Abstract
The skin is the single largest organ in humans, serving as a major barrier to infection, water loss, and abrasion. The functional diversity of skin requires the synthesis of large amounts of lipids, such as triglycerides, wax esters, squalene, ceramides, free cholesterol, free fatty acids, and cholesterol and retinyl esters. Some of these lipids are used as cell membrane components, signaling molecules, and a source of energy. An important class of lipid metabolism enzymes expressed in skin is the Δ9-desaturases, which catalyze the synthesis in Δ9-monounsaturated lipids, primarily oleoyl-CoA (18:1n-9) and palmitoyl-CoA (16:1n-7), the major monounsaturated fatty acids in cutaneous lipids. Mice with a deletion of the Δ9-desaturase-1 isoform (SCD1) either globally (Scd1−/−) or specifically in the skin (skin-specific Scd1-knockout; SKO) present with marked changes in cutaneous lipids and skin integrity. Interestingly, these mice also exhibit increased whole body energy expenditure, protection against diet-induced adiposity, hepatic steatosis, and glucose intolerance. The increased energy expenditure in skin-specific Scd1-knockout (SKO) mice is a surprising phenotype, as it links cutaneous lipid homeostasis with whole body energy balance. This minireview summarizes the role of skin SCD1 in regulating skin integrity and whole body energy homeostasis and offers a discussion of potential pathways that may connect these seemingly disparate phenotypes.
Keywords: Energy Metabolism, Fatty Acid Metabolism, Lipids, Obesity, Skin, Energy Metabolism, Skin
Stearoyl-CoA Desaturase-1
Stearoyl-CoA desaturase (SCD)3 is a Δ9-desaturase anchored in the endoplasmic reticulum membrane via four transmembrane domains (1). As the name implies, this Δ9-desaturase catalyzes the conversion of 12 to 18-carbon saturated fatty acids into monounsaturated fatty acids (MUFAs) through the insertion of the first cis-double bond at the Δ9-position (reviewed in Refs. 2 and 3). These endogenously synthesized MUFAs are important to a variety of cellular functions, including synthesis of complex lipids, such as diacylglycerols, phospholipids, triglycerides (TGs), wax esters, and cholesterol esters. The degree of unsaturation of cellular lipids can also play a role in membrane fluidity and cell signaling. Therefore, SCD is highly conserved, with multiple isoforms providing overlapping but distinct tissue and substrate specificity (2, 3).
In the mouse, there are four known isoforms of SCD. These are all located within a 200-kb region on chromosome 19 and code for 350–360 amino acid proteins with >80% amino acid sequence similarity. SCD1 is ubiquitously expressed, with significant expression in the adult liver, where it is dramatically induced by both dietary carbohydrate and saturated fat (4–11). In addition to the liver, it is expressed in the undifferentiated cells of sebaceous glands in the skin and is critical to sebocyte development (12–14). As will be discussed for the majority of this minireview, skin-specific expression of SCD1 appears to play a role not only in the maintenance of skin integrity but also, unexpectedly, in whole body energy balance. SCD2, which shares significant sequence homology with SCD1, is also ubiquitously expressed but is especially enriched in the murine brain, particularly during myelination in the neonate (15). In the liver, SCD2 expression is reciprocally regulated with SCD1 expression, with expression levels being high during development and decreasing dramatically at weaning, corresponding with an increase in hepatic SCD1 expression. Further evidence of the importance of SCD2 during development comes from the observation that mice lacking SCD2 are born with an impaired skin barrier, resulting in death due to water loss across the skin within hours of birth in a majority of animals (15). Mouse SCD3 is expressed in the Harderian gland and differentiated sebocytes in skin (16, 17). SCD3 is the only isoform that exhibits a preference for palmitate over stearate as a substrate (17). SCD4 is expressed mainly in the murine heart (18).
Humans express two different isoforms of SCD, both of which are microsomal proteins. Human SCD1 is highly homologous to mouse SCD1 and is expressed in adult adipose tissue, liver, lung, brain, heart, pancreas, and skeletal muscle (19–21). The second human SCD isoform is termed SCD5, and it appears to be a primate-specific isoform that is expressed predominantly in the brain and pancreas, with some limited expression in the heart, kidney, lung, and placenta (22–24). The regulation of human SCDs and their role in disease have been reviewed elsewhere (25).
Although the MUFA products of SCD are abundant in the diet, SCD1 is highly regulated, indicating a critical role for endogenously synthesized MUFAs. The regulation of SCD1 has been reviewed extensively elsewhere (2, 3). Briefly, the Scd1 gene is under positive regulation by a variety of dietary and cellular factors, including glucose, fructose, and saturated fatty acids, as well as insulin and transcription factors, such as SREBP-1c (sterol regulatory element-binding protein-1c) and the nuclear receptor liver X receptor. Negative regulation of the Scd1 gene is affected by the actions of the adipokine leptin, as well as polyunsaturated fatty acids (2, 3, 26). Additionally, the SCD1 protein is also subject to degradation by proteases and through the proteasomal pathway (27–30), conferring an additional layer of regulation.
