Abstract
Cholera toxin inhibits chemotaxis of the RAW264 mouse macrophage cell line. The degree of inhibition by cholera toxin increases upon incubation with the cells, suggesting that the entry of the toxin is required for inhibition of chemotaxis. In the absence of guanine nucleotides, cholera toxin catalyzes the [32P]ADP-ribosylation of RAW264 cell membrane proteins of Mr 41,000, Mr 45,000, and a doublet of Mr 48,000-50,000. GTP increases the labeling of the Mr 45,000 protein and the Mr 48,000-50,000 doublet, and it decreases the labeling of the Mr 41,000 protein. Experiments with cholera toxin treatment of intact cells indicate that the Mr 45,000 protein is the major membrane protein ADP-ribosylated by the toxin in vivo. Cholera toxin increases cAMP levels in RAW264 cells, but increased cAMP levels do not correlate with inhibition of chemotaxis, because isoproterenol and forskolin, which also increase cAMP levels, have no effect on chemotaxis.
Full text
PDF
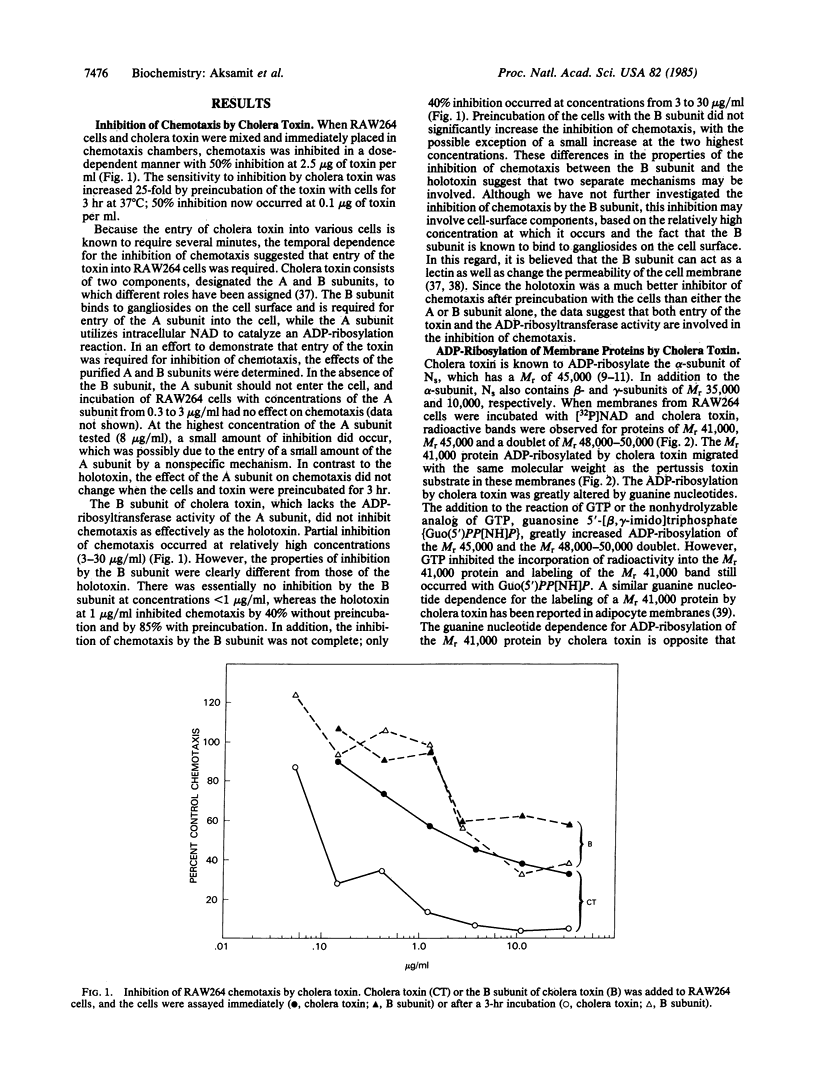

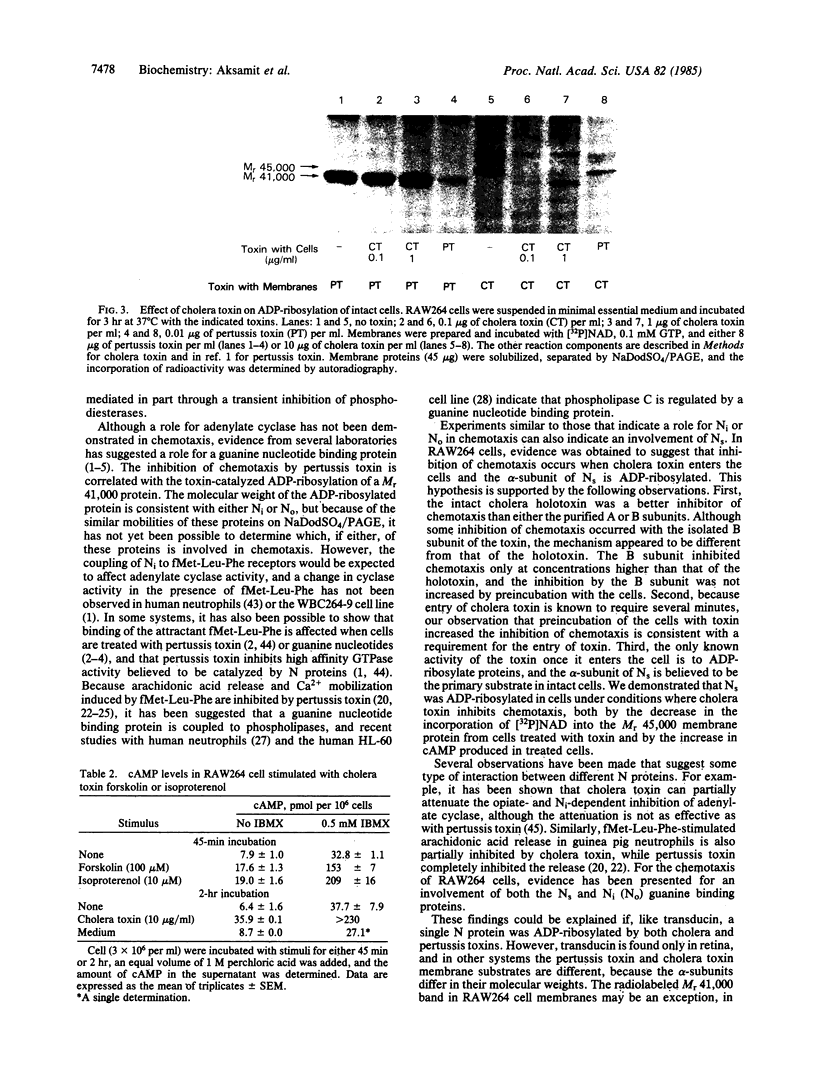

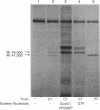
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksamit R. R., Falk W., Leonard E. J. Chemotaxis by mouse macrophage cell lines. J Immunol. 1981 Jun;126(6):2194–2199. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Meade B. D., Manclark C. R., Cantoni G. L., Aksamit R. R. Pertussis toxin inhibition of chemotaxis and the ADP-ribosylation of a membrane protein in a human-mouse hybrid cell line. Proc Natl Acad Sci U S A. 1985 May;82(9):2637–2641. doi: 10.1073/pnas.82.9.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman M. J., Guerrant R. L., Murad F., Richardson S. H., Weaver D., Mandell G. L. Interaction of polymorphonuclear neutrophils with Escherichia coli. Effect of enterotoxin on phagocytosis, killing, chemotaxis, and cyclic AMP. J Clin Invest. 1978 Feb;61(2):227–234. doi: 10.1172/JCI108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Graves C. B., Klaven N. B., McDonald J. M. Effects of guanine nucleotides on cholera toxin catalyzed ADP-ribosylation in rat adipocyte plasma membranes. Biochemistry. 1983 Dec 20;22(26):6291–6296. doi: 10.1021/bi00295a039. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Estensen R. D., Quie P. G., Hogan N. A., Goldberg N. D. Modulation of human neutrophil chemotactic responses by cyclic 3',5'-guanosine monophosphate and cyclic 3',5'-adenosine monophosphate. Metabolism. 1975 Mar;24(3):447–456. doi: 10.1016/0026-0495(75)90124-9. [DOI] [PubMed] [Google Scholar]
