Abstract
Objective:
We hypothesized that total brain volume, white matter volume, and lobar cortical thickness would be different in epilepsy patients. We studied valproate relative to nonvalproate by using patients with epilepsy and healthy controls.
Methods:
Patients with focal intractable epilepsy from a tertiary epilepsy center were the primary group for analysis. A confirmatory analysis was carried out in an independent group of subjects imaged as part of a community-based study of childhood-onset epilepsy. Total brain volume; white matter volume; and frontal, parietal, occipital, and temporal lobe thickness were measured by processing whole-brain T1-weighted MRI using FreeSurfer 5.1.
Results:
Total brain volume, white matter volume, and parietal thickness were reduced in the valproate group relative to controls and nonvalproate users (valproate, n = 9; nonvalproate, n = 27; controls, n = 45; all male). These findings were confirmed in an independent group (valproate, n = 7; nonvalproate, n = 70; controls, n = 20; all male).
Conclusions:
Sodium valproate use in epilepsy is associated with parietal lobe thinning, reduced total brain volume, and reduced white matter volume.
Level of evidence:
This study provides Class IV evidence that use of valproate in epilepsy is associated with reduced parietal lobe thickness, total brain volume, and white matter volume.
Sodium valproate is a widely used antiepileptic medication. It is a recommended first-line medication for generalized seizures, is effective for treating focal-onset seizures, and has efficacy in treatment of psychiatric disorders and migraines. Rare adverse effects associated with sodium valproate include isolated cases of mental deterioration with reversible brain atrophy.1 In this study, we used quantitative analysis of structural MRI to determine whether there are systematic differences in cortical thickness and brain volume that are associated with valproate use.
We hypothesized that brain volume, white matter volume, and cortical thickness measures would be different in epilepsy patients taking sodium valproate relative to both patients with epilepsy not currently taking valproate and healthy controls. We examined this issue first in a group with focal epilepsy from a tertiary referral center and then confirmed the results in an independent community-based cohort of patients with childhood-onset epilepsy.
METHODS
Participants and MRI acquisition.
Group A.
Subjects with consecutive intractable focal epilepsy were referred for 3 T imaging as part of the Comprehensive Epilepsy Program, Austin Health, Melbourne. Epilepsy cases were characterized according to brain lobe localization and the presence of lesions, based on Comprehensive Epilepsy Program notes interpreted by an experienced epileptologist (G.D.J.). Investigations included clinical semiology, video monitoring of EEG, 1.5-T MRI, neuropsychologic evaluation, and PET/SPECT where appropriate. Daily valproate dose and other antiepileptic medications currently being taken were noted. Participants with epilepsy were grouped into those currently taking valproate (+VPA) and those not currently taking valproate (−VPA). Differences in additional antiepileptic medication use between the +VPA and −VPA groups were investigated (sequential χ2 tests of each additional antiepileptic medication). Imaging was performed on a 3-T Siemens Tim Trio (Malvern, PA) scanner between 2008 and 2012. Whole-brain T1-weighted 3D magnetization-prepared rapid acquisition with gradient echo MRI was acquired with the following acquisition parameters: echo time, 2.6 ms; repetition time, 1,900 ms; flip angle, FA = 9°; inversion time, 900 ms; and voxel resolution, 0.9 mm isotropic.
Group B.
Community-based subjects with childhood-onset epilepsy were recruited as part of the Connecticut Study of Epilepsy in Children. Cohort B was imaged when case subjects were invited to take part in a comprehensive reassessment which included a research MRI scan. Controls were recruited to represent the sex and age distribution of all cases being scanned for research purposes. Current and past antiepileptic medication use was recorded. Recruitment, characterization, and follow-up of this cohort have been described in detail elsewhere.2,3 Imaging was performed on a 1.5-T Siemens Sonata MRI scanner between 2008 and 2012. Whole-brain coronal T1-weighted magnetization-prepared rapid acquisition with gradient echo MRI was acquired with the following image parameters: echo time, 3.05 ms; repetition time, 1,730 ms; flip angle, 15°; inversion time, 1,100 ms; and voxel size, 0.94 × 0.94 × 1.6 mm.
All controls were neurologically normal, without any history of epilepsy, neurologic disorders, or other major medical illness. Standard clinical MRI interpretation of controls was normal.
Standard protocol approvals, registrations, and patient consents.
