Background: IL-8 promotes angiogenesis and metastasis in ovarian cancer.
Results: Proteasome inhibition induces specific recruitment of IKKβ, EGR-1, and S536P-p65 to the IL-8 promoter.
Conclusion: The increased IKKβ, EGR-1, and S536P-p65 recruitment results in the increased IL-8 expression and release in ovarian cancer cells.
Significance: The BZ-increased IL-8 release may be responsible for the BZ-limited effectiveness in ovarian cancer treatment.
Keywords: Chemokines, Chromatin Immunoprecipitation (ChIP), Egr-1, Immunology, NF-κB (NF-KB), Ovarian Cancer, Proteasome, Transcription Regulation, Interleukin-8
Abstract
Proinflammatory and pro-angiogenic chemokine interleukin-8 (IL-8, CXCL8) contributes to ovarian cancer progression through its induction of tumor cell proliferation, survival, angiogenesis, and metastasis. Proteasome inhibition by bortezomib, which has been used as a frontline therapy in multiple myeloma, has shown only limited effectiveness in ovarian cancer and other solid tumors. However, the responsible mechanisms remain elusive. Here, we show that proteasome inhibition dramatically increases the IL-8 expression and release in ovarian cancer cells. The responsible mechanism involves an increased nuclear accumulation of IκB kinase β (IKKβ) and an increased recruitment of the nuclear IKKβ, p65-phosphorylated at Ser-536, and the transcription factor early growth response-1 (EGR-1) to the endogenous IL-8 promoter. Coimmunoprecipitation studies identified the nuclear EGR-1 associated with IKKβ and with p65, with preferential binding to S536P-p65. Both IKKβ activity and EGR-1 expression are required for the increased IL-8 expression induced by proteasome inhibition in ovarian cancer cells. Interestingly, in multiple myeloma cells the IL-8 release is not increased by bortezomib. Together, these data indicate that the increased IL-8 release may represent one of the underlying mechanisms responsible for the decreased effectiveness of proteasome inhibition in ovarian cancer treatment and identify IKKβ and EGR-1 as potential new targets in ovarian cancer combination therapies.
Introduction
Ovarian cancer is among the leading causes of cancer death in women. Because most ovarian cancers relapse and become drug-resistant, the survival rates remain low. Progression of ovarian cancer has been associated with the increased expression of proinflammatory and proangiogenic chemokines, such as IL-8, which contribute to cancer development through their induction of tumor cell proliferation, survival, migration, and angiogenesis (1–6). At the transcriptional level, IL-8 expression is regulated by the transcription factor NFκB (7, 8). NFκB activity is constitutively increased in aggressive ovarian cancers, and inhibition of NFκB activity suppresses angiogenesis and tumorigenicity of ovarian cancer cells and increases their sensitivity to chemotherapy and apoptosis (9–12).
The increased activity of NFκB in ovarian cancer cells is mediated by the enzymes of the IκB kinase (IKK)2 complex, which phosphorylate IκBα, resulting in the proteasomal degradation of IκBα and nuclear translocation and accumulation of NFκB subunits (13–16). In addition to phosphorylating IκBα, IKKs can also phosphorylate the NFκB subunits, particularly p65 (17, 18). Although the cytoplasmic degradation of IκBα, resulting in the nuclear translocation of NFκB subunits, represents a general step in NFκB activation, the specificity of NFκB-regulated responses is mediated by the subunit composition of NFκB complexes and their post-translational modifications (19, 20).
Inhibition of the proteasomal degradation of IκBα has been used as a rationale for development of anti-cancer therapies targeting the expression of NFκB-dependent proinflammatory and anti-apoptotic genes (21–24). Bortezomib (BZ, Velcade, PS-341) is the first Food and Drug Administration-approved proteasome inhibitor that has been developed as a selective inhibitor of the chymotrypsin-like activity of the 26 S proteasome and has shown remarkable anti-tumor activity in multiple myeloma and other hematological malignancies (21–26). In solid tumors, including ovarian carcinoma, BZ has been less effective as a single agent; however, the mechanisms remain largely unknown (27–30). Nevertheless, BZ has been considered in combination with other therapies, especially cisplatin, as it prevents the cisplatin-induced degradation of cisplatin influx transporter, resulting in enhanced cisplatin uptake and tumor killing (31, 32). Thus, understanding the mechanisms responsible for the resistance of ovarian carcinoma and other solid tumors to proteasome inhibition may lead to the development of more effective combination therapies.
We have recently shown that in metastatic prostate cancer cells, bortezomib increases the IL-8 expression (33). This study was undertaken to investigate the mechanism of BZ resistance in ovarian cancer cells. Here we show that in ovarian carcinoma cells, proteasome inhibition also increases the expression and release of IL-8. However, the mechanism is different from prostate cancer cells and involves an increased nuclear accumulation of IKKβ and a gene-specific recruitment of IKKβ, Ser-536-phosphorylated p65, and the transcription factor early growth response-1 (EGR-1) to the endogenous IL-8 promoter. Interestingly, however, we found that in contrast to prostate and ovarian cancer cells, BZ does not increase the IL-8 release in multiple myeloma cells. Taken together, these data indicate that the increased IL-8 release induced by proteasome inhibition may represent one of the mechanisms responsible for the decreased effectiveness of BZ in ovarian cancer and other solid tumors and suggest that targeting IKKβ and EGR-1may lead to new combination therapies for ovarian cancer.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Purified polyclonal antibodies against human IκBα (sc-371), p65 NFκB (sc-372), Ser-536-phosphorylated p65 NFκB (sc-33020), p50 NFκB (sc-7178), EGR-1 (sc-189), IKKα (SC-7218), IKKβ (sc-8014), and lamin B (sc-6216) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Purified polyclonal antibody against lactate dehydrogenase (LDH; 20-LG22) was from Fitzgerald Industries International (Concord, MA), and actin antibody was from Sigma. Horseradish peroxidase-conjugated anti-rabbit, anti-mouse, and anti-goat secondary antibodies were from Santa Cruz Biotechnology.
Bortezomib was obtained from ChemieTek (Indianapolis, IN). MG132 and the IKK inhibitors PS-1145 and Bay-117082 were purchased from Sigma. All other reagents were molecular biology grade and were from Sigma.
Cell Culture
All cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA). Ovarian cancer OVCAR3 and SKOV3 cells were cultured in RPMI 1640 medium (Invitrogen) supplemented with 20% heat inactivated fetal bovine serum (FBS; Invitrogen) and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). Multiple myeloma IM-9 and RPMI-8226 were grown in RPMI 1640 medium with 10% FBS and antibiotics. Before treatment, cells were seeded (5 × 105 cells/ml) for 24 h in 6-well plates and grown at 37 °C with 5% CO2. Bortezomib, MG132, and IKK inhibitors were dissolved in DMSO and stored at −80 °C. An equivalent volume of DMSO was used in all experiments as a solvent control.
