Abstract
Objective:
The underlying pathophysiology of tremor in Parkinson disease (PD) is unclear; however, it is known that tremor does not appear to be as responsive to dopaminergic medication as bradykinesia or rigidity. It is suggested that serotonergic dysfunction could have a role in tremor development.
Methods:
Using 11C-DASB PET, a marker of serotonin transporter binding, and clinical observations, we have investigated function of serotonergic terminals in 12 patients with tremor-predominant and 12 with akinetic-rigid PD. Findings were compared with those of 12 healthy controls.
Results:
Reductions of 11C-DASB in caudate, putamen, and raphe nuclei significantly correlated with tremor severity on posture and action, but not with resting tremor. The tremor-predominant group also showed reductions of 11C-DASB in other regions involved in motor circuitry, including the thalamus and Brodmann areas 4 and 10.
Conclusions:
Our findings support a role for serotonergic dysfunction in motor circuitries in the generation of postural tremor in PD.
Tremor is a cardinal feature of Parkinson disease (PD) affecting approximately 70% of patients and generally manifesting asymmetrically.1 Rest tremor has a characteristic frequency of 3 to 5 Hz whereas postural and action tremors are of higher frequency (4–8 Hz). Postmortem2 studies have shown loss of serotonergic (5-HT) projections in PD, although to a lesser extent than dopaminergic (DAergic) neurons. 11C-DASB (11C-labeled 3-amino-4-[2-[(di(methyl)amino)methyl]phenyl]sulfanylbenzonitrile) PET imaging, a marker of serotonin transporter (SERT) binding, has shown that 5-HT dysfunction occurs early in PD3 and that levels correlate with nonmotor symptoms such as depressive symptoms and alterations of body weight.4,5
Whereas l-dopa and dopamine agonists successfully relieve bradykinesia and rigidity, the response of parkinsonian tremor is variable.6 Moreover, 18F-dopa7 and 11C-raclopride8 PET have failed to associate tremor scores with either pre- or postsynaptic DAergic mechanisms. These observations suggest that PD tremor may, at least in part, be generated by non-DAergic mechanisms. Early clinical trials in humans have suggested that agents acting on the 5-HT system, such as HT2 agonists, can help attenuate tremor in PD.9,10 Identifying the underlying mechanisms of tremor will allow the development of new optimized therapies for the management of this poorly managed, troublesome symptom for patients with PD.
We hypothesized that PD tremor, unlike bradykinesia and rigidity, could be related to dysfunctions of the 5-HT system in areas related to motor function and we sought to investigate this in vivo using 11C-DASB PET, a specific marker of the 5-HT transporter that can be used as an index of presynaptic 5-HT terminal integrity,11,12 together with clinical observations.
METHODS
Clinical evaluation.
Twenty-four patients with idiopathic PD and 12 healthy controls were included in this study and provided written informed consent (see table for subject characteristics). Twelve of the patients had tremor-predominant PD and 12 had akinetic-rigid PD. Symptom predominance was defined according to subscale scores of Unified Parkinson’s Disease Rating Scale motor part III (UPDRS-III). All patients were assessed with the UPDRS-III Motor Subscale on the day of the PET scan in an “OFF” medication state. Part III of the UPDRS is designed to assess the severity of the cardinal motor findings in patients with PD (e.g., tremor, rigidity, bradykinesia, postural instability). It contains 27 questions (for 14 items) for different body regions, scored from 0 (normal) to 4 (maximal severity) with a maximum (worst) score of 108.
Table.