Written informed consent of adult subjects or written parental permission and assent of minors were obtained as required by the Institutional Review Board of each site. Ethics approval for study participants was obtained from the relevant authorities, and study procedures were approved by institutional ethical standards committees on human experimentation.
Image analysis.
Whole-brain T1-weighted MRI was acquired for each participant. MRI scans were processed by using FreeSurfer version 5.1 (developed by the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown).4 Cortical thickness values were averaged over each major brain lobe (frontal, parietal, occipital, and temporal). Total brain volume and white matter volume were measured. Image processing was fully automated and masked with respect to subject allocation (disease and treatment status).
The general linear model was used to investigate total brain volume, white matter volume, frontal lobe thickness, parietal lobe thickness, occipital lobe thickness, and temporal lobe thickness in subjects with epilepsy currently taking valproate relative to those not currently taking valproate and healthy controls. Age was included as a covariate in all analyses. Group A and group B analyses were conducted separately. Two-sided statistical analyses were used for the group A analysis. One-sided statistical analyses were used for the group B analysis, since the direction (increase or decrease) of any effect of valproate on volume or thickness was determined by the group A analysis.
The primary research question for this study was to determine whether sodium valproate use in epilepsy is associated with differences in total brain volume, white matter volume, and lobar cortical thickness. The study design provided Class IV evidence for these hypotheses.
We conducted a secondary analysis to determine whether past valproate use was associated with changes in brain morphometric properties. Group B nonvalproate users were subdivided into past valproate users and patients with epilepsy who had never used valproate. The 6 brain volume measures in group B past valproate users were compared with those in group B participants who had never used valproate.
RESULTS
Group A.
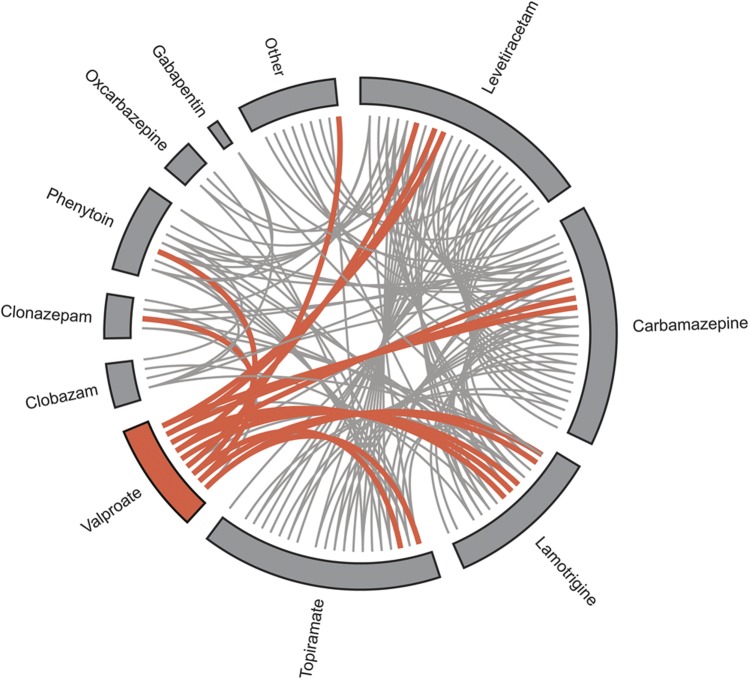
Nine subjects with epilepsy currently taking valproate (age, 33 ± 8.1 years), 27 subjects with epilepsy not currently taking valproate (age, 34.7 ± 10.3 years), and 45 healthy controls (age, 29.5 ± 10.2 years) were included. All subjects were male; nonvalproate and control groups were sex-matched to the valproate group. The median dose of sodium valproate was 1,400 mg/d (8 subjects, 1,000–2,000 mg/d; ninth subject, 400 mg/d). There was no difference in the number of patients on monotherapy compared with polytherapy in patients on valproate compared with patients with epilepsy not on valproate (table 1, χ2 test; p = 0.87). Localization information is provided in table 1. For subjects with epilepsy on polytherapy, there was no significant difference between other antiepileptic medication use in the +VPA and –VPA groups (figure 1, p > 0.05; for all additional antiepileptic medications, see table e-1 on the Neurology® Web site at www.neurology.org).
Table 1.