Western Analysis of Cytoplasmic and Nuclear Extracts
Nuclear (NE) and cytoplasmic extracts (CE) were prepared as described previously (34–36). Contamination of nuclear and cytoplasmic fractions by cytoplasmic and nuclear proteins, respectively, was determined by Western analysis using LDH and lamin B as specific markers. Denatured proteins were separated on 12% denaturing polyacrylamide gels, and immunoblotting analysis was performed as described (36).
Proteasome Activity Assay
Activity of the 20 S catalytic proteasomal core unit was measured in whole cell extracts by using the 20 S Proteasome Activity Assay kit (Chemicon, APT280; Temecula, CA) as described by the manufacturer. Briefly, cells were lysed in a lysis buffer (50 mm Hepes, pH 7.5, 5 mm EDTA, 150 mm NaCl, 1% Triton X-100) for 30 min on ice, and the lysates were collected by centrifugation (10,000 × g, 15 min, 4 °C). The cell lysates were incubated (2 h, 37 °C) with a labeled substrate, LLVY-7-amino-4-methylcoumarin, and the cleavage activity was monitored by detection of the free fluorophore 7-amino-4-methylcoumarin using a fluorescence plate reader (Berthold Mithras LB940, Berthold Technologies) at 355/460 nm.
Real Time PCR
Total RNA was isolated by using RNeasy mini-kit (Qiagen, Valencia, CA). The iScript one-step RT-PCR kit with SYBR Green (Bio-Rad) was used as a supermix, and 20 ng/μl RNA was used as template on a Bio-Rad MyIQ Single Color Real-time PCR Detection System (Bio-Rad). The primers used for quantification of IL-8, CCL2, CXCL5, IL-6, TNFα, and actin mRNA were purchased from SA Biosciences (Frederick, MD).
ELISA
Cytokine release was measured in cell culture supernatants by commercially available ELISA kits (R&D, Minneapolis, MN) as previously described (36).
Transfection with siRNA
Human EGR-1 (sc-29303) and non-silencing (sc-37007) small interfering RNAs (siRNAs) were obtained from Santa Cruz Biotechnology. Before transfection, cells were seeded into a 6-well plate and incubated in a humidified 5% CO2 atmosphere at 37 °C in antibiotic-free RPMI medium supplement with 20% FBS for 24 h to 80% confluence. For each transfection, 80 pmol of either non-silencing siRNA-A control or EGR-1 siRNA (Santa Cruz Biotechnology) were used. The cells were transfected for 6 h in siRNA transfection medium (sc-36868) with siRNA transfection reagent (sc-29528) according to manufacturer's instructions (Santa Cruz Biotechnology). After transfection, fresh medium with antibiotics was added, and the cells were grown for 24 h before BZ treatment.
Chromatin Immunoprecipitation (ChIP)
ChIP analyses were performed as described (37). Proteins and DNA were cross-linked by adding formaldehyde to the growth medium to a final concentration of 1% for 15 min at 37 °C, and glycine was added at a final concentration of 0.125 m to neutralize formaldehyde. Cells were washed with PBS containing protease inhibitors and collected by centrifugation. Cells were then resuspended in SDS lysis buffer, incubated at 4 °C for 10 min, and sonicated. The lysates were centrifuged at 15,000 × g for 10 min at 4 °C, and the supernatant extracts were diluted with ChIP dilution buffer and precleared with protein A/G-agarose (Santa Cruz, CA) for 30 min at 4 °C. Immunoprecipitation was performed overnight at 4 °C with specific antibodies. After immunoprecipitation, the samples were incubated with protein A/G-agarose for 1 h, and the immune complexes were collected by centrifugation (150 × g at 4 °C), washed, and eluted with 1% SDS, 0.1 m NaHCO3. After reversing the cross-linking, proteins were digested with proteinase K, and the samples were extracted with phenol/chloroform followed by precipitation with ethanol. The pellets were resuspended in nuclease-free water and subjected to real time PCR. Immunoprecipitated DNA was analyzed by real-time PCR (25 μl reaction mixture) using the iQ SYBR Green Supermix and the Bio-Rad MyIQ Single Color Real-Time PCR Detection System (Bio-Rad). Each immunoprecipitation was performed four times using different chromatin samples, and the occupancy was calculated by using the ChIP-qPCR Human IGX1A Negative Control Assay (SA Biosciences) as a negative control and corrected for the efficiency of the primers, which detect specific genomic DNA sequences within ORF-free intergenic regions or “promoter deserts” lacking any known or predicted structural genes. The primers used for real time PCR were the following: IL-8, forward (5′-GGGCCATCAGTTGCAAATC-3′) and reverse (5′-GCTTGTGTGCTCTGCTGTCTC-3′); TNFα, forward (5′-CGCTTCCTCCAGATGAGCTT-3′) and reverse (5′-TGCTGTCCTTGCTGAGGGA-3′); IL-6, forward (5′-CCTCAGACATCTCCAGTCCT-3′) and reverse (5′-AATGACGACCTAAGCTGCAC-3′); CCL2, forward (5′-CCCAGTATCTGGAATGCAGG-3′) and reverse (5′-TCTGCCTCCCACTTCTGCT-3′); CXCL5, forward (5′-CCCAACCTCTTCTTTCCACAC-3′) and reverse (5′-GGAGCGAAGATTGGAGGATC-3′).
Immunoprecipitation
Nuclear extracts were prepared by using Active Motif's Nuclear Complex Co-IP kit (Active Motif, 54001; Carlsbad, CA) as described previously (34). The nuclear extracts were incubated for 12 h at 4 °C with EGR-1 (sc-189X) or control pre-immune IgG (sc-2027) antibodies that had been cross-linked with A/G Plus Agarose (sc-2003). After washing, the bound proteins were eluted with 1 m glycine buffer, pH 2.5, followed by neutralization with 1 m Tris-HCl, pH 8.0. The samples were boiled for 5 min in 5× SDS sample buffer, resolved on 10% SDS gels, and detected by using EGR-1-, p65-, S536P-p65-, IKKβ-, and IKKα-specific antibodies.
Statistical Analysis
The results represent at least three independent experiments. Numerical results are presented as the means ± S.E. Data were analyzed by using an InStat software package (GraphPAD, San Diego, CA). Statistical significance was evaluated by using the Mann-Whitney U test with Bonferroni correction for multiple comparisons, and p < 0.05 was considered significant.