Characteristics of the patients with Parkinson disease and healthy controls
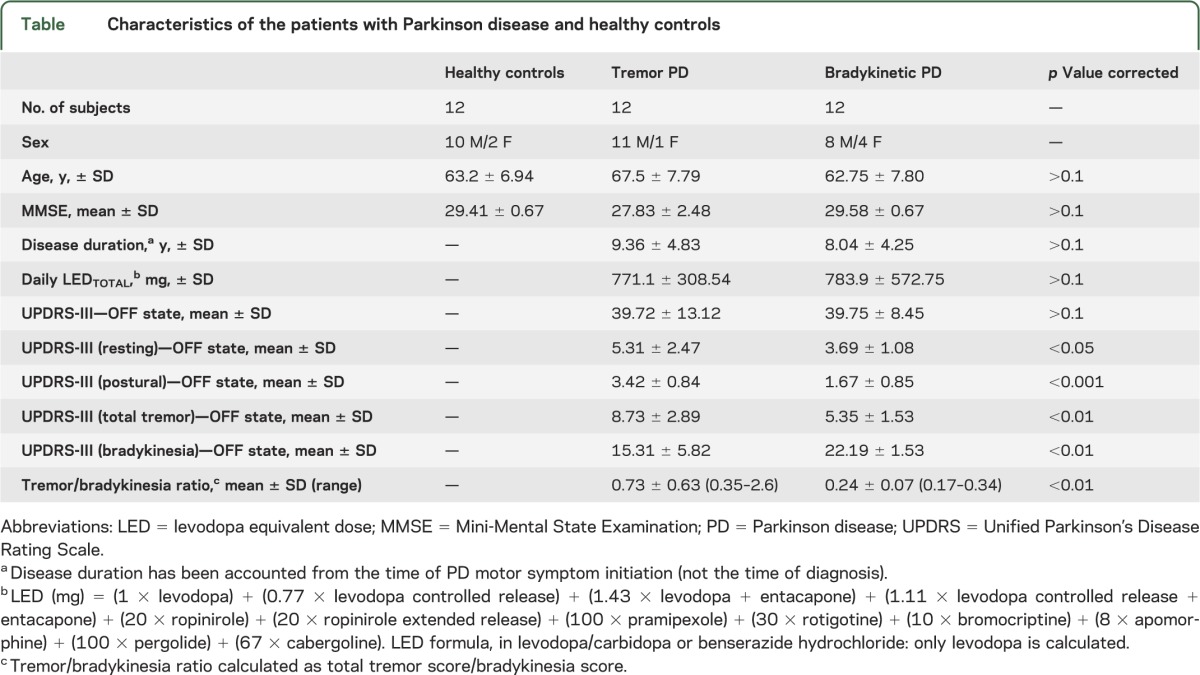
Tremor and bradykinesia symptoms were assessed separately combining the appropriate UPDRS subitem scores. The tremor score comprised the sum of the items “tremor at rest” for face-lip-chin, right and left hand, right and left foot, and “action or postural tremor” for right and left hand (maximum score 32). “Tremor at rest” and “action or postural tremor,” however, were also evaluated separately to take into account different types of parkinsonian tremor or possible coexistence of essential tremor (ET). The bradykinesia score was the sum of the subitems “facial expression,” “body bradykinesia,” “arising from chair,” right and left “finger taps,” right and left “hand pronation/supination,” and right and left “leg agility” (maximum score 36).
Depression scores for all patients were below the cutoff point for PD depression as assessed by the Beck Depression Inventory–II (scores <17) and the Hamilton Rating Scale for Depression (scores <14) and nondemented as assessed by the Mini-Mental State Examination.
Other clinical assessments included Hoehn and Yahr staging, calculation of the daily levodopa equivalent dose (LED).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the local ethics committee, and written informed consent was obtained from each participant.
Scanning procedures.
All PET scans were performed using an ECAT HR+ (CTI/Siemens 962) 3-dimensional (3D) PET tomography scanner, which covers a 15.5-cm axial field of view. The camera has a mean image transaxial resolution (3D mode) of 6.0 + 0.5 mm and an axial resolution of 5.0 + 0.8 mm at 10 cm distance from the center.13 The 11C-DASB scanning protocol involved a 3D acquisition over 90 minutes initiated 30 seconds after a mean bolus injection of 450 MBq of 11C-DASB. Twenty-eight time frames of tissue data were generated. Patients underwent a volumetric T1 MRI using a 1.5-tesla MRI (Picker Eclipse) for the purposes of image registration and to facilitate anatomical localization of the regions of interest (ROIs). Head position relative to the camera laser light was monitored throughout the scans and was repositioned if movement was detected. Patients were scanned in a room with low light and no noise and while in a resting state. All patients had their medication stopped at least 18 hours before scanning.
