Abstract
Ectotherms are considered to be particularly vulnerable to climate warming. Descriptions of habitat temperatures and predicted changes in climate usually consider mean monthly, seasonal or annual conditions. Ectotherms, however, do not simply experience mean conditions, but are exposed to daily fluctuations in habitat temperatures. Here, we highlight how temperature fluctuation can generate ‘realized’ thermal reaction (fitness) norms that differ from the ‘fundamental’ norms derived under standard constant temperatures. Using a mosquito as a model organism, we find that temperature fluctuation reduces rate processes such as development under warm conditions, increases processes under cool conditions, and reduces both the optimum and the critical maximum temperature. Generalizing these effects for a range of terrestrial insects reveals that prevailing daily fluctuations in temperature should alter the sensitivity of species to climate warming by reducing ‘thermal safety margins’. Such effects of daily temperature dynamics have generally been ignored in the climate change literature.
Keywords: Anopheles stephensi, climate change, conservation, diurnal temperature fluctuation, ectotherm fitness, Jensen's inequality, thermal fitness curve, thermal reaction norm
Introduction
The relationship between ectotherm life-history traits and temperature is typically characterized by a nonlinear asymmetric curve, defining the optimum temperature (To) and the operative temperature range between the critical minimum temperature (CTmin) and the critical maximum temperature (CTmax) (Fig. 1). These curves are often used to assess the sensitivity of ectotherm species to climate warming, sometimes considering individual life-history traits (Amarasekare & Savage, 2012), or composite fitness metrics such as the intrinsic rate of increase (Deutsch et al., 2008; Tewksbury et al., 2008). Approaches include simple use of curves to track expected changes in overall fitness (Deutsch et al., 2008; Tewksbury et al., 2008), in CTmin or CTmax (Piyaphongkul et al., 2012; Ribeiro et al., 2012) or in thermal performance breadth (Angert et al., 2011) as a result of changing thermal environments. In addition, studies use measures such as the Thermal Safety Margin (TSM), which defines the amount of warming possible before habitat temperatures (Th) reach and ultimately exceed the thermal optimum (To) (Deutsch et al., 2008; Huey et al., 2009; Hoffmann, 2010; Hofmann & Todgham, 2010; Clusella-Trullas et al., 2011; Bonebrake & Deutsch, 2012; Krenek et al., 2012).
Figure 1.
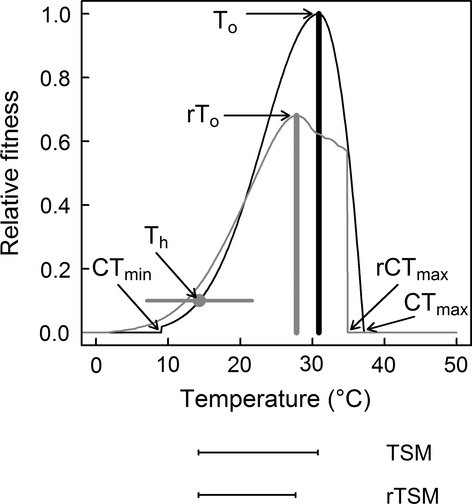
Effect of daily temperature variation on ectotherm fitness. Fundamental performance curve (relative fitness; black line) under constant temperature conditions and realized performance when appropriate daily temperature variation is considered (grey line) for the temperate terrestrial insect Muscidifurax zaraptor. Gray data point and horizontal grey line represent the mean habitat temperature (Th) and the average habitat temperature range, respectively. CTmin, critical minimum temperature; (r)To, (realized) optimum temperature; (r)CTmax, (realized) critical maximum temperature; and (r)TSM, (realized) thermal safety margin.
Thermal reaction norms and fitness curves tend to be derived from constant temperature experiments conducted under controlled laboratory conditions (e.g., see species listed in Deutsch et al., 2008). However, temperature is highly dynamic (Geerts, 2003; Paaijmans et al., 2010) and numerous studies provide evidence of insects and other ectotherms ‘integrating’ the effects of temperature during the daily cycle (Liu et al., 1995; Kingsolver et al., 2009; Paaijmans et al., 2010; Bozinovic et al., 2011; Duncan et al., 2011; Estay et al., 2011; Folguera et al., 2011). Accordingly, short-term environmental variance has the potential to affect life-history traits and fitness above and beyond the effects of mean temperatures alone (Ruel & Ayres, 1999; Martin & Huey, 2008; Folguera et al., 2009, 2011; Terblanche et al., 2010; Bozinovic et al., 2011; Clusella-Trullas et al., 2011). As climate change will not only alter mean temperatures, but also the daily temperature ranges (Easterling et al., 1997, 2000), understanding these effects is necessary to define the ‘realized’ thermal reaction norms (i.e., the actual fitness curves observed under variable conditions in nature) for different species and to quantify vulnerability to climate warming.