Much of what we know regarding the physiological function of SCD1 comes from studies conducted in mice with a targeted deletion of the Scd1 gene. These mice are characterized by a lean hypermetabolic phenotype, which includes resistance to diet-induced and genetically induced obesity and insulin resistance, as well as significant changes in nonshivering thermogenesis (6–11, 31–37). In addition to these alterations in energy metabolism, SCD1-deficient mice also have a marked cutaneous phenotype. Indeed, SCD1 was first identified in mice with a natural occurring mutation in the Scd1 gene (Table 1). These “asebia” mice are characterized by the presence of dry flaky skin and severe alopecia resulting from sebocyte atrophy, underscoring a critical role for SCD1 in sebocyte development (12, 13, 34, 38, 39). Subsequent studies in these naturally SCD1-deficient asebia mice and in mice with a targeted deletion of the Scd1 gene (Scd1−/− mice) have revealed that SCD1 is in fact expressed in the sebaceous glands of mouse skin and is regulated throughout the hair cycle (13, 34).
TABLE 1.
Comparison of cutaneous and whole body phenotypes of SCD1-deficient mouse models
A comparison of various SCD1-deficient mouse models with regard to primary cutaneous phenotypes and resistance to obesity is shown below. SC, stratum corneum; FC, free cholesterol; WDEs, wax diesters; TEWL, transepidermal water loss.
| Model | Mutation | Scd1 expression | Skin phenotype | Resistance to obesity | Skin lipid composition | Skin barrier function |
|---|---|---|---|---|---|---|
| Asebia J1 | Spontaneous deletion spanning exons 1–4 (13) | None (13) | Alopecia; sebocyte hypoplasia; slight thickening and decreased hydration of SC (42) | Resistant to leptin-deficiency induced obesity (6) | Increased FC; decreased wax monoesters, sterol esters, WDEs, and glycerol (42) | Normal (38, 42) |
| Asebia 2J | Spontaneous 18-bp deletion in exon 2/intron 2 boundary (13) | Aberrantly spliced 687-, 738-, and 828-bp transcripts (13) | Alopecia; sebocyte hypoplasia; markedly thicker SC (38) | Reduced sterol esters, FC, and WDEs (38) | Increased TEWL (38) | |
| Scd1−/− | Targeted germ-line deletion of Scd1 exons 1–6 (50) | None (50) | Alopecia; sebocyte hypoplasia (50) | Resistant to HFD-induced obesity (7) | Increased FC; decreased TGs, WDEs, and cholesterol esters (50) | Increased TEWL (44) |
| Scd1 SKO | Targeted deletion of Scd1 exons 1–6 under keratin-14 promoter (34) | No expression in skin (34) | Alopecia; sebocyte hypoplasia (34) | Resistant to HFD-induced obesity (34) | Increased FC and ceramides; decreased TGs and WDEs (34) | |
In an effort to understand tissue-specific contributions of SCD1 to the whole body energy metabolism phenotype observed in Scd1−/− mice, a series of tissue-specific Scd1−/− mice were generated and characterized (11, 35, 40). Although it was clear from studies in the global Scd1−/− model that SCD1 regulates skin integrity, the generation of a skin-specific Scd1−/− animal model (SKO mouse) has revealed surprising connections between skin-specific expression of SCD1, maintenance of the integrity of the skin, and whole body energy metabolism (34, 36).
Sebaceous and Epidermal Lipids Regulate Skin Permeability Barrier
As the largest organ in the body, the skin serves a variety of functions, the most important of which is to serve as a barrier against external insults. The skin is a highly structured organ and consists of various different cell types with specific functions that help maintain the integrity of the tissue. The skin also houses a variety of specialized structures (such as hair follicles, sweat glands, and sebaceous glands) that impart functional diversity to the organ (14, 41–43).
One of the primary functions of the skin is to serve as the permeability barrier, preventing heat and water loss across the surface of the skin (14, 41, 42–44). Therefore, maintenance of the integrity of the skin is critical in preventing dehydration and in regulating core body temperature in homeotherms. The permeability barrier of the skin is comprised of various different cell types, including keratinocytes and corneocytes in the stratum corneum of the epidermis (42, 43). In addition, skin cells are bathed in a rich array of lipids, including free cholesterol, TGs, ceramides, and free fatty acids. It is now clear that these lipids, many of which are actively synthesized in the skin, play an important role in the maintenance of the skin barrier (14, 15, 34, 41, 42–44).