Summary demographic data for patients with epilepsy taking valproate (+VPA), nonvalproate epilepsy cases (−VPA), and healthy controls
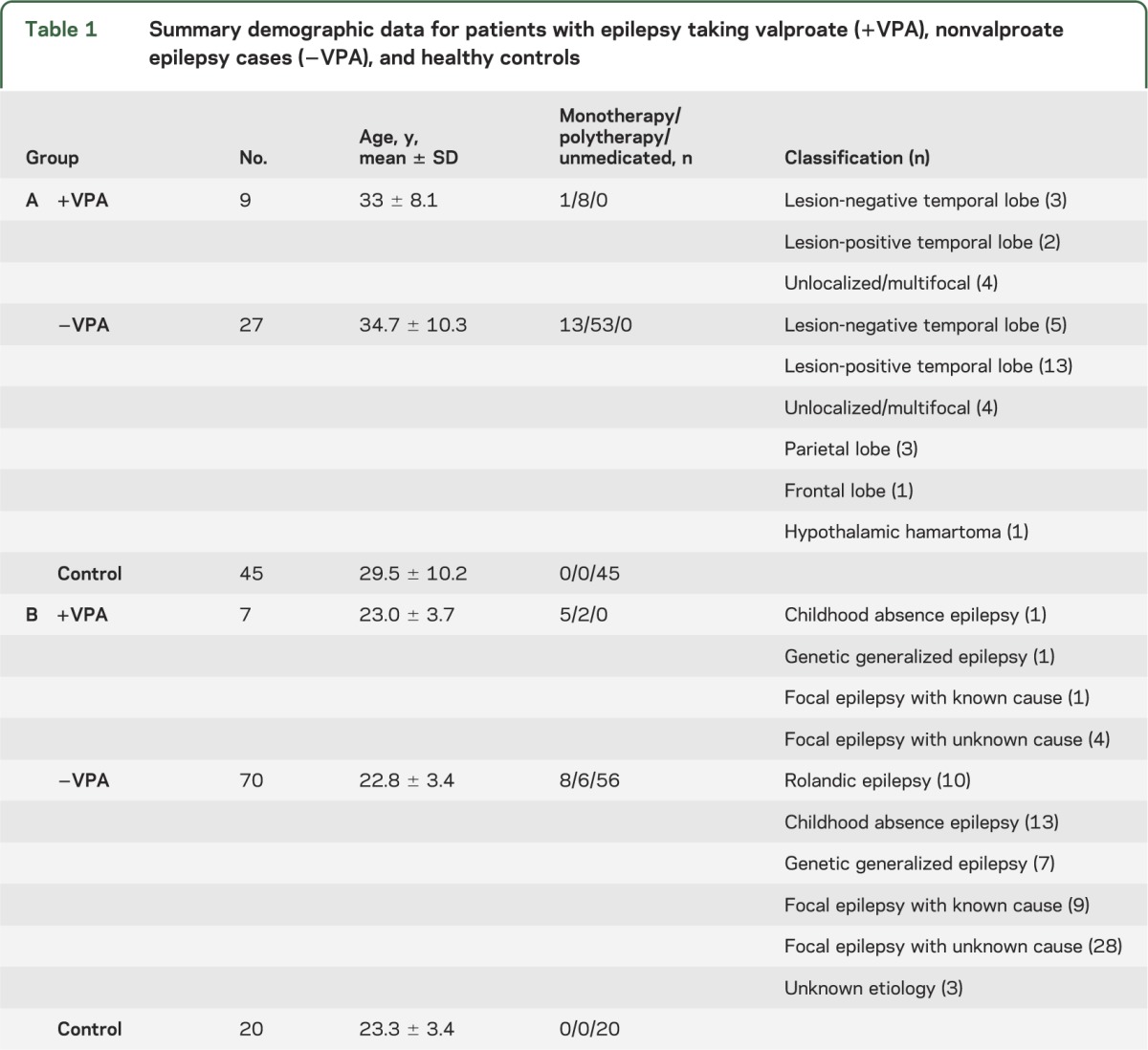
Figure 1. Antiepileptic drug combinations in intractable focal epilepsy.
Antiepileptic drug combinations in intractable focal epilepsy cases (group A). Valproate cases are shown in orange, and nonvalproate cases are shown in gray. A line between the 2 drugs indicates the individual was taking the drugs as polytherapy. The “other” category includes nonantiepileptic medications.