Here, using the mosquito Anopheles stephensi as a model organism, we ask whether the ‘fundamental’ thermal reaction norms established under constant temperature conditions differ from those derived under more natural fluctuating temperatures. We illustrate how daily temperature fluctuations can lower both the optimum and critical maximum temperatures of thermal reaction norms. We then generalize these effects for a range of terrestrial insects using a rate summation modeling approach. This analysis reveals that daily temperature fluctuations will tend to reshape the fundamental fitness curve, reducing the temperature optimum and hence, the TSM. Together, the empirical and theoretical data demonstrate that predicting the impacts of climate on ectotherm fitness requires a better understanding of the effects of short-term temperature dynamics.
Materials and methods
Empirical studies exploring the effects of temperature variation on life-history traits
Experiments were carried out in incubators (Percival Scientific Inc., Perry, IA, USA and Conviron, Canada, accuracy: ±0.5 °C), at 90 ± 10% relative humidity and a 12L : 12D photoperiod (L: 12:00 hours to 0:00 hours). Temperature was monitored closely with temperature loggers (OM-62; Omega, Stamford, CT, USA) at 15 min intervals. To exclude the potential effect of incubator, incubator programs were changed between experiments (e.g., an incubator running at a low but constant temperature was then programmed to fluctuate around a high mean temperature, etc.).
Immature An. stephensi (first instar larvae; <24 h old) were reared in plastic cups with 3 cm of distilled water (diameter 7 cm; 115 mL of water) and 50 larvae per cup (or 2.3 larvae cm−2). A small water volume was chosen to ensure water temperature tracked the temperature in the incubators. Live larvae and pupae were counted and cups cleaned daily. Immatures were placed back in clean water that was stored overnight at the respective temperature, to avoid temperature variation due to daily changing of the water. Larvae were fed 0.3 mg of tropical fish food (Tetrafin®) per larva per day. Emerged adult mosquitoes were counted (at the end of the night cycle) and removed. Daily development rate (reciprocal of time until adult emergence) and survival to adult were measured during different temperature experiments, with six replicate cups per temperature treatment.
Utilizing these general methods we conducted a suite of experiments to explore effects of mean temperature and daily temperature variation on specific aspects of thermal reaction norms.
The fundamental thermal reaction norm
Larvae were reared at constant temperatures, ranging from 16 to 36 °C, with 2 °C increments, to obtain the fundamental thermal reaction norms (and a measure of the optimum, To, and the critical maximum temperature, CTmax) for development and survival. This temperature range was selected on the basis of pilot data and those published for An. gambiae (Bayoh & Lindsay, 2003). Note that critical minimum (CTmin) and maximum (CTmax) temperatures are commonly derived from experiments whereby temperature is slowly decreased or increased, and some kind of short-term physiological or behavioral response is recorded (e.g., ability of an insect to right itself). In our experiments, we define CTmin and CTmax more ecologically as those temperatures at which mosquito survival through to adulthood is zero (which could still mean larvae surviving for many days but failing to emerge from pupae). This measure is not equivalent to the traditional upper or low lethal temperatures, which again tend to be defined based on mortality following single short-term exposures to temperature extremes (Piyaphongkul et al., 2012; Ribeiro et al., 2012).
Effects of fluctuation at different points on the reaction norm
Based on the fundamental reaction norms, we examined the effects of diurnal temperature variation on larval development and survival at 18 °C (near CTmin), 32 °C (near To for development), and 26 °C (an intermediate temperature). We evaluated constant temperatures and diurnal temperature ranges (DTRs) of 8 °C (i.e., ±4) and 12 °C (i.e., ±6) around the same means, with the daily temperature profiles described by the Parton & Logan temperature model (see model details below). These temperature ranges are commonly experienced by anopheline mosquitoes (Paaijmans et al., 2008).
Effects of temperature variation on the temperature optimum
Here, we investigated the effects of a DTR of 12 °C around subtly different means of 28, 30, and 32 °C to examine more precisely whether daily fluctuations in temperature affected the optimum temperature for development and survival.
For this set of experiments, we ran a full factorial generalized linear model (GZLM) analysis to assess how mean temperature, diurnal temperature fluctuation, and replicate influence larval development time (days) and the number of emerging adult mosquitoes. We assumed a linear distribution (identity link function) and a Poisson distribution (log link function) for the GZLMs with development time and number of emerging adult mosquitoes as response variables, respectively. We assessed goodness of fit of the final models through model deviance/d.f. scores and model residuals. Reduced models were achieved by eliminating the highest order, nonsignificant interactions through backward elimination. All post hoc tests were Bonferroni corrected, and analyses were run in spss 20.0 (IBM Corporation, Armonk, NY, USA). In addition, for the temperature optima experiment, development times were transformed to meet the assumption of normality.
Effects of temperature variation on the critical maximum temperature
To investigate the effects of temperature variation on CTmax, we measured survival at a range of high mean temperatures with and without different DTRs. Specifically we tested 33 and 35 °C with a DTR of 8, 31 and 33 °C with a DTR of 12, and 29.5 and 31.5 °C with a DTR of 16 °C. In addition, we ran a further assay to examine the impact of smaller fluctuations (DTRs of 3, 5, and 7 °C) around 35 °C only. Our aim in these experiments was to determine the temperature combinations at which mosquito survival through to adulthood was zero.
