Abstract
Objective:
The objective of this randomized, double-blind, placebo-controlled, crossover study was to examine if patients with optic neuropathy would derive a therapeutic benefit from 4-aminopyridine (4-AP) treatment. Furthermore, the study was intended to determine if patients with certain P100 latencies or retinal nerve fiber layer (RNFL) measures would be more likely to respond to therapy.
Methods:
Patients were enrolled in a randomized, placebo-controlled, double-blind, crossover study of 10 weeks duration. Patients underwent visual evoked potentials (VEP), optical coherence tomography (OCT), and visual acuity before starting 5 weeks of either placebo or 4-AP. After 5 weeks, they completed a second evaluation (VEP, OCT, and visual acuity) and were crossed over between treatment arms. Five weeks later, they had their final evaluation. All investigators were blinded to treatment arm until after data analysis.
Results:
On average, patients had faster P100s on 4-AP when compared to placebo. A subset of patients had distinct responses to 4-AP as measured by improvements in visual acuity. Finally, eyes with an RNFL measure between 60 and 80 µm had the highest response rate.
Conclusions:
4-Aminopyridine is useful for improving vision in patients with demyelinating optic neuropathy. Future clinical trials may be able to enrich a patient population for potential responders using OCT and VEP measures. Selecting patients for future trials should use RNFL measures as part of inclusion/exclusion criteria.
Classification of evidence:
This study provides Class IV evidence supporting the use of 4-AP in certain patients with optic neuropathy to improve visual function (patients with RNFL between 60 and 80 µm).
Visual dysfunction secondary to optic neuritis is common in multiple sclerosis (MS).1,2 Despite apparent recovery, visual function, as measured by low-contrast vision, can remain impaired, as quantified with low-contrast visual acuity (VA) Sloan charts.3–9 Patients may remain impaired for edge detection (e.g., walking stairs), night vision, stereopsis, and motion detection. Further, when factors known to precipitate Uhthoff phenomena are present, VA can be compromised.10,11
Low-contrast vision correlates with retinal nerve fiber layer (RNFL) thinning as measured by optical coherence tomography (OCT).12,13 The RNFL consists of unmyelinated axons of ganglion cells and is a reproducible measure of axonal integrity. Additionally, patients often have delayed latencies on visual evoked potential (VEP) testing secondary to demyelination.
While deficits secondary to axonal damage are potentially irreversible, it may be possible to modify the deficits that result from demyelination and reversible axonal dysfunction with 4-aminopyridine (4-AP). Acting as a potassium channel blocker, 4-AP increases the fidelity of axonal excitability and augments conduction along demyelinated neurons.14 Studies have demonstrated a beneficial effect of 4-AP on various MS symptoms.15–19
In this randomized, double-blind, placebo-controlled study, our objective was to determine what parameters would define patients most likely to exhibit improvements in objective visual system metrics while on 4-AP.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was a prospective, double-blind, placebo-controlled crossover design of the effect of 4-AP in patients with MS with optic neuropathy. The protocol was approved by the UT Southwestern Institutional Review Board (study number 092010-085), and all subjects gave written informed consent.
Patients.
We recruited 22 subjects with MS between the ages of 18 and 70 years. We included subjects with evidence of optic neuropathy (defined as an RNFL of less than or equal to 80 µm in at least one eye, or a greater than 10-µm difference between the two eyes). Patients were more than 6 months from their last clinical optic neuritis event and steroid exposure. Patients were excluded if they were pregnant, were already taking 4-AP or dalfampridine, received steroids during the study, or had any contraindications to the medication (history of seizures or renal insufficiency). Subjects were also excluded if they had other diseases that might confound visual testing results (e.g., glaucoma or nystagmus).