Skin SCD1 Regulates Skin Integrity and Energy Balance
Although cutaneous abnormalities were initially described in mice with a global deletion of SCD1, a potential link between the favorable metabolic phenotype and the skin abnormalities of Scd1−/− mice was not suspected until the generation of mice lacking SCD1 in the skin (34). Skin-specific Scd1−/− (SKO) mice were generated by targeting the deletion of Scd1 using a keratin-14 promoter. Skin-specific deletion of Scd1 recapitulated the cutaneous abnormalities observed in Scd1−/− mice (34). Interestingly, in addition to severe alopecia and sebocyte hypoplasia (Table 1), SKO mice were also lean and protected from diet-induced obesity, similar to Scd1−/− mice (34). This lean phenotype was accompanied by a significant up-regulation of metabolic rate, similar to Scd1−/− mice. Similar to global Scd1−/− mice, SKO mice were hyperphagic on both chow and high-fat diets (HFDs) but were almost completely protected from weight gain on either of these diets. Although WT animals go on to become insulin-resistant when fed a HFD, both Scd1−/− and SKO mice are protected from this secondary complication of obesity (34). One of the phenotypes of global SCD1 deficiency is a lack of the de novo lipogenesis response in the liver to insults such as high-carbohydrate or saturated fat feeding (5, 8, 10). However, this hepatic lipogenesis response is not affected in SKO mice, suggesting that skin-specific deletion of SCD1 is not sufficient to impair hepatic lipogenesis (34). Indeed, it appears that liver-specific deletion of SCD1 impairs hepatic lipogenesis to a similar extent as observed in global Scd1−/− mice (11).
Although hepatic lipogenesis was unaffected in SKO mice, these animals presented a coordinated set of metabolic changes in peripheral tissues, indicating increased lipid catabolism and activation of thermogenesis. This included an up-regulation of uncoupling genes in the liver, skeletal muscle, and white and brown adipose tissues. In addition, genes of fatty acid oxidation were increased in the liver and adipose tissues of SKO mice, indicating increased lipid catabolism in these animals (34).
One of the most striking phenotypes of global SCD1 deficiency is an extreme cold sensitivity, such that Scd1−/− mice do not survive longer than 2–4 h when exposed to a 4 °C environment (31). Similar to Scd1−/− mice, SKO mice also displayed significant cold sensitivity (Fig. 1), with most animals dying of hypoglycemia within 3 h of cold exposure (34). This extreme cold susceptibility was brought on by an inability to maintain plasma glucose levels during cold exposure and a precipitous depletion of hepatic glycogen stores in SKO mice exposed to cold for as little as 90 min. Interestingly, if these animals were fed a HFD for 3 weeks prior to cold exposure, their cold sensitivity was dramatically improved, with SKO animals being able to tolerate over 24 h of cold exposure. After 90 min of cold exposure, HFD-fed SKO mice were able to maintain plasma glucose levels and were spared from the near complete depletion of hepatic glycogen that was observed in chow-fed SKO mice upon 90 min of cold exposure. This protection from energy depletion upon cold exposure occurred in HFD-fed SKO mice even though they did not gain any measurable amount of body weight due to 3 weeks of HFD feeding (34). These results suggest that although body weight may not be rescued by dietary intervention in SKO mice, it is possible to rescue at least some of their energetic deficit by feeding a hypercaloric diet.
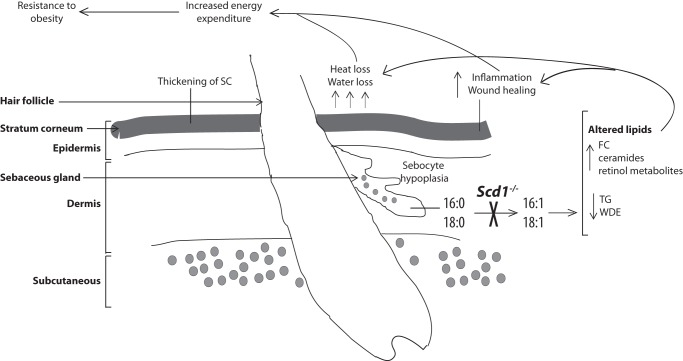
FIGURE 1.
Cutaneous changes in SCD1-deficient mice and relationship to whole body energy expenditure: a model. The major cutaneous changes observed in SCD1-deficient animals are depicted. The inability to desaturate fatty acids, such as palmitate and stearate, results in hypoplastic sebaceous glands and a thickened stratum corneum (SC). A concurrent increase in free cholesterol (FC), ceramides, and retinol metabolites, along with a significant depletion of TG and wax diesters (WDE), in the skin of SCD1-deficient mice accompanies various cutaneous phenotypes, including increased inflammation and heat and water loss across the surface of the skin. These cutaneous phenotypes appear to be causally linked to the favorable metabolic phenotype of SCD1-deficient mice, potentially due to an increase in energetic demand for body temperature maintenance or due to the energy-intensive nature of cutaneous wound healing.