Theoretical analysis of thermal safety margins under current and future climate conditions
We now extend our approach to explore the effects of temperature fluctuation on the TSM of 29 terrestrial insect species. These species represent a subset of those presented in an earlier study by Deutsch et al. (2008) and cover diverse taxa from a range of temperate and tropical habitats. We used rate summation (Liu et al., 1995) to accumulate fitness rates at hourly intervals using the ‘fundamental’ fitness curves for each species and the relevant diurnal temperature cycles for the local environment. This approach generates a ‘realized’ thermal fitness curve based on the average daily temperature variation experienced by the insect in nature. We then compare the TSMs based on the fundamental thermal fitness curve with TSMs based on the realized curves.
The asymmetric thermal curves for relative insect fitness (the intrinsic rate of increase scaled to 1 as a maximum) were calculated using a Gaussian quadratic function described by Deutsch et al. (2008). In this earlier study, the authors calculated the critical maximum (CTmax) and optimum temperature (To) for 38 terrestrial insect species using available empirical data on intrinsic growth rate at several temperatures. However, rather than calculating the critical temperature (CTmin) from the quadratic function, the authors provided an operational definition of CTmin. While this was appropriate for their study, for our rate summation approach we needed the full thermal fitness curves so we refitted Gaussian quadratic models to the empirical data from the original source literature. When the original fitness data were presented as a figure only, the figure was digitized using Engauge Digitizer to obtain the values. To increase the robustness of our model, we used two additional criteria: (1) there should be at least three fitness data points below To and (2) the relative fitness value of one of these data points had to be lower than 0.5. Based on these criteria, nine of the original 38 species were omitted from our analysis of TSM (species 2, 7, 8, 10, 15, 16, 17, 21, and 36).
The appropriate variation in habitat temperature for each species was estimated using the mean monthly minimum and maximum temperatures obtained from WorldClim, version 1.4 (release 3; http://www.worldclim.org, Hijmans et al., 2005). Mean monthly minimum and maximum temperature surfaces were generated from the period 1960–1990 (referred to as contemporary temperatures) using weather station records containing at least 10 years of data in this period. These surfaces were imported into ESRItm ArcGIS ArcView 9.3 and used to obtain the climatic data for the geographical locations of the 29 insect species. Daily minimum and maximum temperature data for 2080 were obtained for UKMO_HadCM3 (Hadley Centre for Climate Prediction and Research, Met Office, UK) using SRES – A2A from http://www.ccafs-climate.org/data/, version IPCC 4, at a spatial resolution of 2.5 min. This climate model and scenario is one of the several possible combinations but has been used in a number of recent ecological studies examining possible effects of climate change (Heikkinen et al., 2010; Lassalle et al., 2010; Milanovich et al., 2010; Jaramillo et al., 2011). Climate data were again imported in ArcView 9.3 and temperature information extracted for the locations of the 29 insect species, as above.
We considered only months in which individual species were expected to be active and where physiological processes such as growth and reproduction are likely to occur, i.e., mean habitat temperature >CTmin, the critical minimum temperature for fitness, and months where the minimum temperature is greater than 0 °C, which sometimes excluded the winter months. Applying some form of seasonal constraint is consistent with other studies (e.g., Clusella-Trullas et al., 2011), but differs from the original approach of Deutsch et al. (2008), who considered year-round mean temperatures. How habitat temperature is characterized will influence the value of the TSM. However, our focus here is not on the absolute values of TSM but the relative change that results from the use of fluctuating temperatures compared with mean temperatures.
We used a standard method for generating realistic variations in the daily air temperature, whereby the phase and form of the diurnal rhythm of air temperature are given by a sinusoidal progression during daytime and a decreasing exponential curve during the night (Parton & Logan, 1981). The air temperature was modeled at 1 h intervals using the contemporary and the forecast minimum and maximum temperatures, assuming a day length of 12 h.
Combining the fundamental fitness curves for each species and the relevant diurnal temperature cycles for the local environment, fitness was calculated using either the mean habitat temperature, or using rate summation to accumulate fitness at hourly intervals. The rate summation approach generates a realized thermal fitness curve with a realized optimum temperature (rTo), based on the average daily temperature variation experienced by the insect in nature. We compared TSMs based on the fundamental fitness curves (To–Th) with those based on the realized fitness curves (rTo–Th).
All model calculations and figures were produced in the statistical package R (R Development Core Team, 2012).