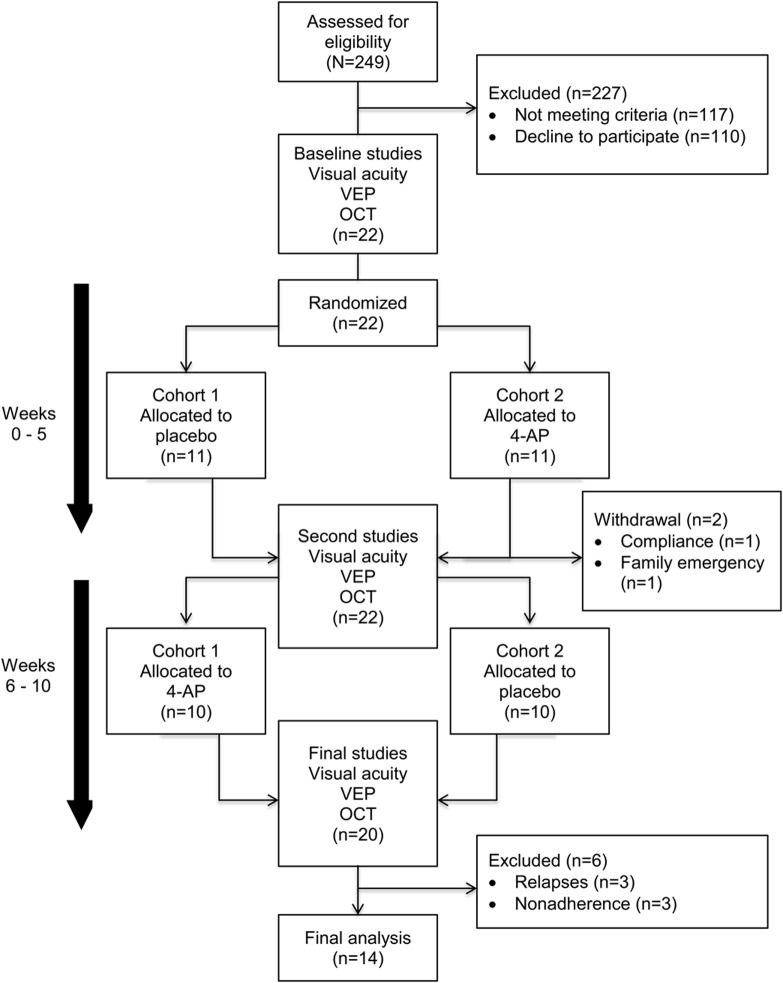
A total of 249 patients were prescreened via chart reviews and telephone interviews. Patients who met the inclusion and exclusion criteria were scheduled for a screening visit. During each visit, subjects underwent VA testing, OCT, and VEPs (VERIS with FMS 3 stimulator using VERIS Science 3.2 software, Electrodiagnostic Imaging, Redwood City, CA; 2 repetition per second conventional VEP with 60-minute arc checks). All VEP studies occurred in the same room with the same setup throughout the study. Study visits and assessments are represented in figure 1.
Figure 1. Study visits and assessments.
4-AP = 4-aminopyridine; OCT = optical coherence tomography; VEP = visual evoked potential.
High-contrast VA was measured using an Early Treatment Diabetic Retinopathy Study chart in front of a backlit cabinet. Low-contrast VA was measured using Sloan low-contrast VA charts at 2.5% and 1.25% contrast levels. OCT measurements of the average RNFL thickness were obtained using high-speed, high-definition, spectral domain OCT with Spectralis (Heidelberg Engineering, Heidelberg, Germany) 5.3c with n-site software. Conventional pattern-reversal VEPs were measured using VERIS software. Scalp gold cup electrodes were placed according to the International 10/20 system.
Compounded 4-AP and placebo capsules were obtained from a compounding pharmacy (Pharmacy Solutions, Arlington, TX). The capsules were packaged in identical bottles. The patients were instructed to increase the dose over each 5-week period according to a titration schedule beginning with 5 mg twice a day and ending in a dose of 10 mg 3 times a day.
At completion, patients were asked about compliance and capsules were counted. Compliance was defined as consuming greater than or equal to 80% of the doses and taking the medication within 24 hours of a study visit.
Statistics and data analysis.