During routine investigations, mice are housed at 25 °C, which is well below thermoneutrality and has been documented to contribute to changes in energy metabolism (45–49). Given the cutaneous phenotype of SKO mice, it was hypothesized that SKO mice may be in a constant state of increased energy expenditure to maintain body temperature when housed at 25 °C. Indeed, markers of cold exposure, including β3-adrenergic receptor signaling in brown adipose tissue, were elevated in SKO mice, suggesting increased cold perception in these mice even at room temperature (34). To follow up on this hypothesis, WT and SKO mice were housed at 33 °C, and food intake and body weight gain on a HFD were measured (36). Surprisingly, SKO mice still consumed more food than WT counterparts but were resistant to weight gain during the feeding period (36). Interestingly, although SKO mice were almost completely protected from weight gain on a HFD even when maintained at 33 °C for the duration of feeding, hyperphagia in SKO mice appeared to be less pronounced at 33 °C compared with mice housed in conventional rooms at 25 °C (34, 36). When housed at 25 °C, SKO mice ate over 50% more HFD by weight compared with WT counterparts (34). At 33 °C, although SKO mice were still hyperphagic, they only ate ∼30% more food than WT counterparts (36). This attenuation of hyperphagia in SKO mice at thermoneutrality indicates increased metabolic efficiency and is suggestive of cold sensing playing an important role in the hypermetabolic phenotype of SCD1-deficient animals. Although these studies indicated that body weight was not rescued by maintaining animals at thermoneutrality, changes in other metabolic features, such as hepatic glycogen stores and fat mass, were not examined in these mice maintained at 33 °C. Given the apparent attenuation of the hyperphagic response in SKO mice at 33 °C, it is possible that partial rescue of alternative metabolic phenotypes may occur prior to rescue of body weight in these animals.
It is important to note that the thermoneutrality experiments reviewed above were performed in animals over 6 weeks of age and during a relatively short duration of 7–9 weeks (36). However, changes in cold sensing and metabolic adaptations could occur well before adulthood in these animals. Indeed, the cutaneous abnormalities in SKO and Scd1−/− mice are apparent before weaning. Therefore, the question of whether sustained rescue of cold sensing in SCD1-deficient mice can reverse the lean metabolic phenotype was not addressed by this study.
SCD1-induced Changes in Skin Lipid Composition and Permeability Barrier
Prevention of transepidermal water loss is one of the key barrier functions of the skin, and cutaneous lipids play an important role in preserving the skin water barrier (41–43). Alterations in transepidermal water loss and barrier permeability have been reported in mice with a global deletion of SCD1 (38, 44), and one set of investigations suggested that artificial occlusion of the skin in SCD1-deficient animals could potentially confer cold resistance and abolish the protection from obesity observed in Scd1−/− mice (44). In the thermoneutrality experiments described above, although ambient temperatures were maintained close to thermoneutral conditions, relative humidity was maintained at 30–40% (36). Increased water loss across the surface of the skin could therefore still have accounted for the obesity resistance observed in SKO mice.
The cutaneous permeability barrier is significantly affected by the lipid composition of the skin. In this regard, both global SCD1 deficiency and skin-specific deletion of SCD1 result in striking changes in cutaneous lipids, including a large increase in free cholesterol levels, increased ceramide levels, and decreased levels of other key skin lipids (such as free fatty acids, TGs, and wax diesters) (Table 1) (34, 49). This set of studies and more recent investigations revealed a coordinated increase in expression of key cholesterol biosynthetic genes, including HMG-CoA synthase, HMG-CoA reductase, and squalene epoxidase, which may account for the increase in skin free cholesterol levels in SKO mice (34, 36).
The increase in skin free cholesterol levels may be due to an inability to esterify cholesterol in the absence of sufficient monounsaturated fatty acids, which are important components of the skin cholesterol ester pool. The increase in skin free cholesterol content is likely to be a significant cause of the skin pathology observed in SKO mice (Fig. 1). Other mouse models that display an increase in skin free cholesterol content, including mice overexpressing human APOC1 (apolipoprotein C1) and mice deficient in the cholesterol esterification enzyme acyl-CoA:cholesterol acyltransferase-1, also display cutaneous abnormalities similar to SCD1-deficient mice (50–52). Interestingly, the APOC1-overexpressing mouse also displays a similar lean metabolic phenotype (51), like SCD1-deficient mice, but any potential link between the cutaneous changes and metabolic alterations in this model has not been investigated.
Inflammation and Wound Healing in Skin of SCD1-deficient Mice
Among the asebia mouse strains that harbor a natural mutation in the Scd1 gene, the asebia J1 strain has been shown to have no impairment in skin permeability (42) despite significant sebocyte atrophy (Table 1). However, asebia 2J mice have been reported to have increased skin permeability in conjunction with altered cutaneous lipid content (38). These results suggest that ancillary factors, in addition to the sebaceous lipid content of skin, may regulate the maintenance of an intact skin barrier and thereby exert effects on whole body energy homeostasis.