Results
Empirical studies
The ‘fundamental’ thermal reaction norms established under constant temperature conditions showed the operative range for mosquito larval development and survival to extend from around 15 °C (estimated CTmin) to 36 °C (CTmax), with an optimum temperature (for development) of around 32 °C (Fig. 2a and b). The addition of realistic daily temperature variation changed the shape of these fundamental reaction norms. Temperature variations around a cool mean temperature (18 °C) significantly increased development rate (DTR 8 °C, P < 0.0001; DTR 12 °C, P < 0.0001) and survival (DTR 8 °C, P < 0.0001; DTR 12 °C, P < 0.0001), compared with the constant baseline temperature (Fig. 2c and d). In contrast, temperature variation around a warm mean temperature (32 °C) significantly reduced development rate (DTR 8 °C, P < 0.0001; DTR 12 °C, P < 0.0001) and survival probability (DTR 8 °C, P < 0.0001; DTR 12 °C, P < 0.0001). The effects of the larger DTR were significantly greater than the smaller DTR (P < 0.0001) for development and survival at fluctuation around 18 °C and for survival at fluctuation around 32 °C. At the intermediate temperature (26 °C), fluctuation had no significant effects relative to the baseline constant temperature.
Figure 2.
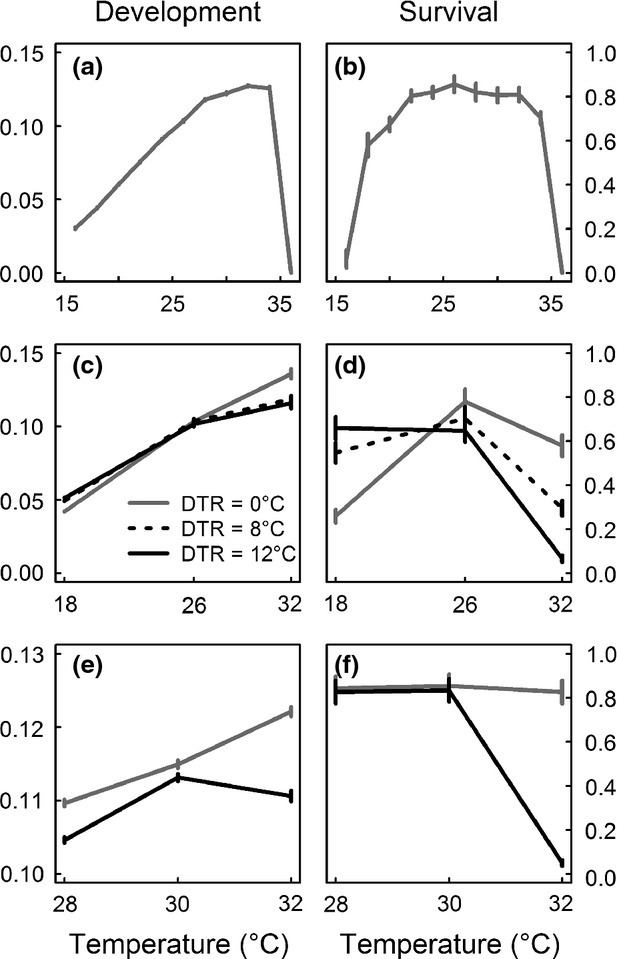
Impact of constant and variable temperatures on insect life-history traits. (a, b) Fundamental thermal reaction norm for (a) daily development rate and (b) survival of Anopheles stephensi mosquito immatures measured at 11 constant temperatures. (c, d) Effects of fluctuation at different points on the fundamental curve: Estimated marginal mean (c) development rate and (d) survival at mean temperatures of 18, 26, or 32 °C, combined with daily temperature ranges (DTRs) of 0, 8, or 12 °C. (e, f) Effects of temperature variation on the temperature optimum: Estimated marginal mean (e) development rate and (f) survival at mean temperatures of 28, 30 or 32 °C, combined with DTRs of 0 or 12 °C. The vertical error bars in all panels represent the standard error of the mean.
Daily temperature variation also affected the estimate of the temperature optimum. In the absence of any variation, larval development rate increased as mean temperatures shifted from 28 to 30 and then to 32 °C (P < 0.0001), although there was no effect on survival (Fig. 2e and f). With no evidence of a turnover, these data suggest a temperature optimum ≥32 °C. With the addition of daily temperature variation, however, both development rate and survival were significantly reduced at 32 °C compared with 30 °C (P = 0.014 and P < 0.0001 for development and survival, respectively). This means that the temperature optimum in a real-world fluctuating environment (what we call the ‘realized’ optimum temperature, rTo) will be lower than the ‘fundamental’ optimum temperature (To) estimated under constant conditions.
Similar patterns occurred with the critical maximum temperature, CTmax (Fig. 3a–c). At a constant temperature of 35 °C, larvae survive through to adulthood (Fig. 3a and d), indicating a CTmax clearly >35 °C. With the addition of a DTR of 8 °C, however, survivorship was reduced to zero indicating a ‘realized’ critical maximum temperature (rCTmax) <35 °C. With increasing DTRs of 12 and 16 °C, the rCTmax values were reduced further to <33 and <31.5 °C, respectively. Varying DTR around a single mean temperature of 35 °C yielded similar results. With a DTR of 7 °C, no mosquitoes survived to adult eclosion. As the extent of the DTR reduced from 5 to 3 to 0 °C, survivorship gradually increased indicating a shift in rCTmax (Fig. 3d).
Figure 3.