RESULTS
Proteasome Inhibition Induces Nuclear Accumulation of p65 NFκB and IκBα in Ovarian Cancer Cells
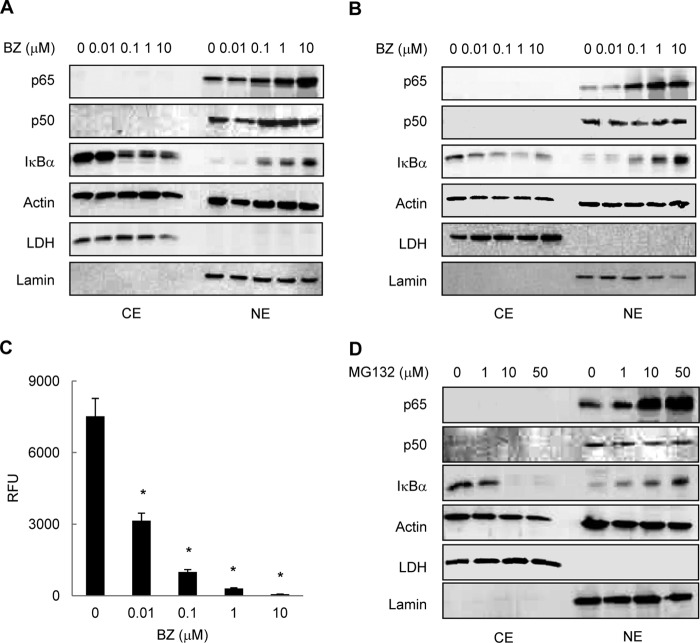
To understand the mechanism of how proteasome inhibition regulates the NFκB-dependent transcription in ovarian cancer cells, we first analyzed the nuclear-cytoplasmic distribution of NFκB proteins in human ovarian cancer OVCAR3 and SKOV3 cells treated with increasing concentrations of bortezomib. Both NFκB p65 and p50 proteins were localized predominantly in the nucleus in OVCAR3 (Fig. 1A) and SKOV3 (Fig. 1B) cells; this is consistent with the high constitutive NFκB activity in these cells. Cell incubation with increasing BZ concentrations for 24 h further increased the nuclear levels of p65 both in OVCAR3 (Fig. 1A) and SKOV3 (Fig. 1B) cells, likely caused by inhibition of the previously reported p65 proteasomal degradation (38). In addition to p65, BZ induced the nuclear translocation and accumulation of IκBα in OVCAR3 (Fig. 1A) and SKOV3 (Fig. 1B) cells. This was consistent with our previous studies demonstrating that in prostate cancer and leukemia cells, proteasome inhibition induces translocation of IκBα from the cytoplasm to the nucleus, resulting in a gene-specific repression of NFκB-dependent transcription (33–35). In contrast to p65 or IκBα, BZ did not have any pronounced effect on the nuclear levels of p50 in OVCAR3 and SKOV3 cells (Fig. 1, A and B). BZ also did not have any effect on actin levels or on the nuclear-cytoplasmic distribution of LDH and lamin, which were used as cytoplasmic and nuclear markers, respectively (Fig. 1, A and B).
FIGURE 1.
Proteasome inhibition induces nuclear accumulation of p65 NFκB and IκBα in ovarian cancer cells. Shown is Western blotting of CEs and NEs prepared from OVCAR3 (A) and SKOV3 (B) cells incubated with increasing concentrations of BZ for 24 h and analyzed by using p65, p50, and IκBα antibodies. To confirm equal protein loading, the membranes were stripped and re-probed with actin antibody. The presence of cytoplasmic proteins in nuclear fraction was evaluated by re-probing the membrane with LDH antibody. Nuclear contamination in the cytoplasmic fraction was assessed by using lamin B-specific antibody. Each lane corresponds to ∼5 × 104 cells. C, The 26 S proteasome activity was measured in whole cell extracts prepared from OVCAR3 cells incubated 24 h with increasing BZ concentrations. The activity is expressed as relative fluorescence units (RFU) of BZ-treated cells compared with untreated cells. The values represent the mean ± S.E. of four experiments; asterisks denote a statistically significant (p < 0.05) inhibition compared with control untreated cells. D, Western blotting of CE and NE prepared from OVCAR3 cells incubated 24 h with increasing concentrations of MG132 and analyzed by using p65, p50, IκBα, and control actin, LDH, and lamin B antibodies as described above. Each lane corresponds to ∼5 × 104 cells.
To verify that BZ inhibits the proteasome activity in ovarian cancer cells, we measured the activity of the 20 S proteasome catalytic subunit in whole cell extracts prepared from OVCAR3 cells incubated 24 h with increasing concentrations of BZ. As shown in Fig. 1C, 0.1 μm BZ, which approximately corresponds to the clinically used BZ concentrations in cancer patients (39), inhibited ∼90% of the proteasome activity. Importantly, the nuclear accumulation of p65 NFκB and IκBα induced by proteasome inhibition in ovarian cancer cells was not limited to BZ, as MG132 had a similar effect (Fig. 1D).
Proteasome Inhibition Increases Gene Expression of Chemokines IL-8, CCL2, and CXCL5, Whereas It Does Not Affect Expression of TNFα and IL-6 in Ovarian Cancer Cells
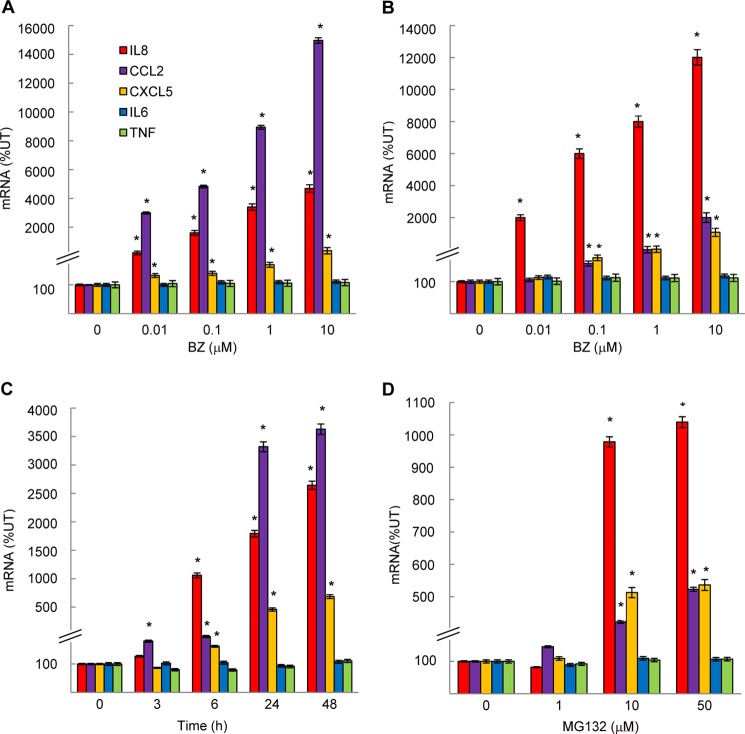
To determine whether the increased nuclear p65 accumulation induced by proteasome inhibition was associated with increased expression of NFκB-dependent genes in ovarian cancer cells, we first analyzed mRNA levels of IL-8, IL-6, and TNFα. BZ significantly increased the IL-8 expression in OVCAR3 (Fig. 2A) and SKOV3 (Fig. 2B) cells without having any significant effect on mRNA levels of TNFα or IL-6. Compared with untreated cells, the IL-8 expression induced by 0.1 μm BZ (24 h) increased ∼20-fold in OVCAR3 cells (Fig. 2A) and 60-fold in SKOV3 cells (Fig. 2B). The increased IL-8 mRNA expression induced by proteasome inhibition was time-dependent (Fig. 2C).