This pilot study was designed to determine if physiologic parameters could be determined that would inform future trial designs for patients with optic neuropathy. An improvement of 5 or more letters on the 2.5% VA chart was the primary outcome, while an improvement in the P100 latencies was a secondary outcome measure.
Data analysis was conducted while blinded to study drug allotment (L.H. and B.M.G.). Comparisons of baseline characteristics among the randomized cohort and the analyzed cohort utilized paired t tests and Mann-Whitney tests. The change in correct number of letters on the high-contrast and low-contrast charts during the medication arm and the placebo arm of the study was analyzed using a paired t test. The change in VEP P100 latency on placebo compared to medication was also analyzed using a one-tailed paired t test. Significance was measured as p < 0.05 for all tests.
Classification of evidence.
This study provides Class IV evidence supporting the use of 4-AP in certain patients with optic neuropathy to improve visual function (patients with RNFL between 60 and 80 µm).
RESULTS
The table summarizes the baseline demographics for all subjects enrolled in the study (22 subjects). After randomization, 2 subjects withdrew from the study, leaving 20 patients to complete the study (figure 1). There was one serious adverse event during this study; a subject was hospitalized secondary to hypokalemia thought to be unrelated to the study medication (she had started another medication with a known risk of hypokalemia). Only 14 patients (28 eyes) out of the 20 patients completing the study were included in the analyses (figure 1).
Table.
Baseline demographic informationa
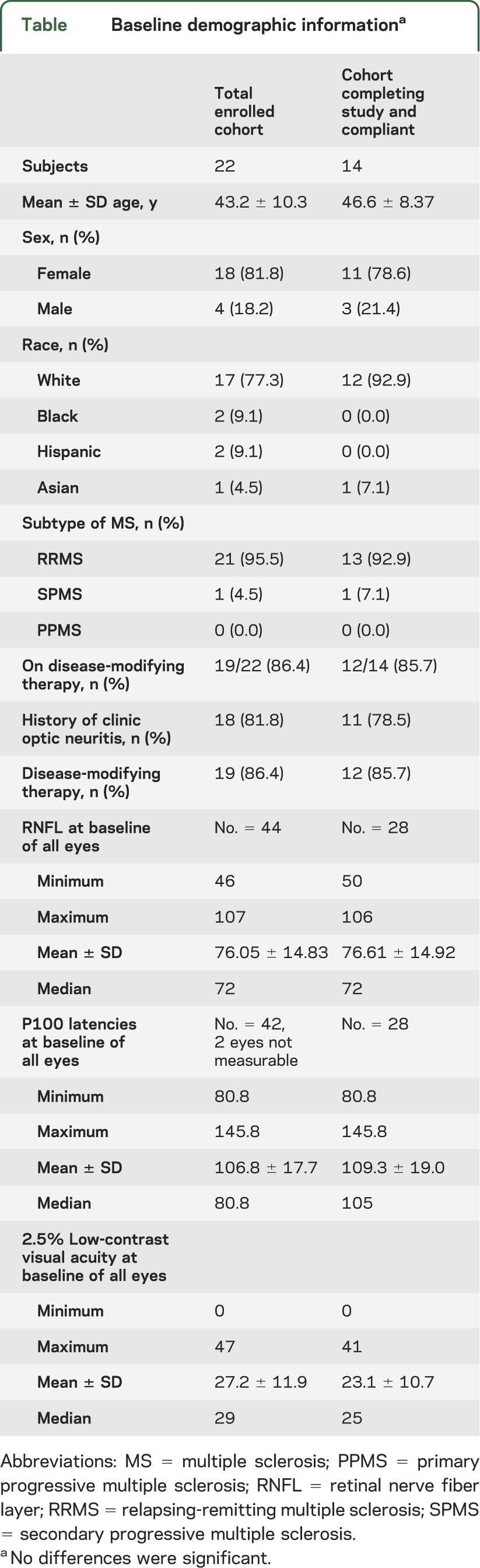
The mean RNFL of the original 44 eyes (22 patients) was 76.05 (SD ± 14.83), which was not significantly different from the mean RNFL of the 28 eyes analyzed at the conclusion of the study (76.61; SD ± 14.92) (p = 0.94).