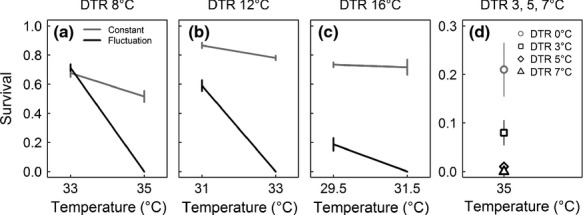
Impact of variable temperatures on the critical maximum temperature. Survival of Anopheles stephensi mosquito immatures at various mean temperatures (x-axis) and daily temperature ranges (DTRs) of (a) 8 °C, (b) 12 °C, or (c) 16 °C. Note that rCTmax is lower than the fundamental CTmax of 36 °C in a constant environment (Fig. 2b). (d) Effect of smaller DTRs around a mean temperature of 35 °C. Vertical error bars represent the standard error of the mean.
Theoretical studies
We examined the consequences of daily temperature variation for 29 terrestrial insect species using rate summation (i.e., summing fitness at hourly intervals as temperature fluctuates across the standard fitness curve in line with the average DTR experienced by the insect). In Fig. 1, we present an illustrative example for one temperate species (note fitness is scaled to 1 to enable comparisons of relative fitness). The rate summation approach yields qualitatively similar patterns to our empirical studies, whereby fluctuation at low mean temperatures increases fitness, while fluctuation around high mean temperatures reduces fitness. Fluctuation also reduces the temperature optimum (rTo < To) (together with the maximum attainable fitness at this optimum), as well as the critical maximum temperature (rCTmax < CTmax). Commensurate with the lower temperature optimum, the TSM is reduced in the fluctuating environment from 16.6 to 13.5 °C.
Extending this approach to the full set of species results in reductions in TSMs ranging from 0.8 to 4.5 °C (Fig. 4a). The decrease in TSM applies to all species across latitudes; because daily temperature variation lowers the realized temperature optimum and brings it closer to the prevailing habitat temperature, we predict species to have increased sensitivity to climate warming.
Figure 4.
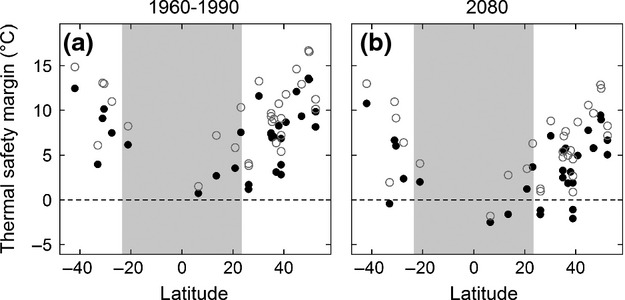
Effects of daily temperature variation and climate warming on thermal safety margins across latitude. Thermal safety margins for 29 terrestrial insect across latitude, as estimated for (a) the period 1960–1990 or (b) the period 2080. Open gray circles represent estimates based on the mean habitat temperature, black solid circles estimates based on the average habitat temperature range. The gray area represents the tropics.
The increased sensitivity is confirmed by substituting the contemporary climate data with projections from the HadCM3 climate scenario for 2080 (considering not only changes in mean temperature but also the forecast changes in temperature variation for species-specific locations, see Figure S1). As expected, with warmer habitat temperatures across the board, the TSMs of all species are reduced. However, with mean temperatures and the fundamental fitness curves, only one of the TSMs was reduced to zero or below, indicating some remaining buffer against negative impacts on fitness for the majority of species. With daily temperature fluctuations and the realized fitness curves, on the other hand, effects for all species were more severe and seven species had negative TSMs (Fig. 4b).
Discussion
Our empirical and theoretical investigations demonstrate that key elements of thermal sensitivity (To, CTmax, TSM), as well as the overall shape of the thermal reaction norms, depend not only on mean temperatures but also on the extent of daily temperature variation.
The empirical and theoretical effects of temperature variation follow a number of previous studies (Siddiqui et al., 1973; Worner, 1992; Liu et al., 1995; Mironidis & Savopoulou-Soultani, 2008; Paaijmans et al., 2010; Bozinovic et al., 2011) and are consistent with Jensen's inequality (Ruel & Ayres, 1999), whereby fluctuation over a concave function (i.e., the cold end of a reaction norm) results in a net increase in a rate process (or fitness), fluctuation over a convex function (i.e., the warmer part of a reaction norm) a net decrease, and fluctuation over a linear function, no net change. There is evidence from a range of taxa that short-term temperature dynamics can influence both the ecology (Rohr & Raffel, 2010; Bozinovic et al., 2011; Estay et al., 2011; Folguera et al., 2011; Hamilton et al., 2012; Raffel et al., 2013) and evolution (Martin & Huey, 2008; Kingsolver et al., 2009; Asbury & Angilletta, 2010) of ectotherm life-history traits (and note that temporal variation in temperature can also affect hibernating endotherms (Boyles & McKechnie, 2010)). The extent to which such effects derive from rate summation alone remains unclear. Additional physiological mechanisms such as production and breakdown of heat shock proteins (McMillan et al., 2005) could further exacerbate the influence of temperature variation, especially toward the extremes of thermal reaction norms.