11C-DASB PET image analyses.
ROI analysis.
After reconstruction of the 11C-DASB image volumes, the mean 11C-DASB image volume was created from the entire dynamic data set using an inhouse software package. A standardized template of high-contrast ROIs were aligned and resliced to match the dimensions of the PET images, and 11C-DASB ROI time-activity curves (TACs) were extracted. Motion correction was applied using a frame-by-frame realignment procedure as previously described.14,15 Subject MRIs were transformed to a voxel size of 1 mm3, centered on the anterior commissure, and aligned to the anterior-posterior commissure line. MRIs were coregistered to the mean PET volume using the Mutual Information Registration algorithm and realign function in Statistical Parametric Mapping (SPM)2 (Wellcome Department of Cognitive Neuroscience, Institute of Neurology) implemented in MATLAB 6.5 (The MathWorks Inc., Natick, MA). Definition of ROIs was performed on the coregistered MRI aided by the Duvernoy 3D sectional atlas.14 ROIs were defined manually on both hemispheres for cerebellum, ventral and dorsal raphe nuclei, caudate nucleus, and putamen. To account for possible partial volume effects, ROI volumes were standardized across subjects. Sampling of ROIs produced new TACs that were checked against intrascan notes and for movement correction improvement. ANALYZE medical imaging software (version 8.1, Mayo Foundation) was used for processing the ROIs and TACs. 11C-DASB nondisplaceable binding potential (BPND) (distribution volume ratio of a receptor-containing region to a reference region minus 1)11,15 was estimated in each ROI using the Logan method.16 The cerebellum, excluding the vermis, was used as a reference region for nonspecific binding.17
SPM analysis.
The PET data for the tremor-predominant PD group were also analyzed by SPM in order to confirm the ROI analysis findings. SPM is able to delineate small regions more accurately than the voxel-by-voxel ROI method. SPM analysis was conducted using SPM99. The BP images were normalized into a standard stereotaxic Montreal Neurological Institute space with a normal 11C-DASB PET template using SPM2 software implemented in MATLAB 6.5. All BP images were then smoothed to 6 × 6 × 6 mm full width at half maximum to improve the signal-to-noise ratio. Smoothed and normalized BP images were then entered into a 1-sample t test. By specifying a contrast of “1,” decreases in 11C-DASB binding could be visualized on a canonical T1 MRI.
Meteorologic data.
Meteorologic data (London Office, UK) for the periods of the assessments were also acquired to account for confounding factors of weather and seasonal changes on 11C-DASB binding as previously described (data not shown).5
Statistical analyses.
Statistical analyses were performed using SPSS software package (version 16; SPSS Inc., Chicago, IL) for Windows. For all comparisons, SDs were checked for statistical differences and Gaussian distributions with Bartlett, Kolmogorov, and Smirnov methods for applying corrections (Welch). Using Pearson correlation statistics (r), we investigated whether total tremor and tremor subtype scores correlated with 11C-DASB binding (corrected for multiple comparisons) in the patients with PD. Analysis of variance was used to test for 11C-DASB differences between the PD subgroups and normal controls, applying the Tukey-Kramer posttest correction.
RESULTS
Clinical findings.
PD patient groups were comparable for age, disease duration, medication intake, and UPDRS-III score in the practically defined OFF medication state (table). The tremor-predominant PD group had significantly higher resting and action-postural tremor scores. The akinetic-rigid PD group had significantly higher bradykinesia scores and tremor/bradykinesia ratios (table).
ROI analysis.