FIGURE 2.
Proteasome inhibition increases gene expression of chemokines IL-8, CCL2, and CXCL5, whereas it does not affect expression of TNFα and IL-6 in ovarian cancer cells. Shown is real time RT-PCR analysis of mRNA levels of IL-8, CCL2, CXCL5, IL-6, and TNFα genes measured in OVCAR3 (A) and SKOV3 (B) cells incubated with 0–10 μm BZ for 24 h. C, real time RT-PCR analysis of IL-8, CCL2, CXCL5, IL-6, and TNFα mRNA levels in OVCAR3 cells incubated with 0.1 μm BZ for up to 48 h. D, RT-PCR of IL-8, CCL2, CXCL5, IL-6, and TNFα mRNA levels in OVCAR3 cells incubated 24 h with increasing concentrations of MG132. The values represent the mean ± S.E. of four experiments; asterisks denote a statistically significant (p < 0.05) inhibition compared with control untreated (UT) cells.
Because the proximal NFκB binding site in human IL-8 promoter (GGAATTTCC) differs from the NFκB binding sites in IL-6 (GGGATTTTC) and TNFα (GGGTATCCT) promoters by having A instead of G in the third position, we searched for other NFκB-regulated genes that would have the same NFκB binding site as IL-8. We found two genes that have the same NFκB binding sequence as IL-8: the pro-survival and pro-angiogenic chemokines CCL2 (also called monocyte chemoattractant protein-1; MCP-1) and CXCL5 (epithelial-neutrophil activating peptide (ENA-78)). Interestingly, both CCL2 and CXCL5 mRNA levels were also significantly increased by BZ in OVCAR3 (Fig. 2, A and C) and SKOV3 (Fig. 2B) cells.
To determine whether other proteasome inhibitors also increase the IL-8, CCL2, and CXCL5 mRNA levels in ovarian cancer cells, we tested the effect of MG132 on cytokine gene expression in OVCAR3 cells. Although BZ is a specific inhibitor of the β5 subunit chymotrypsin-like activity, MG132 targets all three proteasomal β subunits and their activities (25, 26). As shown in Fig. 2D, 10 and 50 μm MG132 significantly increased the IL-8, CCL2, and CXCL5 mRNA levels in OVCAR3 cells even though to a lesser extent than BZ (Fig. 2A). IL-6 and TNFα mRNA levels were again unaffected (Fig. 2D). These results demonstrate that the increased chemokine expression is not limited to BZ, but other proteasome inhibitors induce chemokine mRNA levels as well. Interestingly, however, the effect of MG132 on CCL2 expression in OVCAR3 cells (Fig. 2D) was only modest compared with the BZ effect (Fig. 2A). Because in addition to having different specificity, BZ and MG132 have different chemical and metabolic stability (40, 41), these data suggest that different proteasome activities and/or different kinetics are important for the increased IL-8, CCL2, and CXCL5 gene expression in ovarian cancer cells.
Proteasome Inhibition Specifically Increases IL-8 Release in Ovarian Cancer Cells
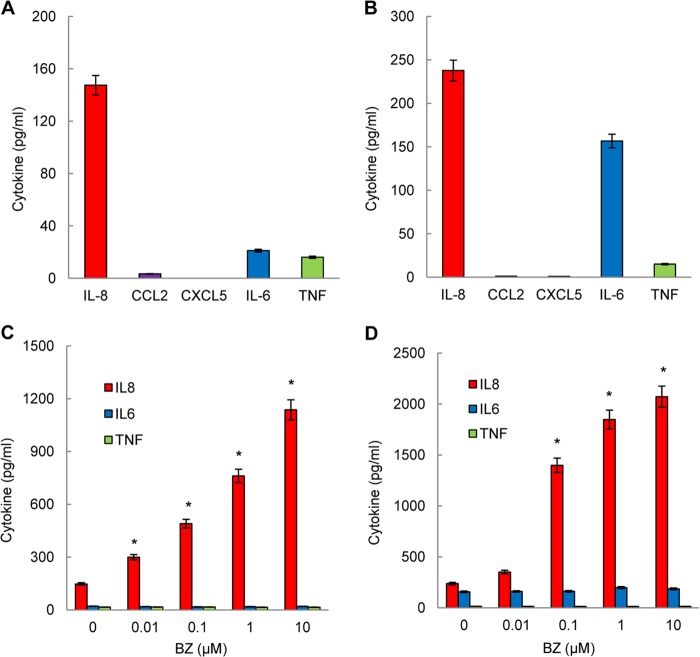
Because proteasome inhibition greatly increased the IL-8, CCL2, and CXCL5 mRNA levels in ovarian cancer cells, we analyzed whether these chemokines are secreted by OVCAR3 (Fig. 3A) and SKOV3 (Fig. 3B) cells and whether BZ increases their release (Fig. 3, C and D). Both untreated ovarian cancer cell lines released considerable amounts of IL-8; SKOV3 cells (Fig. 3B) released approximately two times more IL-8 than OVCAR3 cells (Fig. 3A). Interestingly, however, neither cell type released appreciable amounts of CCL2 or CXCL5, whereas they did release IL-6 and TNFα (Fig. 3, A and B). Proteasome inhibition by BZ significantly increased the IL-8 release both in OVCAR3 (Fig. 3C) and SKOV3 (Fig. 3D) cells. However, in contrast to IL-8, BZ did not induce CCL2 or CXCL5 release (data not shown) and did not affect the IL-6 and TNFα secretion in both cell types (Fig. 3, C and D). Thus, proteasome inhibition by BZ specifically increases the IL-8 release in human ovarian cancer cells.
FIGURE 3.
Proteasome inhibition specifically increases IL-8 release in ovarian cancer cells. Cytokine release was measured in cell culture supernatants of untreated (24 h) OVCAR3 (A) and SKOV3 (B) cells (5 × 105 cells/ml) by ELISA. ELISA of cytokine release measured in cell culture supernatants from OVCAR3 (C) and SKOV3 (D) cells were treated (24 h) with increasing BZ concentrations. The values represent the mean ± S.E. of four experiments; asterisks denote a statistically significant (p < 0.05) change compared with control (no BZ) cells.
