegan C., Murray J., Moss P. The number of cytomegalovirus-specific CD4+ T cells is markedly expanded in patients with B-cell chronic lymphocytic leukemia and determines the total CD4+ T-cell repertoire. Blood. 2010;116:2968–2974. doi: 10.1182/blood-2009-12-257147. [DOI] [PubMed] [Google Scholar]
- 27.Marashi S.M., Raeiszadeh M., Enright V., Tahami F., Workman S., Chee R. Influence of cytomegalovirus infection on immune cell phenotypes in patients with common variable immunodeficiency. Journal of Allergy and Clinical Immunology. 2012;129:1349–1356. doi: 10.1016/j.jaci.2012.02.011. [DOI] [PubMed] [Google Scholar]