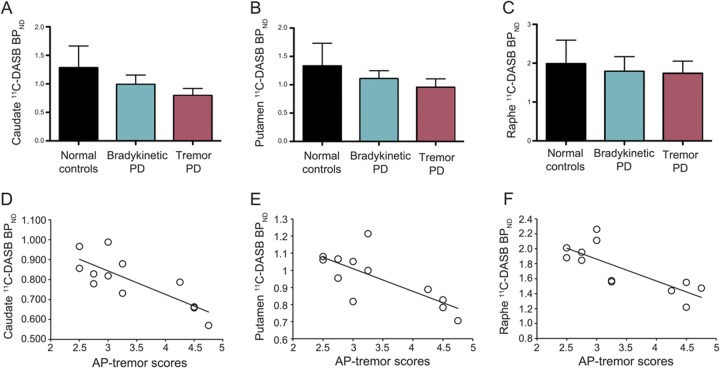
The tremor-predominant PD group showed significantly lower 11C-DASB BPND values compared with the akinetic-rigid PD group and normal controls in caudate (average = 0.794 vs 0.988, p < 0.001, F = 11.46) and putamen (average = 0.954 vs 1.107, p < 0.005, F = 6.39). The tremor-predominant group showed a trend for lower raphe nuclei 11C-DASB BPND values compared with the akinetic-rigid PD group and normal controls although statistical significance was not reached. Reduced levels of 11C-DASB BPND in caudate, putamen, and raphe nuclei in the group of patients with tremulous PD significantly correlated with higher action-postural tremor scores (figure 1) but not with resting or total tremor scores. No correlations were found between 11C-DASB BPND and tremor scores in the akinetic-rigid PD group or between 11C-DASB BPND and bradykinesia or rigidity scores in either PD group (all p > 0.1).
Figure 1. 11C-DASB binding in normal controls and patients with tremor-predominant PD and akinetic-rigid PD and correlation of 11C-DASB binding and action-postural tremor in the tremor-predominant PD group.
11C-DASB BPND values of 12 healthy controls, 12 patients with bradykinetic PD, and 12 patients with tremor-predominant PD in (A) caudate, (B) putamen, and (C) raphe nuclei. Significant correlations between increased AP tremor scores and reduced 11C-DASB BPND values in (D) caudate (r = −0.80, p < 0.001), (E) putamen (r = −0.74, p < 0.005), and (F) raphe nuclei (r = −0.78, p < 0.005) in 12 patients with tremor-predominant PD. *p < 0.005. AP = action-postural; BPND = nondisplaceable binding potential; 11C-DASB = 11C-labeled 3-amino-4-[2-[(di(methyl)amino)methyl]phenyl]sulfanylbenzonitrile; PD = Parkinson disease.
Voxel-based SPM.
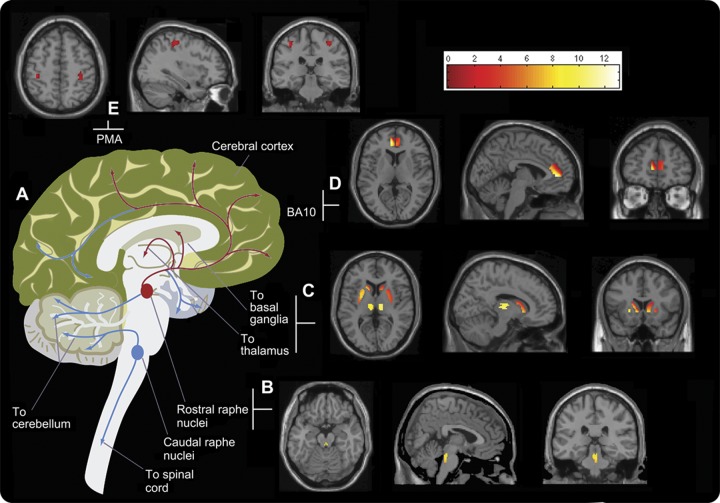
Following our a priori hypotheses, several regions were drawn in ANALYZE medical imaging software (version 8.1, Mayo Foundation) to mask the SPM analysis. The voxel-based analysis confirmed findings from the ROI analysis showing significant decreases in 11C-DASB BPND values in raphe nuclei, caudate, and putamen (p < 0.05). It revealed additional 11C-DASB BPND reductions in the thalamus, and Brodmann areas 4 and 10 (p < 0.05) in the tremor-predominant PD group compared with the akinetic-rigid group (figure 2).
Figure 2. Motor circuitries in the human brain.