The Increased IL-8 Expression Induced by Proteasome Inhibition in Ovarian Cancer Cells Is Mediated by IKKβ
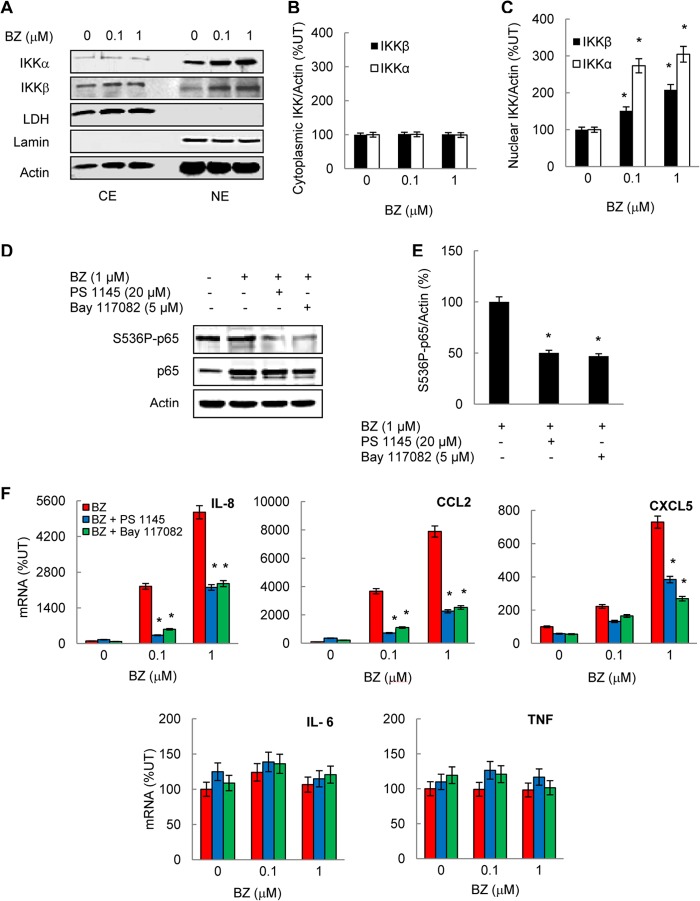
To understand the mechanism of how proteasome inhibition increases the IL-8 expression in ovarian cancer cells, we first investigated the IKK involvement and analyzed the cytoplasmic and nuclear levels of IKKα and IKKβ by Western blotting. Both IKKα and IKKβ were localized in the cytoplasm and in the nucleus (Fig. 4A). Interestingly, however, whereas the cytoplasmic levels of IKKα and IKKβ were not changed (Fig. 4B), BZ significantly increased their nuclear levels (Fig. 4C), indicating that proteasome inhibition may prevent the nuclear degradation of IKKα and IKKβ in ovarian cancer cells.
FIGURE 4.
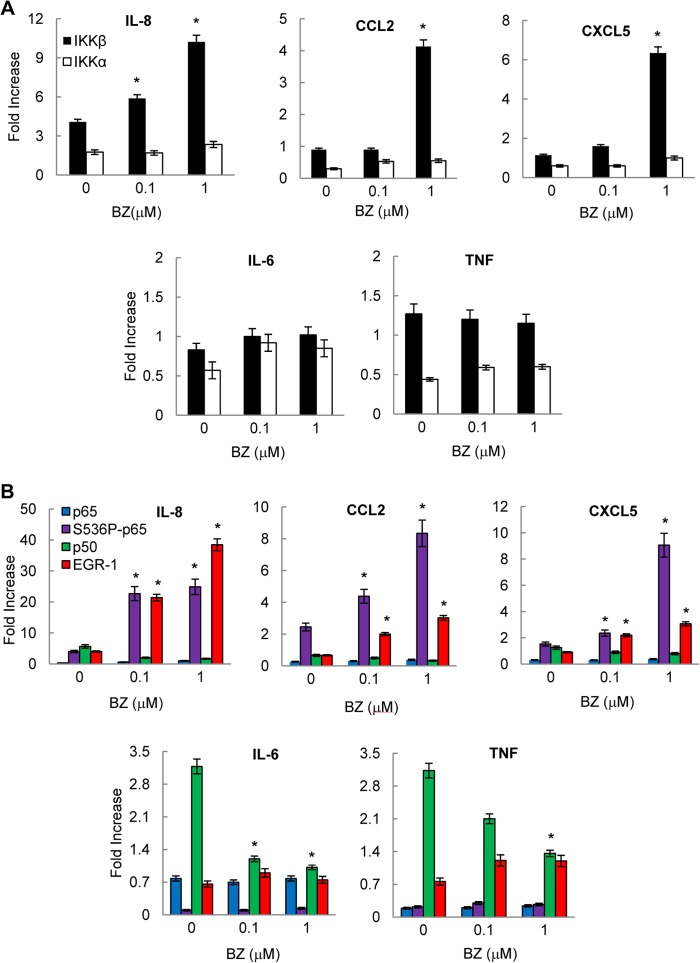
The increased IL-8, CCL2, and CXCL5 gene expression induced by proteasome inhibition in ovarian cancer cells is mediated by IKKβ. A, Western blotting of CE and NE prepared from OVCAR3 cells treated with 0, 0.1, and 1 μm BZ for 24 h and analyzed by using IKKα, IKKβ, and control LDH, lamin B, and actin antibodies. Each lane corresponds to ∼5 × 104 cells. Densitometric evaluation of IKKβ and IKKα levels in cytoplasmic (B) and nuclear (C) extracts of BZ-treated OVCAR3 cells is shown. IKK bands were scanned, and their densities were normalized to actin. The values for CEs and NEs of untreated cells were arbitrarily set to 100%, and the other values are presented relative to these values. The data represent the means of three experiments ±S.E. D, Western blotting of NE prepared from OVCAR3 cells pretreated 12 h with 20 μm PS-1145 or 5 μm Bay-117082 before 24 h incubation with 0 and 1 μm BZ, analyzed by using S536P-p65, p65 and actin antibodies. Each lane corresponds to ∼5 × 104 cells. E, densitometric evaluation of S536P-p65 levels in nuclear extracts analyzed by Western blotting in panel D. The values for PS1145 and Bay117082 inhibitors were compared with the value for BZ alone, which was considered 100%. F, real time RT-PCR analysis of IL-8, CCL2, CXCL5, IL-6, and TNFα mRNA levels in OVCAR3 cells pretreated 12 h with 20 μm PS-1145 or 5 μm Bay-117082 before 24 h of incubation with 0, 0.1, and 1 μm BZ. The values represent the mean ± S.E. of four experiments; asterisks denote a statistically significant (p < 0.05) inhibition compared with control BZ-treated cells. UT, untreated.
To determine whether the increased nuclear levels of IKKα and/or IKKβ are associated with the increased mRNA levels of IL-8 (and CCL2 and CXCL5) induced by proteasome inhibition, we analyzed the effect of IKK inhibition on the gene expression of IL-8, CCL2, and CXCL5 in ovarian cancer cells. We used two inhibitors of IKK: PS-1145, which is a specific inhibitor of IKKβ, and Bay-117082, which is a broad-spectrum inhibitor of IKK kinases (21, 42). Both inhibitors suppressed Ser-536 phosphorylation of p65 in the nucleus (Fig. 4D), which is in agreement with a previous study demonstrating that both IKKα and IKKβ can phosphorylate p65 at Ser-536 (43). In OVCAR3 cells incubated for 24 h with 1 μm BZ, a 12 h preincubation with 20 μm PS-1145 or 5 μm Bay-117082 inhibited p65 phosphorylation by ∼50% (Fig. 4E). This is consistent with the previously reported PS-1145 and Bay-117082 IC50 values for IκBα phosphorylation and NFκB-dependent transcription in prostate cancer cells (42, 44, 45). Both inhibitors also significantly suppressed the BZ-induced IL-8, CCL2, and CXCL5 mRNA levels in OVCAR3 (Fig. 4F) and SKOV3 (data not shown) cells, whereas they did not have any substantial effect on TNFα or IL-6 expression (Fig. 4F). In cells treated with 1 μm BZ, 20 μm PS-1145, and 5 μm Bay-117082 suppressed ∼50% of IL-8 mRNA levels. These data indicated that the increased IL-8 expression induced by proteasome inhibition in ovarian cancer cells is mediated by IKKβ.