The correlation between average RNFL and the P100 latency as measured by VEP had a Pearson r value of −0.4230. While there was not a significant difference in the average improvement of VA (as measured by the 2.5% low-contrast VA chart) between the placebo and 4-AP treatment regimens (p = 0.24, 95% confidence interval [CI] −2.6 to 1.29), there were more responders to the 4-AP arm of the study. Eight of 28 (28.6%) of the eyes exposed to 4-AP improved by 7 or more letters on the low-contrast VA test compared to only 3 of the 28 (10.7%) eyes exposed to placebo. A significant treatment effect was seen in 7 of 28 eyes while on 4-AP, compared to 1 of 28 on placebo.
When comparing the average change in P100 latencies, there was a statistically significant difference between the 4-AP and placebo arms. For the entire cohort of 28 eyes, the mean change in P100 latency while on placebo was 1.57 compared to −1.20 while on 4-AP (p = 0.004). When the RNFL measurements considered were restricted to 60–80 µm (19 eyes), the mean change while on placebo was 1.45 (95% CI −1.46 to 4.36) compared to −1.89 (95% CI −3.71 to −0.07) while on 4-AP (p = 0.01). When the RNFL measurements considered were restricted to 60–70 µm (12 eyes), the mean change while on placebo was 2.57 (95% CI −1.78 to 6.92) compared to −1.88 (95% CI −3.81 to 0.06) while on 4-AP (p = 0.009).
A linear regression analysis identified a higher variance of change in P100 in eyes on placebo compared to eyes on 4-AP, especially when focused on eyes with a RNFL between 60 and 80 (figure 2). Two-way analysis of variance found that both treatment and RNFL values were significantly associated with change in P100 (p 0.01 and <0.0001, respectively).
Figure 2. Linear regression of P100 latency change per RNFL measure during placebo and 4-AP treatment.
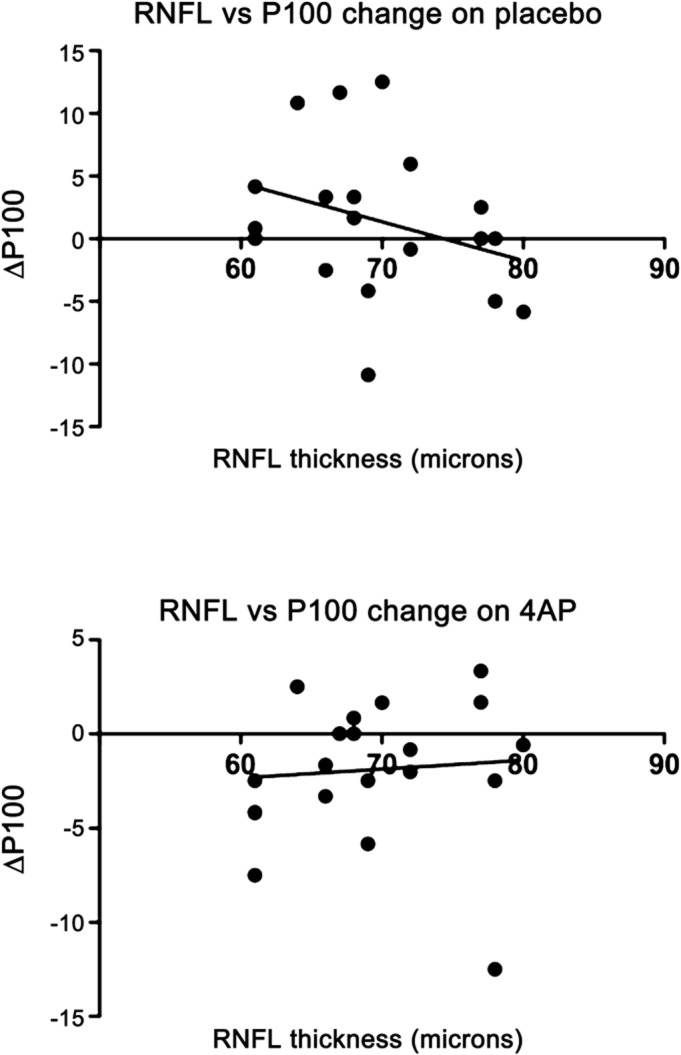
4-AP = 4-aminopyridine; RNFL = retinal nerve fiber layer.