- Hudson T. H., Johnson G. L. Peptide mapping of adenylate cyclase regulatory proteins that are cholera toxin substrates. J Biol Chem. 1980 Aug 10;255(15):7480–7486. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee W. A., Koski G., Tocque B., Simonds W. F. On the mechanism of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:153–159. [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. Guanine nucleotides modulate the binding affinity of the oligopeptide chemoattractant receptor on human polymorphonuclear leukocytes. J Clin Invest. 1983 Sep;72(3):748–753. doi: 10.1172/JCI111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Smiley P. A. Association of the N-formyl-Met-Leu-Phe receptor in human neutrophils with a GTP-binding protein sensitive to pertussis toxin. Proc Natl Acad Sci U S A. 1985 Feb;82(3):869–873. doi: 10.1073/pnas.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Rapiejko P. J., Garciá-Sáinz J. A. Pertussis toxin catalyzes the ADP-ribosylation of two distinct peptides, 40 and 41 kDa, in rat fat cell membranes. FEBS Lett. 1984 Oct 29;176(2):301–306. doi: 10.1016/0014-5793(84)81184-9. [DOI] [PubMed] [Google Scholar]
- Marx R. S., McCall C. E., Bass D. A. Chemotaxin-induced changes in cyclic adenosine monophosphate levels in human neutrophils. Infect Immun. 1980 Jul;29(1):284–286. doi: 10.1128/iai.29.1.284-286.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade B. D., Kind P. D., Ewell J. B., McGrath P. P., Manclark C. R. In vitro inhibition of murine macrophage migration by Bordetella pertussis lymphocytosis-promoting factor. Infect Immun. 1984 Sep;45(3):718–725. doi: 10.1128/iai.45.3.718-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Molski T. F., Naccache P. H., Marsh M. L., Kermode J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits the rise in the intracellular concentration of free calcium that is induced by chemotactic factors in rabbit neutrophils: possible role of the "G proteins" in calcium mobilization. Biochem Biophys Res Commun. 1984 Oct 30;124(2):644–650. doi: 10.1016/0006-291x(84)91603-6. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983 Sep 25;258(18):11369–11376. [PubMed] [Google Scholar]