Defining the temperature extremes and critical thermal limits is important for understanding species distribution limits and responses to climate change (Deutsch et al., 2008; Huey et al., 2009; Santos et al., 2011; Piyaphongkul et al., 2012; Ribeiro et al., 2012). Estimates of CTmax are highly sensitive to the methodology used and can vary depending on factors such as heating rates, insect age, and body mass (Bowler & Terblanche, 2008; Santos et al., 2011; Ribeiro et al., 2012), as well as the specific response parameter studied (Santos et al., 2012). The observed reduction in CTmax under fluctuating temperature conditions adds another layer of complexity to accurately defining this critical parameter.
Exactly how measures such as TSM influence vulnerability to climate change is unclear. A species' vulnerability to climate change will depend on a range of factors, and unraveling the relative importance of these creates a number of research challenges. For example, we have focused on temperature as a single variable but other abiotic (e.g., rainfall, humidity, CO2) and biotic stressors (intra- and interspecific competitors, predators and parasites etc.) can interact with temperature to determine overall fitness (Clusella-Trullas et al., 2011; Hamilton et al., 2012). In addition, while it is generally true that smaller terrestrial ectotherms conform to ambient temperature (Stevenson, 1985a), certain ectotherms may limit temperature extremes via thermal behavior (Stevenson, 1985b; Huey et al., 2012) and hence, modulate the short-term influence of temperature variability. In the longer term, ectotherms can potentially modify responses through genotypic adaptation and/or phenotypic plasticity (Atkins & Travis, 2010; Chevin et al., 2010), further altering thermal reaction norms. Moreover, overall fitness is a composite metric and it is important to partition temperature–fitness relationships into component parts, such as fecundity, development, and survivorship (Folguera et al., 2011; Amarasekare & Savage, 2012). In our experiments, we focused on two immature life-history traits (development time and survival) to demonstrate the effects of fluctuation on thermal reaction norms, yet overall insect fitness is determined by a suite of traits, including adult longevity and life-time reproductive output, each with potentially different reaction norms (e.g., see Mordecai et al., 2013). How multiple reaction norms combine is unclear but given the potential for trade-offs, temperature optima for overall fitness might differ from those of individual traits.
These issues notwithstanding, we follow the argument of Huey et al. (2012) that thermal fitness curves provide a convenient, fundamental descriptor of how temperature influences fitness of ectotherms. In this regard, TSM represents a comparative metric to characterize sensitivity (Deutsch et al., 2008; Jaramillo et al., 2009; Clusella-Trullas et al., 2011; Krenek et al., 2012). Other proxies of sensitivity, such as warming tolerance, tolerance range, and fitness breadth rely similarly on attributes of the thermal fitness curve (Huey et al., 2012).
Climate change can lead to phenological change, species' range shifts and even extinction of plants and animals (Easterling et al., 2000). Most studies to date use the standard fitness curves derived from constant temperature experiments combined with mean habitat temperatures to assess such climate-induced risks. Our results indicate that inclusion of daily temperature dynamics can generate substantial shifts in these fitness profiles. The general lowering of TSMs and CTmax, together with the potential for altered ecological interactions under fluctuating temperature conditions (e.g., Duncan et al., 2011; Hamilton et al., 2012), might provide some explanation of the observation that terrestrial ectotherms are shifting much faster in response to climate change than previously predicted (Chen et al., 2011). To better understand the role of environmental temperature, there is a clear need to integrate ecologically realistic thermal regimes into a wider range of empirical studies (Rohr et al., 2011; Niehaus et al., 2012), matching thermal regimes with the operative body temperatures experienced by organisms in the field.
Acknowledgments
We thank Silvie Huijben for her help with R-coding, and Janet Teeple and Katey Glunt for assistance in the laboratory. This study was supported by a NSF-EID program grant (#EF-0914384). The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views of the National Science Foundation. This project was also assisted by a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Mean temperatures and daily temperature ranges, and changes therein as a result of climate warming. (a) mean habitat temperature (Th; as recorded in the 1960–1990 period) and daily temperature range (DTR), and (b) the change in mean habitat temperature (Th) and DTR as a result of warming. The gray area represents the tropics.