Proteasome Inhibition Specifically Increases IKKβ, S536P-p65, and EGR-1 Recruitment to IL-8 Promoter
The above data suggested that the nuclear IKKβ regulates the IL-8 expression in ovarian cancer cells. We hypothesized that if the nuclear IKKβ is involved in the BZ-increased IL-8 (and CCL2 and CXCL5) mRNA expression, then it would be recruited to IL-8, CCL2, and CXCL5 promoters but not to TNFα or IL-6 promoters. Thus, we analyzed IKKβ and IKKα occupancy at the endogenous IL-8, CCL2, CXCL5, IL-6, and TNFα promoters in OVCAR3 cells treated 24 h with 0, 0.1, and 1 μm BZ. As shown in Fig. 5A, proteasome inhibition significantly increased IKKβ, but not IKKα, recruitment to IL-8, CCL2, and CXCL5 promoters, whereas it did not have any significant effect on IKKβ or IKKα recruitment to IL-6 or TNFα promoters (Fig. 5A).
FIGURE 5.
Proteasome inhibition specifically increases IKKβ, S536P-p65, and EGR-1 recruitment to the NFκB binding site in human IL-8, CCL2, and CXCL5 promoters. Recruitment of IKKβ and IKKα (A) and p65, S536P-p65, p50, and EGR-1 (B) to endogenous IL-8, CCL2, CXCL5, IL-6, and TNFα promoters was analyzed by ChIP and quantified by real time PCR in OVCAR3 cells treated 24 h with 0, 0.1, and 1 μm BZ. The data are presented as the change in occupancy over the human IGX1A (SA Biosciences) sequence control and represent the mean ± S.E. of four experiments. Asterisks denote a statistically significant (p < 0.05) inhibition compared with control untreated cells.
Because IKKβ phosphorylates p65 at Ser-536 (43), we wanted to determine whether the increased IKKβ occupancy at IL-8 promoter is associated with elevated S536P-p65 recruitment. In addition to Ser-536-phosphorylated p65 NFκB, we analyzed recruitment of p65, p50, and the transcription factor EGR-1, which was shown to regulate IL-8 expression in other cell types (46, 47). As shown in Fig. 5B, proteasome inhibition did not change p65 recruitment to any of these promoters, whereas it inhibited p50 recruitment to IL-6 and TNFα promoters. In contrast to p65, proteasome inhibition greatly increased the recruitment of S536P-p65 to IL-8 (and CCL2 and CXCL5) promoters, but it did not affect the low S536P-p65 occupancy at IL-6 and TNFα promoters (Fig. 5B). Interestingly, proteasome inhibition also significantly increased EGR-1 recruitment to IL-8, CCL2, and CXCL5 promoters but not to IL-6 or TNFα promoters (Fig. 5B). Together, these data demonstrated that in ovarian cancer cells the proteasome inhibition by BZ specifically increases IKKβ, S536P-p65, and EGR-1 recruitment to IL-8, CCL2, and CXCL5 promoters.
The Nuclear EGR-1 Associates with p65, S536P-p65, and IKKβ in Ovarian Cancer Cells, and Proteasome Inhibition Further Increases the Nuclear EGR-1, S536P-p65, and IKKβ Interaction
The increased IKKβ, S536P-p65, and EGR-1 occupancy at the IL-8 promoter indicated that IKKβ, S536P-p65, and EGR-1 interact in the nucleus of ovarian cancer cells. To investigate this possibility, we analyzed the nuclear EGR-1, S536P-p65, and IKKβ interaction by co-immunoprecipitation using EGR-1 antibody. As shown in Fig. 6A, EGR-1 was immunoprecipitated from the nuclear extracts of untreated and BZ (1 μm; 24 h)-treated OVCAR3 cells by using EGR-1 but not pre-immune control IgG antibody. The nuclear EGR-1 was complexed with p65 NFκB. However, although the nuclear levels of p65 were increased by BZ (Figs. 6A and 1A), the amount of co-immunoprecipitated p65 in BZ-treated cells was ∼50% lower compared with untreated cells (Fig. 6B). In contrast, the amount of co-immunoprecipitated S536P-p65 was increased by BZ by ∼40% compared with untreated cells (Fig. 6B), indicating that EGR-1 preferentially binds p65 phosphorylated at Ser-536, and BZ further increases this interaction. In addition, EGR-1 co-immunoprecipitated with the nuclear IKKβ, but not IKKα (Fig. 6A), and BZ further increased the EGR1-IKKβ interaction by ∼100% (Fig. 6B). These data indicated that BZ increases the nuclear interaction of IKKβ, S536P-p65, and EGR-1, resulting in their increased association with the IL-8 promoter and IL-8 expression.
FIGURE 6.