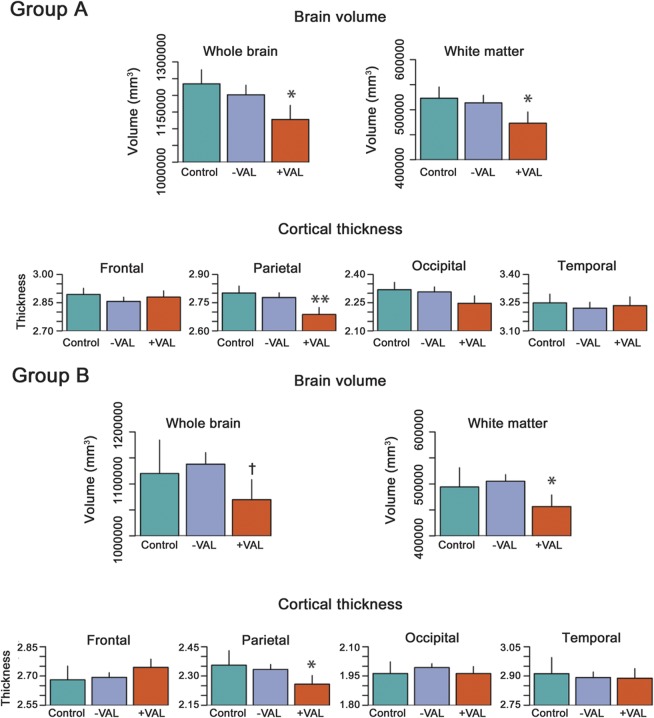
Parietal lobe thickness was reduced in the valproate group relative to controls and the nonvalproate epilepsy group (figure 2, mean decrease of 0.1 mm; p = 0.0033). No thickness differences were observed in other lobes. An effect size map of the spatial distribution of thickness differences between valproate and nonvalproate subjects with epilepsy is shown in figure 3. White matter and total brain volumes were reduced in the valproate group relative to controls and the nonvalproate epilepsy group (mean reduction of 49,924 mm3 white matter volume, p = 0.029; 106,859 mm3 total brain volume; p = 0.014).
Figure 2. Volume and cortical thickness in valproate epilepsy cases, nonvalproate epilepsy cases, and controls.
Epilepsy patients taking valproate (+VPA) have reduced brain volume, white matter volume, and parietal lobe cortical thickness relative to epilepsy patients not on valproate (−VPA) and healthy controls. The figure shows brain volume measurements and average lobar thickness in controls, nonvalproate epilepsy cases (−VPA), and valproate-taking epilepsy cases (+VPA). Group A are presurgical focal epilepsy cases from a tertiary epilepsy center; group B are childhood-onset epilepsy cases. The error bars represent standard errors on the mean. Statistically significant differences are marked with an asterisk (**p < 0.001; *p < 0.05; †p < 0.1).
Figure 3. Valproate-related cortical thickness decreases in focal epilepsy.
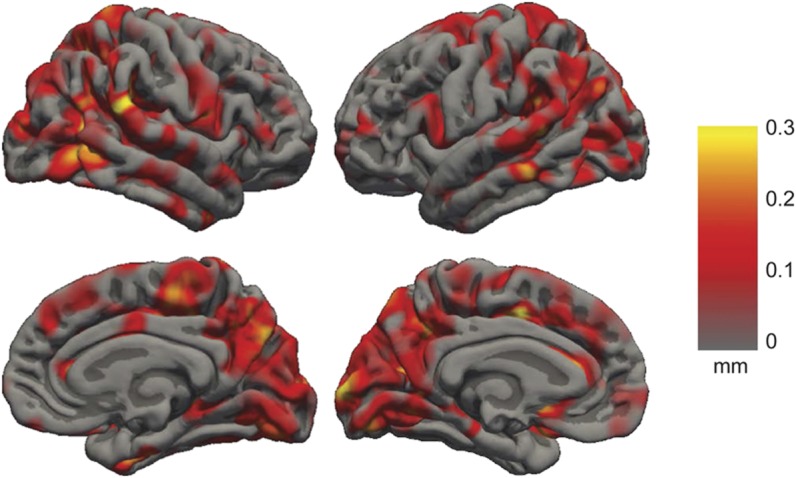
Spatial distribution of cortical thickness decreases in subjects with focal epilepsy taking valproate relative to subjects with epilepsy not taking valproate (group A). The intensity of the color overlay indicates the magnitude of the thickness decrease. Hotter colors indicate reduced cortical thickness in subjects taking valproate.
Group B.