It has been suggested that the extent of epidermal inflammation may help explain some of the differences in skin permeability between various strains of SCD1-deficient mice (38). SKO mice displayed significantly increased skin inflammation, evident from increases in expression of inflammatory genes related to psoriasis, tissue damage, and the wound-healing process (36). Although psoriasis is generally associated with obesity (53–55), the observation of increased markers of wound healing in the skin of SKO mice is potentially significant in explaining the increased energy metabolism in these animals. Wound healing is an extremely energy-intensive process (56–58). Following injuries where skin integrity is severely compromised, including burn injuries that result in destruction of skin structures like sebaceous glands, there is a marked increase in energy expenditure and onset of cold intolerance (58–60), similar to mice deficient in SCD1 (31, 34). Although elevation of ambient temperatures can apparently slow down metabolic rate in patients with extensive burn injuries, patients with more moderate burns do not show any decrease in metabolic rate when exposed to higher ambient temperatures, closer to thermoneutrality (60). These observations are quite similar to the metabolic changes observed in SKO mice (31, 34, 36), suggesting that an increased energetic demand due to activation of wound-healing processes could at least partially account for the increase in energy expenditure observed in SCD1-deficient mice (Fig. 1).
Altered Retinol Metabolism in Skin of SCD1-deficient Mice
In addition to changes in the lipid content of the skin of SKO mice, it has been recently shown that levels of vitamin A metabolites are significantly altered in the skin of these mice relative to WT counterparts (36). Retinol, retinoic acid, and retinyl esters were all significantly increased in the skin of SKO mice, even when placed on a retinol-deficient diet (Fig. 1). This was accompanied by increased expression of genes regulated by retinoic acid activation of the retinoic acid receptor (RAR), including RBP1 (retinol-binding protein-1) and CRAPB2 (cellular retinoic acid-binding protein-2) (36). Additionally, the retinol esterification gene lecithin-retinol acyltransferase was elevated, and genes encoding proteins that oxidize retinol to retinaldehyde were decreased in the skin of SKO mice (36). Changes in retinoic acid-induced gene expression are evident as early as 23 days of age in SKO mice, suggesting that these changes may mediate the subsequent cutaneous abnormalities present in SKO mice.
In addition to transactivating RAR, retinoic acid has also been shown to bind to FABP5 (fatty acid-binding protein-5) and transactivate peroxisome proliferator-activated receptor δ (PPARδ) (61, 62). Commensurate with the increased retinoic acid levels in the skin of SKO mice, Fabp5 gene expression and expression of target genes of PPARδ were also found to be significantly elevated, suggesting increased PPARδ activation in the skin of these mice (36). These results are potentially significant in explaining the cutaneous phenotype of SCD1-deficient mice because PPARδ-overexpressing animals also present with psoriasis and epidermal hyperplasia (63), similar to SCD1-deficient mice. PPARδ has also been shown to be important to the skin wound-healing response (64–67). The increase in PPARδ signaling in SCD1-deficient mice may therefore be a compensatory response to cutaneous injury in these mice, since PPARδ has been shown to be rapidly up-regulated following cutaneous insults (64–67). However, whether or not increased PPARδ signaling in skin is related to the hypermetabolic phenotype of Scd1−/− and SKO mice has yet to be determined.
Conclusion
Although the exact mechanisms whereby SCD1 deficiency in the skin may mediate whole body energy expenditure are still under debate, the studies conducted thus far in mice with a global deletion of SCD1, as well as skin-specific SCD1 ablation, have demonstrated an important link between sebaceous lipids and changes in diverse processes such as retinol metabolism, chronic inflammation, and cold sensing. To understand the contribution of each of these pathways to the hypermetabolic phenotype of SCD1-deficient mice, future studies should be aimed at selective rescue of signaling through each of these pathways, potentially through the use of RAR or PPARδ antagonists or anti-inflammatory compounds. Additionally, experiments involving skin-specific rescue of SCD1 in the setting of global SCD1 insufficiency would provide much needed definitive insight into the contribution of skin SCD1 to whole body energy metabolism.
Footnotes
- SCD
- stearoyl-CoA desaturase
- MUFA
- monounsaturated fatty acid
- TG
- triglyceride
- HFD
- high-fat diet
- RAR
- retinoic acid receptor
- PPARδ
- peroxisome proliferator-activated receptor δ
- SKO
- skin-specific Scd1-knockout.