DISCUSSION
The primary objective of our study was to determine whether 4-AP would improve VA in patients with MS with optic nerve damage. Using OCT and VEP, we also wanted to determine if a structural and functional profile of responders could be identified.
When defining responders as those who improved by 5 or more letters on 2.5% low-contrast acuity testing, a higher percentage of subjects responded while on 4-AP compared to placebo (32% on 4-AP compared to 14% on placebo). This is consistent with previous studies of dalfampridine, the extended release form of 4-AP, which found that there was only a subset of responders to the medication.20
As a secondary outcome measure, we compared change in P100 latency while on placebo vs 4-AP. Patients on 4-AP had a significantly larger decrease in latency on VEP testing compared to those on placebo. When we graphed the change in P100 latency while on placebo vs 4-AP, there was more variance in the change in latency while on placebo. Patients with an RNFL thickness of less than 60 µm did not have as much of a decrease in latency, presumably because the structural damage was too extensive in these patients. This data suggest a more consistent, uniform conduction of signal in 4-AP–exposed optic nerves. Therefore, it appears that this medication improved conduction speed along the demyelinated optic nerve, consistent with its theorized mechanism of action. The P100 measure has limited clinical value, but based on this study, can be used as part of an assessment tool to predict which patients are more likely to visually respond to 4-AP.
Another goal of this study was to develop a structural and functional profile of potential responders to 4-AP. Based on the clinical response and P100 latency data, one would predict that a group of patients with an RNFL thickness of 60–80 µm would experience more of a treatment effect than patients with RNFLs outside of that range. On its own, a change in P100 is not clinically significant, but this observation is consistent with our hypothesis that this medication would have a greater effect in those patients with mostly demyelinating damage compared to those with more structural axonal damage.
The primary limitation of this study was the small sample size; we may have observed a different effect of 4-AP on VA in a larger group of patients. VEP recordings were also not able to be interpreted in all patients because of the multiple factors that can influence the outcome of this test. Poor acuity often results in a poor recording with unidentifiable peak latency. The crossover design was not likely a factor in this study given the short half-life of 4-AP. Future directions include a larger clinical trial of 4-AP in patients with an RNFL thickness of 60–80 µm.
GLOSSARY
- 4-AP
4-aminopyridine
- CI
confidence interval
- MS
multiple sclerosis
- OCT
optical coherence tomography
- RNFL
retinal nerve fiber layer
- VA
visual acuity
- VEP
visual evoked potential
AUTHOR CONTRIBUTIONS
Ms. Horton: acquisition of data, analysis and interpretation. Ms. Conger: acquisition of data. Mr. Conger: acquisition of data. Ms. Remington: Study concept and design. Ms. Frohman: critical revision of the manuscript for important intellectual content. Dr. Frohman: study concept and design, critical revision of the manuscript for important intellectual content. Dr. Greenberg: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
Supported by a grant from the Doris Duke Charitable Foundation to UT Southwestern to fund Clinical Research Fellow Lindsay Horton. This study was also sponsored by the University of Texas Southwestern Foundation.