References
- Amarasekare P, Savage V. A framework for elucidating the temperature dependence of fitness. American Naturalist. 2012;179:178–191. doi: 10.1086/663677. [DOI] [PubMed] [Google Scholar]
- Angert AL, Sheth SN, Paul JR. Incorporating population-level variation in thermal performance into predictions of geographic range shifts. Integrative and Comparative Biology. 2011;51:733–750. doi: 10.1093/icb/icr048. [DOI] [PubMed] [Google Scholar]
- Asbury D, Angilletta M. Thermodynamic effects on the evolution of performance curves. American Naturalist. 2010;176:E40–E49. doi: 10.1086/653659. [DOI] [PubMed] [Google Scholar]
- Atkins KE, Travis JMJ. Local adaptation and the evolution of species' ranges under climate change. Journal of Theoretical Biology. 2010;266:449–457. doi: 10.1016/j.jtbi.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Bayoh MN, Lindsay SW. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae) Bulletin of Entomological Research. 2003;93:375–381. doi: 10.1079/ber2003259. [DOI] [PubMed] [Google Scholar]
- Bonebrake TC, Deutsch CA. Climate heterogeneity modulates impact of warming on tropical insects. Ecology. 2012;93:449–455. doi: 10.1890/11-1187.1. [DOI] [PubMed] [Google Scholar]
- Bowler K, Terblanche JS. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biological Reviews. 2008;83:339–355. doi: 10.1111/j.1469-185x.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- Boyles JG, McKechnie AE. Energy conservation in hibernating endotherms: why “suboptimal” temperatures are optimal. Ecological Modelling. 2010;221:1644–1647. [Google Scholar]
- Bozinovic F, Bastias DA, Boher F, Clavijo-Baquet S, Estay SA, Angilletta MJ. The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiological and Biochemical Zoology. 2011;84:543–552. doi: 10.1086/662551. [DOI] [PubMed] [Google Scholar]
- Chen IC, Hill JK, Ohlemuller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- Chevin L-M, Lande R, Mace GM. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. Plos Biology. 2010;8:e1000357. doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusella-Trullas S, Blackburn TM, Chown SL. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. American Naturalist. 2011;177:738–751. doi: 10.1086/660021. [DOI] [PubMed] [Google Scholar]
- Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AB, Fellous S, Kaltz O. Temporal variation in temperature determines disease spread and maintenance in Paramecium microcosm populations. Proceedings of the Royal Society B-Biological Sciences. 2011;278:3412–3420. doi: 10.1098/rspb.2011.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling DR, Horton B, Jones PD, et al. Maximum and minimum temperature trends for the globe. Science. 1997;277:364–367. [Google Scholar]
- Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. Climate extremes: observations, modelling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- Estay SA, Clavijo-Baquet S, Lima M, Bozinovic F. Beyond average: an experimental test of temperature variability on the population dynamics of Tribolium confusum. Population Ecology. 2011;53:53–58. [Google Scholar]
- Folguera G, Bastias DA, Bozinovic F. Impact of experimental thermal amplitude on ectotherm performance: adaptation to climate change variability? Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology. 2009;154:389–393. doi: 10.1016/j.cbpa.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Folguera G, Bastias DA, Caers J, Rojas JM, Piulachs MD, Belles X, Bozinovic F. An experimental test of the role of environmental temperature variability on ectotherm molecular, physiological and life-history traits: implications for global warming. Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology. 2011;159:242–246. doi: 10.1016/j.cbpa.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Geerts B. Empirical estimation of the monthly-mean daily temperature range. Theoretical and Applied Climatology. 2003;74:145–165. [Google Scholar]
- Hamilton PT, Richardson JML, Govindarajulu P, Anholt BR. Higher temperature variability increases the impact of Batrachochytrium dendrobatidis and shifts interspecific interactions in tadpole mesocosms. Ecology and Evolution. 2012;2:2450–2459. doi: 10.1002/ece3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen RK, Luoto M, Leikola N, et al. Assessing the vulnerability of European butterflies to climate change using multiple criteria. Biodiversity and Conservation. 2010;19:695–723. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Hoffmann AA. Physiological climatic limits in Drosophila: patterns and implications. Journal of Experimental Biology. 2010;213:870–880. doi: 10.1242/jeb.037630. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Todgham AE. Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annual Review of Physiology. 2010;72:127–145. doi: 10.1146/annurev-physiol-021909-135900. [DOI] [PubMed] [Google Scholar]
- Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Álvarez PH, Garland T. Why tropical forest lizards are vulnerable to climate warming. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1939–1948. doi: 10.1098/rspb.2008.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society B-Biological Sciences. 2012;367:1665–1679. doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo J, Chabi-Olaye A, Kamonjo C, Jaramillo A, Vega FE, Poehling H-M, Borgemeister C. Thermal tolerance of the coffee berry borer Hypothenemus hampei: predictions of climate change impact on a tropical insect pest. PLoS ONE. 2009;4:e6487. doi: 10.1371/journal.pone.0006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo J, Muchugu E, Vega FE, Davis A, Borgemeister C, Chabi-Olaye A. Some like it hot: the influence and implications of climate change on coffee berry borer (Hypothenemus hampei) and coffee production in East Africa. PLoS ONE. 2011;6:e24528. doi: 10.1371/journal.pone.0024528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver JG, Ragland GJ, Diamond SE. Evolution in a constant environment: thermal fluctuations and thermal sensitivity of laboratory and field populations of Manduca sexta. Evolution. 2009;63:537–541. doi: 10.1111/j.1558-5646.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- Krenek S, Petzoldt T, Berendonk TU. Coping with temperature at the warm edge – patterns of thermal adaptation in the microbial eukaryote Paramecium caudatum. PLoS ONE. 2012;7:e30598. doi: 10.1371/journal.pone.0030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassalle G, Crouzet P, Gessner J, Rochard E. Global warming impacts and conservation responses for the critically endangered European Atlantic sturgeon. Biological Conservation. 2010;143:2441–2452. [Google Scholar]