- Okajima F., Katada T., Ui M. Coupling of the guanine nucleotide regulatory protein to chemotactic peptide receptors in neutrophil membranes and its uncoupling by islet-activating protein, pertussis toxin. A possible role of the toxin substrate in Ca2+-mobilizing receptor-mediated signal transduction. J Biol Chem. 1985 Jun 10;260(11):6761–6768. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Richards R. L., Moss J., Alving C. R., Fishman P. H., Brady R. O. Choleragen (cholera toxin): a bacterial lectin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1673–1676. doi: 10.1073/pnas.76.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin I., Rosenblatt J., Becker E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975 Oct;115(4):1126–1134. [PubMed] [Google Scholar]
- Shefcyk J., Yassin R., Volpi M., Molski T. F., Naccache P. H., Munoz J. J., Becker E. L., Feinstein M. B., Sha'afi R. I. Pertussis but not cholera toxin inhibits the stimulated increase in actin association with the cytoskeleton in rabbit neutrophils: role of the "G proteins" in stimulus-response coupling. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1174–1181. doi: 10.1016/0006-291x(85)90309-2. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. Induction of a transient elevation in intracellular levels of adenosine-3',5'-cyclic monophosphate by chemotactic factors: an early event in human neutrophil activation. J Immunol. 1980 Mar;124(3):1482–1491. [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. Increased levels of cyclic adenosine-3',5'-monophosphate in human polymorphonuclear leukocytes after surface stimulation. J Clin Invest. 1980 May;65(5):1077–1085. doi: 10.1172/JCI109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Edge S., Lane B. A chemoattractant receptor on macrophages exists in two affinity states regulated by guanine nucleotides. J Cell Biol. 1984 Feb;98(2):444–448. doi: 10.1083/jcb.98.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Van Dop C., Yamanaka G., Steinberg F., Sekura R. D., Manclark C. R., Stryer L., Bourne H. R. ADP-ribosylation of transducin by pertussis toxin blocks the light-stimulated hydrolysis of GTP and cGMP in retinal photoreceptors. J Biol Chem. 1984 Jan 10;259(1):23–26. [PubMed] [Google Scholar]
- Verghese M. W., Fox K., McPhail L. C., Snyderman R. Chemoattractant-elicited alterations of cAMP levels in human polymorphonuclear leukocytes require a Ca2+-dependent mechanism which is independent of transmembrane activation of adenylate cyclase. J Biol Chem. 1985 Jun 10;260(11):6769–6775. [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. P., Rideout J. L., Wolberg G., Duncan G. S., Elion G. B. 2-Fluoroadenosine 3':5'-monophosphate. A metabolite of 2-fluoroadenosine in mouse cytotoxic lymphocytes. J Biol Chem. 1976 Nov 10;251(21):6757–6766. [PubMed] [Google Scholar]