DISCLOSURE
L. Horton, A. Conger, and D. Conger report no disclosures. G. Remington serves on the speaker's bureau for Teva Neurosciences and Biogen Idec. She has received honoraria from Teva Neurosciences, Biogen Idec., Genzyme, Acorda Therapeutics, the Consortium of Multiple Sclerosis Centers, and the National Multiple Sclerosis Society. T. Frohman has received honorarium for participating as a speaker for Biogen and Novartis, as an advisory board participant for Biogen, Novartis, and Questcor, and as a consultant for Biogen, Novartis, Questcor, and Acorda Pharmaceuticals. E. Frohman has received consulting and speaker fees from Biogen Idec, TEVA, Novartis, and Acorda; and consulting fees from Abbott and Genzyme. B. Greenberg has received grant support from the Accelerated Cure Project, The Guthy Jackson Charitable Foundation for NMO, and Amplimmue, Inc. He has received consulting fees from Biogen, Idec, Sanofi-Aventis, DioGenix, The Greater Good Foundation, and Elan Pharmaceuticals. He owns equity in DioGenix, Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Balcer LJ. Clinical practice: optic neuritis. N Engl J Med 2006;354:1273–1280 [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Frohman TC, Zee DS, McColl R, Galetta S. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol 2005;4:111–121 [DOI] [PubMed] [Google Scholar]
- 3.Ashworth B, Aspinall PA, Mitchell JD. Visual function in multiple sclerosis. Doc Ophthalmol 1989;73:209–224 [DOI] [PubMed] [Google Scholar]
- 4.Baier ML, Cutter GR, Rudick RA, et al. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2005;64:992–995 [DOI] [PubMed] [Google Scholar]
- 5.Balcer LJ, Baier ML, Pelak VS, et al. New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Mult Scler 2000;6:163–171 [DOI] [PubMed] [Google Scholar]
- 6.Balcer LJ, Frohman EM. Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology 2010;74(suppl 3):S16–S23 [DOI] [PubMed] [Google Scholar]
- 7.Cleary PA, Beck RW, Bourque LB, Backlund JC, Miskala PH. Visual symptoms after optic neuritis: results from the Optic Neuritis Treatment Trial. J Neuroophthalmol 1997;17:18–23 [DOI] [PubMed] [Google Scholar]
- 8.Fleishman JA, Beck RW, Linares OA, Klein JW. Deficits in visual function after resolution of optic neuritis. Ophthalmology 1987;94:1029–1035 [DOI] [PubMed] [Google Scholar]
- 9.Kupersmith MJ, Nelson JI, Seiple WH, Carr RE, Weiss PA. The 20/20 eye in multiple sclerosis. Neurology 1983;33:1015–1020 [DOI] [PubMed] [Google Scholar]
- 10.Davis SL, Wilson TE, White AT, Frohman EM. Thermoregulation in multiple sclerosis. J Appl Physiol 2010;109:1531–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frohman TC, Davis SL, Frohman EM. Modeling the mechanisms of Uhthoff's phenomenon in MS patients with internuclear ophthalmoparesis. Ann NY Acad Sci 2011;1233:313–319 [DOI] [PubMed] [Google Scholar]
- 12.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006;113:324–332 [DOI] [PubMed] [Google Scholar]
- 13.Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 2005;58:383–391 [DOI] [PubMed] [Google Scholar]
- 14.Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980;283:570–572 [DOI] [PubMed] [Google Scholar]
- 15.Davis FA, Stefoski D, Rush J. Orally administered 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 1990;27:186–192 [DOI] [PubMed] [Google Scholar]
- 16.Bever CT, Jr, Young D, Anderson PA, et al. The effects of 4-aminopyridine in multiple sclerosis patients: results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trial. Neurology 1994;44:1054–1059 [DOI] [PubMed] [Google Scholar]
- 17.Jones RE, Heron JR, Foster DH, Snelgar RS, Mason RJ. Effects of 4-aminopyridine in patients with multiple sclerosis. J Neurol Sci 1983;60:353–362 [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Bertelsmann FW, van Loenen AC, Koetsier JC. 4-aminopyridine in the treatment of patients with multiple sclerosis. Long-term efficacy and safety. Arch Neurol 1994;51:292–296 [DOI] [PubMed] [Google Scholar]
- 19.Stefoski D, Davis FA, Faut M, Schauf CL. 4-Aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 1987;21:71–77 [DOI] [PubMed] [Google Scholar]
- 20.Goodman AD, Brown TR, Krupp LB, et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738 [DOI] [PubMed] [Google Scholar]