REFERENCES
- 1. Man W. C., Miyazaki M., Chu K., Ntambi J. M. (2006) Membrane topology of mouse stearoyl-CoA desaturase 1. J. Biol. Chem. 281, 1251–1260 [DOI] [PubMed] [Google Scholar]
- 2. Sampath H., Ntambi J. M. (2006) Stearoyl-coenzyme A desaturase 1, sterol regulatory element binding protein-1c and peroxisome proliferator-activated receptor-α: independent and interactive roles in the regulation of lipid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 9, 84–88 [DOI] [PubMed] [Google Scholar]
- 3. Sampath H., Ntambi J. M. (2011) The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann. N.Y. Acad. Sci. 1243, 47–53 [DOI] [PubMed] [Google Scholar]
- 4. Miyazaki M., Kim Y. C., Gray-Keller M. P., Attie A. D., Ntambi J. M. (2000) The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J. Biol. Chem. 275, 30132–30138 [DOI] [PubMed] [Google Scholar]
- 5. Miyazaki M., Kim Y. C., Ntambi J. M. (2001) A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J. Lipid Res. 42, 1018–1024 [PubMed] [Google Scholar]
- 6. Cohen P., Miyazaki M., Socci N. D., Hagge-Greenberg A., Liedtke W., Soukas A. A., Sharma R., Hudgins L. C., Ntambi J. M., Friedman J. M. (2002) Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297, 240–243 [DOI] [PubMed] [Google Scholar]
- 7. Ntambi J. M., Miyazaki M., Stoehr J. P., Lan H., Kendziorski C. M., Yandell B. S., Song Y., Cohen P., Friedman J. M., Attie A. D. (2002) Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. U.S.A. 99, 11482–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyazaki M., Dobrzyn A., Man W. C., Chu K., Sampath H., Kim H. J., Ntambi J. M. (2004) Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 279, 25164–25171 [DOI] [PubMed] [Google Scholar]
- 9. Miyazaki M., Dobrzyn A., Sampath H., Lee S. H., Man W. C., Chu K., Peters J. M., Gonzalez F. J., Ntambi J. M. (2004) Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-α. J. Biol. Chem. 279, 35017–35024 [DOI] [PubMed] [Google Scholar]
- 10. Sampath H., Miyazaki M., Dobrzyn A., Ntambi J. M. (2007) Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J. Biol. Chem. 282, 2483–2493 [DOI] [PubMed] [Google Scholar]
- 11. Miyazaki M., Flowers M. T., Sampath H., Chu K., Otzelberger C., Liu X., Ntambi J. M. (2007) Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 6, 484–496 [DOI] [PubMed] [Google Scholar]
- 12. Parimoo S., Zheng Y., Eilertsen K., Ge L., Prouty S., Sundberg J., Stenn K. (1999) Identification of a novel SCD gene and expression of the SCD gene family in mouse skin. J. Investig. Dermatol. Symp. Proc. 4, 320–322 [DOI] [PubMed] [Google Scholar]
- 13. Zheng Y., Eilertsen K. J., Ge L., Zhang L., Sundberg J. P., Prouty S. M., Stenn K. S., Parimoo S. (1999) Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nature genetics 23, 268–270 [DOI] [PubMed] [Google Scholar]
- 14. Sampath H., Ntambi J. M. (2011) The role of fatty acid desaturases in epidermal metabolism. Dermatoendocrinol. 3, 62–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyazaki M., Dobrzyn A., Elias P. M., Ntambi J. M. (2005) Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc. Natl. Acad. Sci. U.S.A. 102, 12501–12506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Y., Prouty S. M., Harmon A., Sundberg J. P., Stenn K. S., Parimoo S. (2001) Scd3–a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics 71, 182–191 [DOI] [PubMed] [Google Scholar]
- 17. Miyazaki M., Bruggink S. M., Ntambi J. M. (2006) Identification of mouse palmitoyl-coenzyme A Δ9-desaturase. J. Lipid Res. 47, 700–704 [DOI] [PubMed] [Google Scholar]
- 18. Miyazaki M., Jacobson M. J., Man W. C., Cohen P., Asilmaz E., Friedman J. M., Ntambi J. M. (2003) Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J. Biol. Chem. 278, 33904–33911 [DOI] [PubMed] [Google Scholar]
- 19. Bené H., Lasky D., Ntambi J. M. (2001) Cloning and characterization of the human stearoyl-CoA desaturase gene promoter: transcriptional activation by sterol regulatory element binding protein and repression by polyunsaturated fatty acids and cholesterol. Biochem. Biophys. Res. Commun. 284, 1194–1198 [DOI] [PubMed] [Google Scholar]
- 20. Hulver M. W., Berggren J. R., Carper M. J., Miyazaki M., Ntambi J. M., Hoffman E. P., Thyfault J. P., Stevens R., Dohm G. L., Houmard J. A., Muoio D. M. (2005) Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang L., Ge L., Tran T., Stenn K., Prouty S. M. (2001) Isolation and characterization of the human stearoyl-CoA desaturase gene promoter: requirement of a conserved CCAAT cis-element. Biochem. J. 357, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beiraghi S., Zhou M., Talmadge C. B., Went-Sumegi N., Davis J. R., Huang D., Saal H., Seemayer T. A., Sumegi J. (2003) Identification and characterization of a novel gene disrupted by a pericentric inversion inv(4)(p13.1q21.1) in a family with cleft lip. Gene 309, 11–21 [DOI] [PubMed] [Google Scholar]