Seven subjects with epilepsy currently taking valproate (age, 24.6 ± 3.7 years), 71 subjects with epilepsy not taking valproate (age, 23 ± 3.1 years), and 20 healthy controls (age, 23.3 ± 3.3 years) were included. Only males were included to maintain sex matching across the 2 groups. There was no difference between +VPA and −VPA in the number of subjects on monotherapy compared with polytherapy (table 1, χ2 test; p = 0.87).
The finding of reduced parietal thickness in the valproate group was confirmed in cohort B (figure 2, 0.09 mm; p = 0.016). No significant thickness reductions were observed in the nonvalproate epilepsy group or other cortical lobes. White matter and total brain volumes were reduced in the valproate group (reductions of 37,639 mm3 white matter volume, p = 0.047; 50,252 mm3 total brain volume; p = 0.098; note p > 0.05). No volume or cortical thickness differences were observed between past valproate users and subjects with epilepsy who had never used valproate.
DISCUSSION
Our study has demonstrated that valproate treatment for seizure control in epilepsy is associated with cortical thinning in the parietal lobes, along with white matter and whole brain volume reduction. It is important to note that we have confirmed these morphologic changes in an independent cohort with different epilepsy types. Our findings are consistent with pseudoatrophic brain changes associated with valproate use that have been reported in isolated epilepsy cases,1 as well as atrophy associated with valproate use in Alzheimer disease.5 Cerebral volume decreases have also been observed in rats exposed to valproate in utero.6 When we compared group B past valproate users with patients with epilepsy who had never taken valproate, no significant volume or thickness differences were observed. This finding suggests that the parietal lobe thinning and volume changes we have observed in this study are transient.
It is possible that the changes reported in this study are a subtle and common manifestation of the rare cases of valproate-associated encephalopathy.1 Future longitudinal studies investigating factors that contribute to the magnitude of valproate-related morphometric changes in individuals would address this hypothesis. Examples of potential factors that could influence valproate-related atrophic brain changes are duration of valproate treatment, dose, and age (as a measure of brain maturation). The relationship between these factors and valproate-related structural brain changes may be clinically relevant.
The parietal thickness changes identified in our study are notable in light of EEG-fMRI studies that consistently identify modified parietal lobe function during spike and wave discharges in idiopathic generalized epilepsy.7–9 The focal epilepsy cases in group A are highly unlikely to have diffuse primary disease pathology confined to the parietal lobe. Therefore, a strength of this study is that the parietal lobe thinning we have identified is likely to be specifically related to valproate use rather than solely a result of epilepsy.
With regard to the white matter volumetric changes identified in our study, recent research10 has described a relationship between valproate use and alterations to human CNS myelination. Our finding of white matter volume reduction associated with valproate use supports this hypothesis. MRI-based methods for investigating white matter changes by using diffusion-weighted imaging may provide further information about the relationship between valproate use and myelination.
The clinical significance of our findings lies in whether the thickness and volume changes we observed reflect an unknown but focal mechanism of action localized to the parietal lobes, and this poses the question as to whether this change is detrimental to the patient. Detrimental effects include cognitive and behavioral changes and adverse quality-of-life measures associated with valproate use.11 The structural changes observed in our study may provide a mechanistic explanation for reported cognitive and associated changes. A particularly topical related example is impaired cognition associated with valproate exposure in utero.12 We note that the cognitive impairments associated with fetal valproate exposure do not appear to be reversible, in contrast to the findings from the secondary analysis presented in our study. However, given that the cortex develops in a stereotypical pattern, following a posterior-to-anterior gradient into adulthood,13 the relationship between brain maturation and the identified posterior valproate-related brain changes would be of particular interest in children and young adults.
Whether our observed effect is a direct result of valproate use or a secondary response to reduced seizures is unknown. Because our study was cross-sectional and sampling was not randomized, a causative link between valproate use and the observed neuroanatomic changes has not been fully established. A causative link between valproate use and brain volume reduction could be addressed with a prospective longitudinal study in which participants are imaged before, during, and after cessation of valproate, along with monitoring seizure frequency. Similarly, a quantitative MRI analysis of brain volume and cortical thickness associated with valproate use in nonepilepsy disorders would provide valuable information regarding our observed brain changes. Because we limited analyses to males only, there can be no confounding from sex effects on cortical thickness or brain volume measures. The main consequence of this restriction in study sample would be to limit the generalizability of the findings to females.
Pharmacologic studies of the mechanism of action of sodium valproate tend to focus on changes at a cellular level.14 Our method was applied noninvasively in vivo over the whole brain in human subjects and demonstrated a reproducible focal effect. This may be a valuable complementary technique to understand drug action at a network or systems level.