- Liu SS, Zhang GM, Zhu J. Influence of temperature variations on rate of development in insects: analysis of case studies from entomological literature. Annals of the Entomological Society of America. 1995;88:107–119. [Google Scholar]
- Martin TL, Huey RB. Why “Suboptimal” is optimal: Jensen's inequality and ectotherm thermal preferences. American Naturalist. 2008;171:E102–E118. doi: 10.1086/527502. [DOI] [PubMed] [Google Scholar]
- McMillan DM, Fearnley SL, Rank NE, Dahlhoff EP. Natural temperature variation affects larval survival, development and Hsp70 expression in a leaf beetle. Functional Ecology. 2005;19:844–852. [Google Scholar]
- Milanovich JR, Peterman WE, Nibbelink NP, Maerz JC. Projected loss of a salamander diversity hotspot as a consequence of projected global climate change. PLoS ONE. 2010;5:e12189. doi: 10.1371/journal.pone.0012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironidis GK, Savopoulou-Soultani M. Development, survivorship, and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under constant and alternating temperatures. Environmental Entomology. 2008;37:16–28. doi: 10.1603/0046-225X(2008)37[16:DSAROH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mordecai EA, Paaijmans KP, Johnson LR, et al. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecology Letters. 2013;16:22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- Niehaus AC, Angilletta MJ, Sears MW, Franklin CE, Wilson RS. Predicting the physiological performance of ectotherms in fluctuating thermal environments. Journal of Experimental Biology. 2012;215:694–701. doi: 10.1242/jeb.058032. [DOI] [PubMed] [Google Scholar]
- Paaijmans KP, Jacobs AFG, Takken W, Heusinkveld BG, Githeko AK, Dicke M, Holtslag AAM. Observations and model estimates of diurnal water temperature dynamics in mosquito breeding sites in western Kenya. Hydrological Processes. 2008;22:4789–4801. [Google Scholar]
- Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proceedings of the National Academy of Sciences. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton WJ, Logan JA. A model for diurnal variation in soil and air temperature. Agricultural Meteorology. 1981;23:205–216. [Google Scholar]
- Piyaphongkul J, Pritchard J, Bale J. Can tropical insects stand the heat? A case study with the brown planthopper Nilaparvata lugens (Stål) PLoS ONE. 2012;7:e29409. doi: 10.1371/journal.pone.0029409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. Available at http://www.R-project.org. [Google Scholar]
- Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. Disease and thermal acclimation in a more variable and unpredictable climate. Nature Climate Change. 2013;3:146–151. [Google Scholar]
- Ribeiro PL, Camacho A, Navas CA. Considerations for assessing maximum critical temperatures in small ectothermic animals: insights from leaf-cutting ants. PLoS ONE. 2012;7:e32083. doi: 10.1371/journal.pone.0032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proceedings of the National Academy of Sciences. 2010;107:8269–8274. doi: 10.1073/pnas.0912883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Dobson AP, Johnson PTJ, et al. Frontiers in climate change-disease research. Trends in Ecology and Evolution. 2011;26:270–277. doi: 10.1016/j.tree.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel JJ, Ayres MP. Jensen's inequality predicts effects of environmental variation. Trends in Ecology and Evolution. 1999;14:361–366. doi: 10.1016/s0169-5347(99)01664-x. [DOI] [PubMed] [Google Scholar]
- Santos M, Castañeda LE, Rezende EL. Making sense of heat tolerance estimates in ectotherms: lessons from Drosophila. Functional Ecology. 2011;25:1169–1180. [Google Scholar]
- Santos M, Castañeda LE, Rezende EL. Keeping pace with climate change: what is wrong with the evolutionary potential of upper thermal limits? Ecology and Evolution. 2012;2:2866–2880. doi: 10.1002/ece3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui WH, Barlow CA, Randolph PA. Effects of some constant and alternating temperatures on population growth of the pea aphid, Acyrthosiphon pisum (Homoptera: Aphididae) The Canadian Entomologist. 1973;105:145–156. [Google Scholar]
- Stevenson RD. Body size and limits to the daily range of body temperature om terrestrial ectotherms. American Naturalist. 1985a;125:102–117. [Google Scholar]
- Stevenson RD. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. American Naturalist. 1985b;126:362–386. [Google Scholar]
- Terblanche JS, Nyamukondiwa C, Kleynhans E. Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the Mediterranean fruit fly (Ceratitis capitata. Entomologia Experimentalis et Applicata. 2010;137:304–315. [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch CA. Putting the heat on tropical animals. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- Worner SP. Performance of phenological models under variable temperature regimes: consequences of the Kaufmann or rate summation effect. Environmental Entomology. 1992;21:689–699. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean temperatures and daily temperature ranges, and changes therein as a result of climate warming. (a) mean habitat temperature (Th; as recorded in the 1960–1990 period) and daily temperature range (DTR), and (b) the change in mean habitat temperature (Th) and DTR as a result of warming. The gray area represents the tropics.