- 23. Wang J., Yu L., Schmidt R. E., Su C., Huang X., Gould K., Cao G. (2005) Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Commun. 332, 735–742 [DOI] [PubMed] [Google Scholar]
- 24. Zhang S., Yang Y., Shi Y. (2005) Characterization of human SCD2, an oligomeric desaturase with improved stability and enzyme activity by cross-linking in intact cells. Biochem. J. 388, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sampath H., Ntambi J. M. (2008) Role of stearoyl-CoA desaturase in human metabolic disease. Fut. Lipidol. 3, 163–173 [Google Scholar]
- 26. Sampath H., Ntambi J. M. (2005) Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 25, 317–340 [DOI] [PubMed] [Google Scholar]
- 27. Heinemann F. S., Korza G., Ozols J. (2003) A plasminogen-like protein selectively degrades stearoyl-CoA desaturase in liver microsomes. J. Biol. Chem. 278, 42966–42975 [DOI] [PubMed] [Google Scholar]
- 28. Heinemann F. S., Mziaut H., Korza G., Ozols J. (2003) A microsomal endopeptidase from liver that preferentially degrades stearoyl-CoA desaturase. Biochemistry 42, 6929–6937 [DOI] [PubMed] [Google Scholar]
- 29. Heinemann F. S., Ozols J. (1998) Degradation of stearoyl-coenzyme A desaturase: endoproteolytic cleavage by an integral membrane protease. Mol. Biol. Cell 9, 3445–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato H., Sakaki K., Mihara K. (2006) Ubiquitin-proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J. Cell Sci. 119, 2342–2353 [DOI] [PubMed] [Google Scholar]
- 31. Lee S. H., Dobrzyn A., Dobrzyn P., Rahman S. M., Miyazaki M., Ntambi J. M. (2004) Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J. Lipid Res. 45, 1674–1682 [DOI] [PubMed] [Google Scholar]
- 32. Dobrzyn P., Sampath H., Dobrzyn A., Miyazaki M., Ntambi J. M. (2008) Loss of stearoyl-CoA desaturase 1 inhibits fatty acid oxidation and increases glucose utilization in the heart. Am. J. Physiol. Endocrinol. Metab. 294, E357–E364 [DOI] [PubMed] [Google Scholar]
- 33. Miyazaki M., Sampath H., Liu X., Flowers M. T., Chu K., Dobrzyn A., Ntambi J. M. (2009) Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem. Biophys. Res. Commun. 380, 818–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sampath H., Flowers M. T., Liu X., Paton C. M., Sullivan R., Chu K., Zhao M., Ntambi J. M. (2009) Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J. Biol. Chem. 284, 19961–19973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hyun C. K., Kim E. D., Flowers M. T., Liu X., Kim E., Strable M., Ntambi J. M. (2010) Adipose-specific deletion of stearoyl-CoA desaturase 1 up-regulates the glucose transporter GLUT1 in adipose tissue. Biochem. Biophys. Res. Commun. 399, 480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flowers M. T., Paton C. M., O'Byrne S. M., Schiesser K., Dawson J. A., Blaner W. S., Kendziorski C., Ntambi J. M. (2011) Metabolic changes in skin caused by Scd1 deficiency: a focus on retinol metabolism. PLoS ONE 6, e19734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu X., Strable M. S., Ntambi J. M. (2011) Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv. Nutr. 2, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sundberg J. P., Boggess D., Sundberg B. A., Eilertsen K., Parimoo S., Filippi M., Stenn K. (2000) Asebia-2J (Scd1ab2J): a new allele and a model for scarring alopecia. Am. J. Pathol. 156, 2067–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stenn K. S. (2001) Insights from the asebia mouse: a molecular sebaceous gland defect leading to cicatricial alopecia. J. Cutan. Pathol. 28, 445–447 [DOI] [PubMed] [Google Scholar]
- 40. Flowers M. T., Ade L., Strable M. S., Ntambi J. M. (2012) Combined deletion of SCD1 from adipose tissue and liver does not protect mice from obesity. J. Lipid Res. 53, 1646–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Elias P. M., Feingold K. R. (1992) Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin. Dermatol. 11, 176–182 [PubMed] [Google Scholar]
- 42. Fluhr J. W., Mao-Qiang M., Brown B. E., Wertz P. W., Crumrine D., Sundberg J. P., Feingold K. R., Elias P. M. (2003) Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J. Invest. Dermatol. 120, 728–737 [DOI] [PubMed] [Google Scholar]
- 43. Feingold K. R. (2009) The outer frontier: the importance of lipid metabolism in the skin. J. Lipid Res. 50, S417–S422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Binczek E., Jenke B., Holz B., Günter R. H., Thevis M., Stoffel W. (2007) Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol. Chem. 388, 405–418 [DOI] [PubMed] [Google Scholar]