(A) Diagrammatic representation of the major motor circuitries in the human brain. The regions and interregional circuitry identified in this study to be associated with action-postural tremor in PD are highlighted in red. Summed 11C-DASB PET images in transverse, sagittal, and coronal planes showing regions of 5-HT loss within the motor pathway: (B) rostral raphe nuclei; (C) caudate, putamen, and thalamus; (D) Brodmann area 10; and (E) Brodmann area 4, the primary motor area (PMA). Color bar reflects z scores (BPND range: 0–12). BPND = nondisplaceable binding potential; 11C-DASB = 11C-labeled 3-amino-4-[2-[(di(methyl)amino)methyl]phenyl]sulfanylbenzonitrile;5-HT = serotonin; PD = Parkinson disease.
A post hoc analysis was performed to evaluate possible effects of clinical parameters on 11C-DASB binding. We found no significant correlations (all p > 0.1) between total tremor or subtype tremor scores with disease duration, age, sex, or DAergic medication intake (daily LEDTOTAL).
DISCUSSION
Using 11C-DASB PET, we have demonstrated in vivo relative reductions in 5-HT terminal function in the caudate, putamen, raphe nuclei, thalamus, and Brodmann areas 4 and 10 in patients with tremor-predominant PD compared with those who had akinetic-rigid PD and normal controls. Furthermore, the loss of 5-HT terminal transporter binding in caudate, putamen, and raphe nuclei in patients with tremor-predominant PD correlated with the severity of postural tremor, highlighting the possible role of presynaptic 5-HT terminal dysfunction in the motor-related regions in generating postural and action tremors in PD. We did not detect correlations between 11C-DASB binding in any brain regions with resting or total tremor scores indicating that postural and resting tremors could have different underlying pathophysiologies.
Degeneration of the DAergic system is considered the pathologic hallmark of PD and tremor is one of the cardinal symptoms of PD. To date, both animal models2 and in vivo studies utilizing 18F-dopa PET9 and 11C-raclopride10 have demonstrated that levels of reduced striatal DA function are not clinically correlated with tremor but rather with bradykinesia and rigidity.
It is known that the 5-HT system is affected early in PD, although this appears to occur at a slower rate and in a nonlinear fashion compared with the DAergic system.3 Only one previous PET investigation has specifically examined the relationship between tremor and the 5-HT system in PD.18 Using 11C-WAY100635 PET, a reduction of midbrain 5-HT1A receptors was found in PD that correlated with the severity of resting tremor. However, 5-HT1A receptors are present both pre- and postsynaptically and in this study,18 only healthy individuals served as the control group, making it difficult to ascertain the exact mechanism. Our findings show that 5-HT dysfunction in the raphe nuclei, which is the source of 5-HT transmission, is implicated in action-postural tremor and the 5-HT dysfunctions are extended to a motor circuitry including the striatum, thalamus, and the primary motor cortex (figure 2) in a group of patients with well-defined tremor-predominant PD. Moreover, motor circuitry 5-HT dysfunctions correlate with severity of tremor on posture and action. It is possible that resting tremor possesses a serotonergic substrate related more specifically to postsynaptic mechanisms, as evidenced by Doder et al.18 Our study utilized 11C-DASB, a marker of presynaptic SERT only; thus, we were unable to detect any influence of the postsynaptic mechanisms involved in resting tremor in our patients.
The striatum, a region strongly associated with PD pathology, is densely innervated with 5-HT projections from the raphe nuclei19 and also forms part of the ganglio-thalamocortical network. This network, which is known to function as part of the motor pathway, also includes the thalamus and the region functionally related to the premotor cortex: Brodmann area 4. The main function of raphe nuclei is to release 5-HT, therefore the loss of raphe neurons, i.e., due to Lewy body pathology, is likely to result in the subsequent disruption of its projections. In this study, we did not detect statistically significant differences in 11C-DASB values within the raphe nuclei among the 3 groups, although a reduction of SERT functioning was detected in the tremor-predominant group. As previously reported,3 reduction of SERT functioning occurs in a nonlinear fashion, with raphe nuclei affected in advanced PD, compared with dorsal striatal and cortical areas, which demonstrate reduced functioning in early and established PD. It is likely that raphe nuclei, as the most densely innervated area of SERT, possesses increased compensatory mechanisms and that the raphe nuclei projections are affected before the reduction of SERT functioning in the raphe nuclei itself. It is this disruption that would ultimately cause a profound denervation in remote areas of the brain such as the striatum and motor cortex, and these changes may have relevance to the development of action-postural tremor.20–22 In this study, we detected clinical correlations of action-postural tremor scores with loss of 5-HT in the raphe nuclei, caudate, and putamen, but the thalamus and Brodmann area 4 denervation was only detected following the more stringent SPM analysis, providing support that degeneration of the 5-HT system starts in the raphe nuclei, extending to remote areas as the disease progresses.