Quantitative MRI-based analyses of brain changes are widely used in epilepsy research. These studies rarely control for medication use. By including epilepsy patients who do not take valproate as well as healthy controls in this study, we have demonstrated that antiepileptic medication has an effect on cortical thickness and brain volumes. Researchers should be aware of this potential confounding factor in studies of diseases for which valproate is a treatment.
Valproate use in epilepsy is associated with parietal lobe thinning and white matter volume reduction. The surprisingly regional nature of the effect poses interesting challenges in terms of understanding drug efficacy and mechanisms of action.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the neurologists and additional staff of the Comprehensive Epilepsy Program, Austin Health Melbourne, including Professor Sam Berkovic, Professor Ingrid Scheffer, Dr. Mark Newton, Dr. John Archer, Dr. Saul Mullen, and Dr. Patrick Carney. Carly Fitzgerald assisted with database searches for study participants. Dr. Amy Brodtmann provided feedback on the manuscript.
GLOSSARY
- −VPA
subjects not taking valproate
- +VPA
subjects taking valproate
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Pardoe contributed to the design of the study, performed image analysis and statistical analysis of the MRI data, contributed to interpretation of the data, and drafted the original manuscript. Dr. Berg contributed to the design of the study, contributed to analysis and interpretation of the data, and contributed to drafting the manuscript. Dr. Berg is Principal Investigator for the Connecticut Study of Epilepsy. Dr. Jackson contributed to the design of the study, contributed to analysis and interpretation of the data, and contributed to drafting the manuscript.
STUDY FUNDING
Supported by the National Health and Medical Research Council, Australia (project grant 318900), NIH (NS-R37-31146; Principal Investigator, Dr. Berg), and Victorian Life Sciences Computational Initiative, Australia (VR0056), Victorian Government's Operational Infrastructure Support Program, Australia.
DISCLOSURE
Dr. Pardoe received support from grant NS-R37-NS31146. Dr. Berg receives support from grant NS-R37-NS31146. She has received travel funding and honoraria from Eisai, the British Pediatric Neurological Association, and the Epilepsy Research Center (Melbourne); travel funding from UCB, the American Epilepsy Society and the International League Against Epilepsy; awards from the American Epilepsy Society and British Pediatric Neurological Association; and consulting fees from Dow Agro Science. She is past Chair of the ILAE's Commission on Classification and Terminology and Steward for the National Institute of Neurological Disorders and Stroke Benchmarks in Epilepsy Research. Dr. Jackson serves on a scientific advisory board for Neurosciences Victoria; receives royalties from the publication of Magnetic Resonance in Medicine, 2nd ed. (Elsevier 2005); and receives/has received research support from the NHMRC and from grant NINDS-R37-NS31146. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Guerrini R, Belmonte A, Canapicchi R, Casalini C, Perucca E. Reversible pseudoatrophy of the brain and mental deterioration associated with valproate treatment. Epilepsia 1998;39:27–32 [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Mathern GW, Bronen RA, et al. Frequency, prognosis and surgical treatment of structural abnormalities seen with magnetic resonance imaging in childhood epilepsy. Brain 2009;132:2785–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia 1999;40:445–452 [DOI] [PubMed] [Google Scholar]
- 4.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleisher AS, Truran D, Mai JT, et al. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology 2011;77:1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisch C, Hüsch K, Angenstein F, et al. Dose-dependent memory effects and cerebral volume changes after in utero exposure to valproate in the rat. Epilepsia 2009;50:1432–1441 [DOI] [PubMed] [Google Scholar]
- 7.Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI "deactivation" of the posterior cingulate during generalized spike and wave. Neuroimage 2003;20:1915–1922 [DOI] [PubMed] [Google Scholar]
- 8.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 2005;102:15236–15240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamandi K, Salek-Haddadi A, Laufs H, et al. EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage 2006;31:1700–1710 [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig I, Vukadinovic Z, Turner AJ, Catani M. Neuroconnectivity and valproic acid: the myelin hypothesis. Neurosci Biobehav Rev 2012;36:1848–1856 [DOI] [PubMed] [Google Scholar]
- 11.Meador KJ, Loring DW, Hulihan JF, Kamin M, Karim R. Differential cognitive and behavioral effects of topiramate and valproate. Neurology 2003;60:1483–1488 [DOI] [PubMed] [Google Scholar]
- 12.Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004;101:8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 2002;16:669–694 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.