- 45. Feldmann H. M., Golozoubova V., Cannon B., Nedergaard J. (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209 [DOI] [PubMed] [Google Scholar]
- 46. Overton J. M., Williams T. D. (2004) Behavioral and physiologic responses to caloric restriction in mice. Physiol. Behav. 81, 749–754 [DOI] [PubMed] [Google Scholar]
- 47. Silva J. E. (2006) Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 86, 435–464 [DOI] [PubMed] [Google Scholar]
- 48. Lodhi I. J., Semenkovich C. F. (2009) Why we should put clothes on mice. Cell Metab. 9, 111–112 [DOI] [PubMed] [Google Scholar]
- 49. Miyazaki M., Man W. C., Ntambi J. M. (2001) Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J. Nutr. 131, 2260–2268 [DOI] [PubMed] [Google Scholar]
- 50. Jong M. C., Gijbels M. J., Dahlmans V. E., Gorp P. J., Koopman S. J., Ponec M., Hofker M. H., Havekes L. M. (1998) Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J. Clin. Invest. 101, 145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jong M. C., Voshol P. J., Muurling M., Dahlmans V. E., Romijn J. A., Pijl H., Havekes L. M. (2001) Protection from obesity and insulin resistance in mice overexpressing human apolipoprotein C1. Diabetes 50, 2779–2785 [DOI] [PubMed] [Google Scholar]
- 52. Yagyu H., Kitamine T., Osuga J., Tozawa R., Chen Z., Kaji Y., Oka T., Perrey S., Tamura Y., Ohashi K., Okazaki H., Yahagi N., Shionoiri F., Iizuka Y., Harada K., Shimano H., Yamashita H., Gotoda T., Yamada N., Ishibashi S. (2000) Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J. Biol. Chem. 275, 21324–21330 [DOI] [PubMed] [Google Scholar]
- 53. Padhi T., Garima (2013) Metabolic syndrome and skin: psoriasis and beyond. Ind. J. Dermatol. 58, 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lotti T., Hercogova J., Prignano F. (2010) The concept of psoriatic disease: can cutaneous psoriasis any longer be separated by the systemic comorbidities? Dermatol. Ther. 23, 119–122 [DOI] [PubMed] [Google Scholar]
- 55. Naldi L., Mercuri S. R. (2010) Epidemiology of comorbidities in psoriasis. Dermatol. Ther. 23, 114–118 [DOI] [PubMed] [Google Scholar]
- 56. Demling R. H. (2009) Nutrition, anabolism, and the wound healing process: an overview. Eplasty 9, e9. [PMC free article] [PubMed] [Google Scholar]
- 57. Guo S., Dipietro L. A. (2010) Factors affecting wound healing. J. Dent. Res. 89, 219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hart D. W., Wolf S. E., Herndon D. N., Chinkes D. L., Lal S. O., Obeng M. K., Beauford R. B., Mlcak R. P. (2002) Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann. Surg. 235, 152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carter E. A., Bonab A. A., Goverman J., Paul K., Yerxa J., Tompkins R. G., Fischman A. J. (2011) Evaluation of the antioxidant peptide SS31 for treatment of burn-induced insulin resistance. Int. J. Mol. Med. 28, 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilmore D. W., Mason A. D., Jr., Johnson D. W., Pruitt B. A., Jr., (1975) Effect of ambient temperature on heat production and heat loss in burn patients. J. Appl. Physiol. 38, 593–597 [DOI] [PubMed] [Google Scholar]
- 61. Berry D. C., Noy N. (2007) Is PPARβ/δ a retinoid receptor? PPAR Res. 2007, 73256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schug T. T., Berry D. C., Shaw N. S., Travis S. N., Noy N. (2007) Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129, 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romanowska M., Reilly L., Palmer C. N., Gustafsson M. C., Foerster J. (2010) Activation of PPARβ/δ causes a psoriasis-like skin disease in vivo. PLoS ONE 5, e9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Di-Poï N., Michalik L., Tan N. S., Desvergne B., Wahli W. (2003) The anti-apoptotic role of PPARβ contributes to efficient skin wound healing. J. Steroid Biochem. Mol. Biol. 85, 257–265 [DOI] [PubMed] [Google Scholar]
- 65. Michalik L., Desvergne B., Tan N. S., Basu-Modak S., Escher P., Rieusset J., Peters J. M., Kaya G., Gonzalez F. J., Zakany J., Metzger D., Chambon P., Duboule D., Wahli W. (2001) Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR) α and PPARβ mutant mice. J. Cell Biol. 154, 799–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Michalik L., Wahli W. (2007) Peroxisome proliferator-activated receptors (PPARs) in skin health, repair and disease. Biochim. Biophys. Acta 1771, 991–998 [DOI] [PubMed] [Google Scholar]
- 67. Peters J. M., Lee S. S., Li W., Ward J. M., Gavrilova O., Everett C., Reitman M. L., Hudson L. D., Gonzalez F. J. (2000) Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ). Mol. Cell. Biol. 20, 5119–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]