Remote 5-HT dysfunction in tremor has been indicated in a recent dopamine transporter (DAT) with FP-CIT (fluoropropyl-carbomethoxy-iodophenyl-tropane) SPECT study of patients with PD and ET, which found that DAT binding levels were similar in both groups but reported a correlation between tremor and DAT binding in the caudate of patients with ET and not the patients with PD.23 This could implicate caudate involvement in postural tremor in ET but that in PD, the DAergic system is not implicated. Also, lesions in the caudate after cerebral infarction have produced tremor in these patients.24,25 In one report, l-dopa was administered, but the patient's unilateral tremor, which had rest, postural, and action components, was unresponsive, thus indicating that the tremor was not influenced by l-dopa–accessible DAergic mechanisms.26
There are early reports of beneficial effects on PD tremor after administration of agents acting on the 5-HT system. For example, global tremor scores were found to improve in 40 patients over 8 weeks after administration of tryptophan, a biochemical precursor of 5-HT, in addition to the l-dopa therapy.9 Additionally, the 5-HT2 receptor agonist, ritanserin, has also produced an improvement of resting tremor in patients with PD.10
Herein, we have demonstrated that there is a generalized 5-HT dysfunction in motor-related regions in patients with tremor-predominant PD, and that 5-HT transporter functional loss in caudate, putamen, and raphe nuclei is clinically related to the development of action-postural tremor in these patients. Therefore, it is conceivable to consider the use of non-DA medications for the treatment of tremor in PD in cases whereby DAergic medication fails to have an effect.
ACKNOWLEDGMENT
The authors thank all of the patients and their families who took part in this study.
GLOSSARY
- BPND
nondisplaceable binding potential
- 11C-DASB
11C-labeled 3-amino-4-[2-[(di(methyl)amino)methyl]phenyl]sulfanylbenzonitrile
- DAergic
dopaminergic
- DAT
dopamine transporter
- ET
essential tremor
- 5-HT
serotonin
- LED
levodopa equivalent dose
- PD
Parkinson disease
- ROI
region of interest
- SERT
serotonin transporter
- SPM
Statistical Parametric Mapping
- TAC
time-activity curve
- 3D
3-dimensional
- UPDRS
Unified Parkinson’s Disease Rating Scale
AUTHOR CONTRIBUTIONS
M.P. conceptualized the experimental design. M.P. and C.L. organized the study. M.P. and K.W. acquired the data. P.B., D.J.B., and P.P. were responsible for the clinical supervision of the patients. C.L. analyzed the data, conducted the statistical analysis, wrote the first draft of the manuscript, and interpreted the findings. All authors gave input and revised the manuscript.