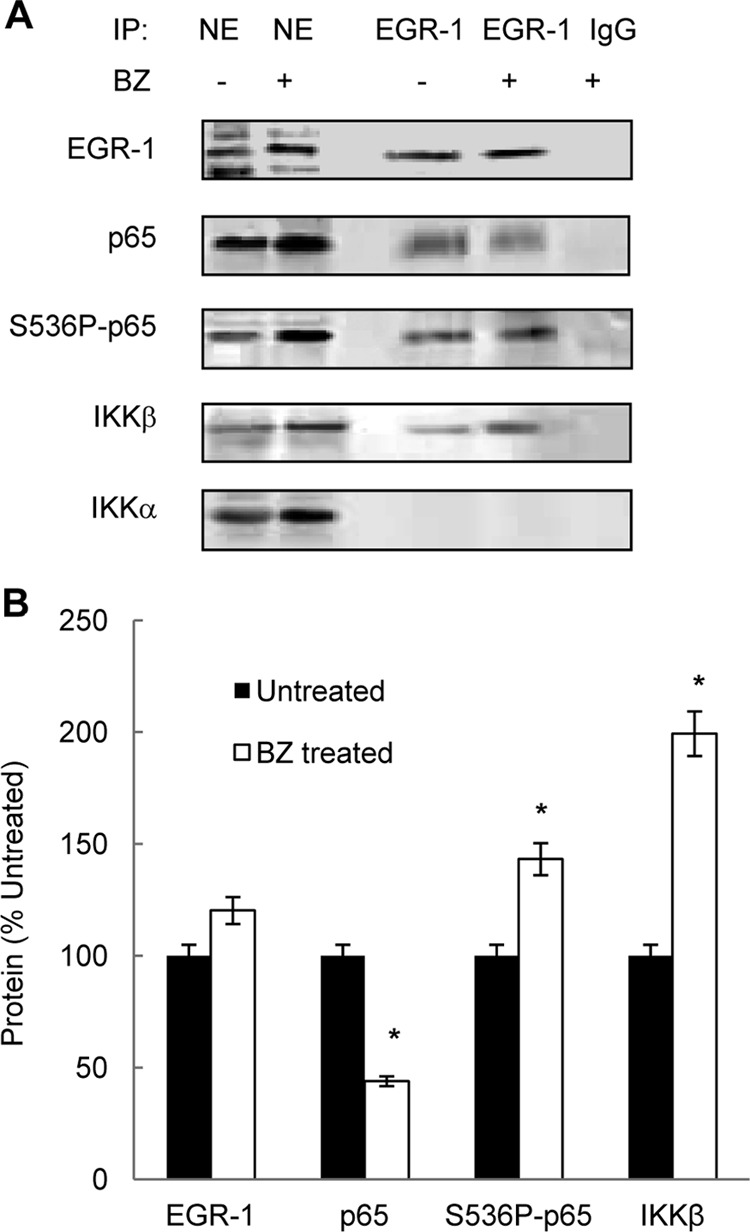
The nuclear EGR-1 associates with p65, S536P-p65, and IKKβ in ovarian cancer cells, and proteasome inhibition further increases the nuclear EGR-1, S536P-p65, and IKKβ interaction. A, nuclear extracts from untreated or BZ-treated (1 μm, 24 h) OVCAR3 cells were immunoprecipitated (IP) using EGR-1 or control pre-immune IgG antibodies and analyzed by Western blotting using EGR-1, p65, S536P-p65, and IKKβ- and IKKα-specific antibodies. B, densitometric evaluation of EGR-1, p65, S536P-p65, and IKKβ levels in the immunoprecipitated samples shown in A. The values represent the mean ± S.E. of four experiments; asterisks denote a statistically significant (p < 0.05) change compared with corresponding immunoprecipitated samples from untreated cells.
The Increased IL-8 Expression Induced by Proteasome Inhibition in Ovarian Cancer Cells Is Mediated by EGR-1
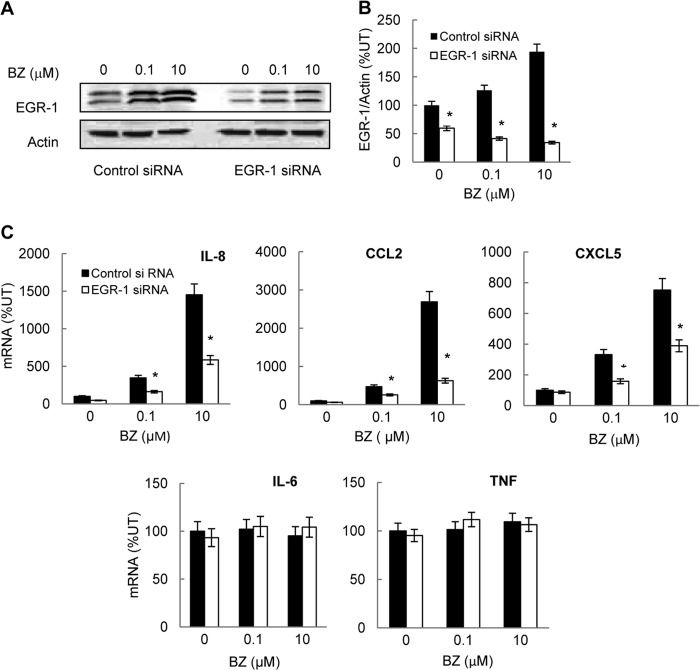
Because proteasome inhibition increased the nuclear binding of EGR-1, S536P-p65, and IKKβ (Fig. 6) and the EGR-1 recruitment to IL-8 promoter (Fig. 5B), we wanted to determine whether EGR-1 is required for the increased IL-8 expression. To this end, OVCAR3 cells were transfected with EGR-1 siRNA or control siRNA and then incubated 24 h with 0, 0.1, and 10 μm BZ. As shown in Fig. 7, A and B, cells transfected with EGR-1-specific siRNA exhibited a significant reduction in EGR-1 protein levels compared with cells transfected with control siRNA. Importantly, the EGR-1 suppression resulted in a greatly reduced IL-8 (and CCL2 and CXCL5) mRNA levels in BZ-treated cells compared with cells transfected with control siRNA (Fig. 7C). In contrast, EGR-1 suppression did not have any considerable effect on the expression of IL-6 or TNFα (Fig. 7C). Thus, the increased IL-8 expression induced by proteasome inhibition in ovarian cancer cells is mediated at least partly by EGR-1.
FIGURE 7.
The increased IL-8, CCL2, and CXCL5 gene expression induced by proteasome inhibition in ovarian cancer cells is mediated by EGR-1. A, Western analysis of EGR-1 and control actin levels in whole cell extracts prepared from OVCAR3 cells transfected with control and EGR-1-specific siRNA and treated 24 h with 0, 0.1, and 10 μm BZ. B, densitometric evaluation of EGR-1 levels in whole cell extracts of OVCAR3 cells transfected with control and EGR-1 siRNA as described in A. The EGR-1 band densities were normalized to actin. The data represent the mean of three experiments ± S.E. C, real time RT-PCR analysis of IL-8, CCL2, CXCL5, IL-6, and TNFα mRNA levels in OVCAR3 cells transfected with control or EGR-1 siRNA and incubated 24 h with 0, 0.1 and 10 μm BZ. The values represent the mean ± S.E. of four experiments; asterisks denote a statistically significant (p < 0.05) inhibition compared with cells transfected with control siRNA. UT, untreated.