STUDY FUNDING
This work was supported by grants from the Michael J. Fox Foundation for Parkinson's Research, United States of America (P14104) and from the Medical Research Council, United Kingdom (Clinical Sciences Center, Neurology Group core grant, 2007–2010).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rajput A. Clinical features of tremor in extrapyramidal syndromes. In: Findley LJ, Koller WC, editors. Handbook of Tremor Disorders. New York: Marcel Dekker; 1995:275–291 [Google Scholar]
- 2.Kish SJ, Tong J, Hornykiewicz O, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson's disease. Brain 2008;131;120–131 [DOI] [PubMed] [Google Scholar]
- 3.Politis M, Wu K, Loane C, et al. Staging of serotonergic dysfunction in Parkinson's disease: an in vivo 11C-DASB PET study. Neurobiol Dis 2010;40:216–221 [DOI] [PubMed] [Google Scholar]
- 4.Politis M, Loane C, Wu K, Brooks DJ, Piccini P. Serotonergic mediated body mass index changes in Parkinson's disease. Neurobiol Dis 2010;43:609–615 [DOI] [PubMed] [Google Scholar]
- 5.Politis M, Wu K, Loane C, et al. Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurology 2010;75:1920–1927 [DOI] [PubMed] [Google Scholar]
- 6.Koller WC, Hubble JP. Levodopa therapy in Parkinson's disease. Neurology 1990;40:40–47 [PubMed] [Google Scholar]
- 7.Brooks DJ, Playford ED, Ibanez V, et al. Isolated tremor and disruption of the nigrostriatal dopaminergic system: an 18F-dopa PET study. Neurology 1992;42:1554–1560 [DOI] [PubMed] [Google Scholar]
- 8.Pavese N, Evans AH, Tai YF, et al. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology 2006;67:1612–1617 [DOI] [PubMed] [Google Scholar]
- 9.Coppen A, Metcalfe M, Carroll JD, Morris JG. Levodopa and L-tryptophan therapy in Parkinsonism. Lancet 1972;1:654–658 [DOI] [PubMed] [Google Scholar]
- 10.Hildebrand J, Delecluse F. Effect of ritanserin, a selective serotonin-S2 antagonist, on Parkinson’s rest tremor. Curr Ther Res 1987;41:298–300 [Google Scholar]
- 11.Ginovart N, Willeit M, Rusjan P, et al. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 2007;27:857–871 [DOI] [PubMed] [Google Scholar]
- 12.Ichise M, Liow JS, Lu JQ, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 2003;23:1096–1112 [DOI] [PubMed] [Google Scholar]
- 13.Brix G, Zaers J, Adam LE, et al. Performance evaluation of a whole-body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J Nucl Med 1997;38:1614–1623 [PubMed] [Google Scholar]
- 14.Montgomery AJ, Thielemans K, Mehta MA, Turkheimer F, Mustafovic S, Grasby PM. Correction of head movement on PET studies: comparison of methods. J Nucl Med 2006;47:1936–1944 [PubMed] [Google Scholar]
- 15.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–1539 [DOI] [PubMed] [Google Scholar]
- 16.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834–840 [DOI] [PubMed] [Google Scholar]
- 17.Kish SJ, Furukawa Y, Chang LJ, et al. Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region? Nucl Med Biol 2005;32:123–128 [DOI] [PubMed] [Google Scholar]
- 18.Doder M, Rabiner EA, Turjanski N, Lees AJ, Brooks DJ. Tremor in Parkinson's disease and serotonergic dysfunction: an 11C-WAY 100635 PET study. Neurology 2003;60:601–605 [DOI] [PubMed] [Google Scholar]
- 19.van der Kooy D, Hattori T. Dorsal raphe cells with collateral projections to the caudate-putamen and substantia nigra: a fluorescent retrograde double labeling study in the rat. Brain Res 1980;186:1–7 [DOI] [PubMed] [Google Scholar]
- 20.Jellinger K. Overview of morphological changes in Parkinson's disease. Adv Neurol 1987;45:1–18 [PubMed] [Google Scholar]
- 21.Halliday GM, Blumbergs PC, Cotton RGH, Blessing WW, Geffen LB. Loss of brain stem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res 1990;510:104–107 [DOI] [PubMed] [Google Scholar]
- 22.Lalley PM. Analysis of Pharmacologically-activated Tremor Mechanisms in the Caudate Nucleus. Ann Arbor, MI: Univsity Microfilms; 1971 [Google Scholar]
- 23.Isaias IU, Marotta G, Hirano S, et al. Imaging essential tremor. Mov Disord 2010;6:679–686 [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ. Delayed onset hand tremor caused by cerebral infarction. Stroke 1992;23:292–294 [DOI] [PubMed] [Google Scholar]
- 25.Grimes JD, Hassan MN, Quarrington AM, et al. Delayed onset posthemiplegic dystonia: CT demonstration of basal ganglia pathology. Neurology 1982;32:1033–1035 [DOI] [PubMed] [Google Scholar]
- 26.Dethy S, Luxen A, Bidaut LM, et al. Hemibody tremor related to stroke. Stroke 1993;24:2064–2096 [DOI] [PubMed] [Google Scholar]