IKKβ Activity Is Required for the Increased EGR-1, IKKβ, and S536P-p65 Recruitment to IL-8 Promoter in Ovarian Cancer Cells
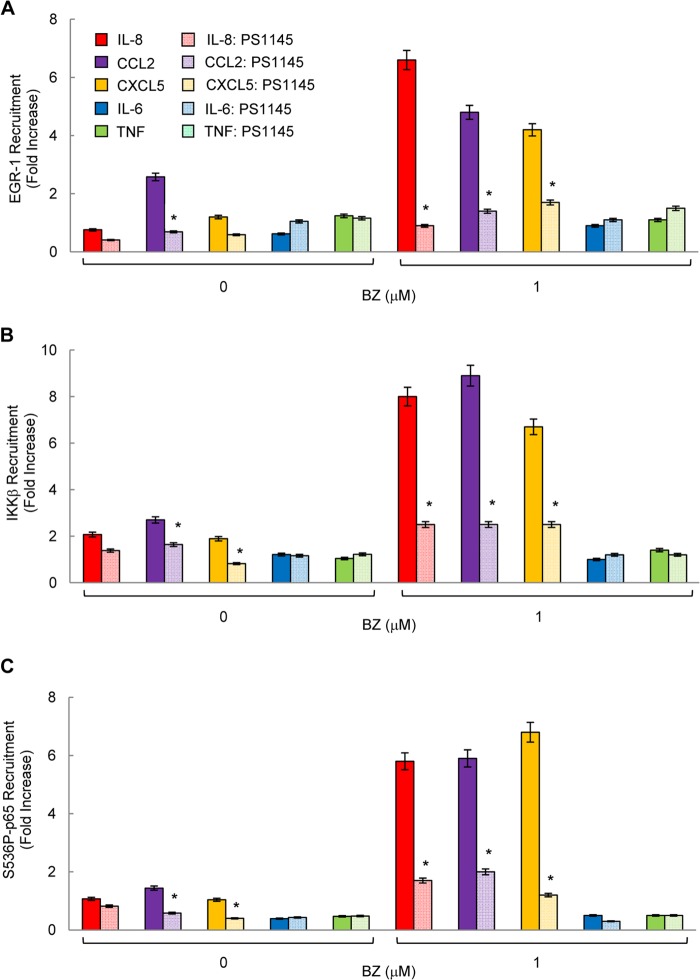
To analyze whether the proteasome inhibition-induced recruitment of IKKβ, S536P-p65, and EGR-1 to IL-8 promoter requires the kinase activity of IKKβ, OVCAR3 cells were preincubated with the IKKβ inhibitor PS-1145 before 24 h of treatment with 1 μm BZ. Inhibition of IKKβ activity by PS-1145 significantly attenuated the BZ-induced EGR-1 (Fig. 8A), IKKβ (Fig. 8B), and S536P-p65 (Fig. 8C) recruitment to IL-8, CCL2, and CXCL5 promoters, whereas it did not have any significant effect on EGR-1, IKKβ, or S536P-p65 recruitment to IL-6 or TNFα promoters. Together, these data demonstrated that in ovarian cancer cells, proteasome inhibition specifically increases S536P-p65, IKKβ, and EGR-1 recruitment to IL-8 promoter and that both IKKβ activity and EGR-1 expression are required for the BZ-induced IL-8 expression in ovarian cancer cells.
FIGURE 8.
IKKβ activity is required for the increased EGR-1, IKKβ, and S536P-p65 recruitment to IL-8, CCL2, and CXCL5 promoters in ovarian cancer cells. Recruitment of EGR-1 (A), IKKβ (B), and S536P-p65 (C) to IL-8, CCL2, CXCL5, IL-6, and TNFα promoters was analyzed by ChIP and quantified by real time PCR in OVCAR3 cells pretreated 12 h with 20 μm PS-1145 before 24 h incubation with 0 or 1 μm BZ. The data are presented as the change in occupancy over the human IGX1A (SA Biosciences) sequence control and represent the mean ± S.E. of four experiments. Asterisks denote a statistically significant (p < 0.05) inhibition compared with control BZ-treated cells.
Bortezomib Does Not Increase IL-8 Release in Multiple Myeloma Cells
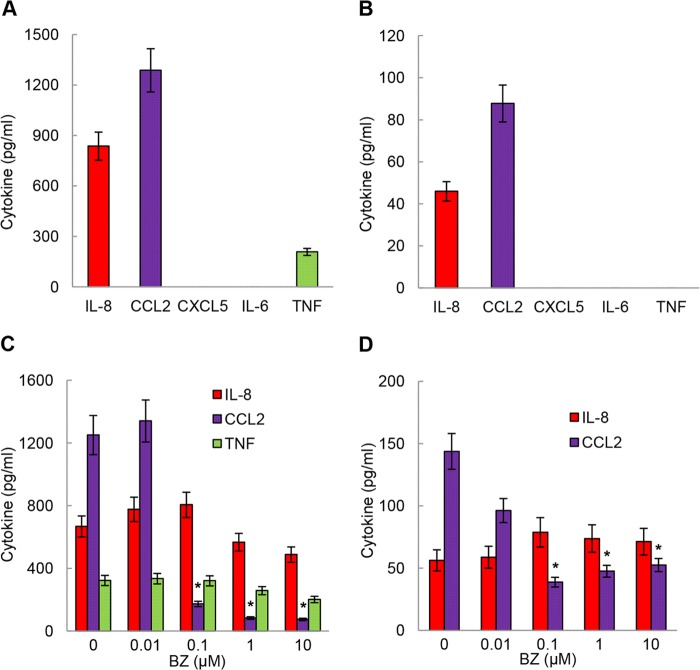
We have recently shown that proteasome inhibition specifically induces IL-8 release also in metastatic prostate cancer cells, albeit by a different mechanism that involves IKKα but not IKKβ (33). Despite the limited effectiveness of BZ as a single agent therapy in ovarian cancer, metastatic prostate cancer, and other solid tumors, proteasome inhibition has been remarkably effective in the treatment of multiple myeloma (25, 26). Thus, we wanted to determine whether BZ increases IL-8 release in multiple myeloma cells. We used two multiple myeloma cell lines: IM-9 cells that release high levels of IL-8 and CCL2 (Fig. 9A) and RPMI-8226 cells that release only low levels of these chemokines (Fig. 9B). Interestingly, BZ did not increase IL-8 release in either cell line, whereas the CCL2 release was actually inhibited (Fig. 9, C and D). Together, these data suggest that the BZ-induced IL-8 release is unique to ovarian, prostate, and perhaps other solid tumors and may represent one the mechanisms responsible for the BZ limited effectiveness in solid cancers.
FIGURE 9.
Bortezomib does not increase IL-8 release in multiple myeloma cells. Shown is ELISA of IL-8, CCL2, CXCL5, IL-6, and TNFα protein levels measured in supernatants of untreated (24 h) IM-9 (A) and RPMI-8226 (B) cells (106 cells/2 ml). ELISA of IL-8, CCL2, and TNFα release from IM-9 (C) and RPMI-8226 (D) cells treated (24 h) with increasing BZ concentrations is shown. The values represent the mean ± S.E. of four experiments; asterisks denote a statistically significant (p < 0.05) change compared with untreated (no BZ) cells.
DISCUSSION
The key finding of this study is that in ovarian cancer cells, proteasome inhibition specifically increases expression and release of IL-8. Our data indicate that the responsible mechanism involves the increased nuclear accumulation of IKKβ and the increased recruitment of IKKβ, S536P-p65, and EGR-1 to the NFκB binding site in IL-8 promoter (Fig. 10). The nuclear EGR-1 associates with IKKβ and with p65, with preferential binding to S536P-p65. Both IKKβ activity and EGR-1 expression are required for the increased expression of IL-8 induced by proteasome inhibition in ovarian cancer cells. These data provide the first evidence for the gene-specific recruitment of IKKβ, S536P-p65, and EGR-1 to the NFκB binding site in IL-8 promoter and for the increased release of IL-8 in ovarian cancer cells.
FIGURE 10.
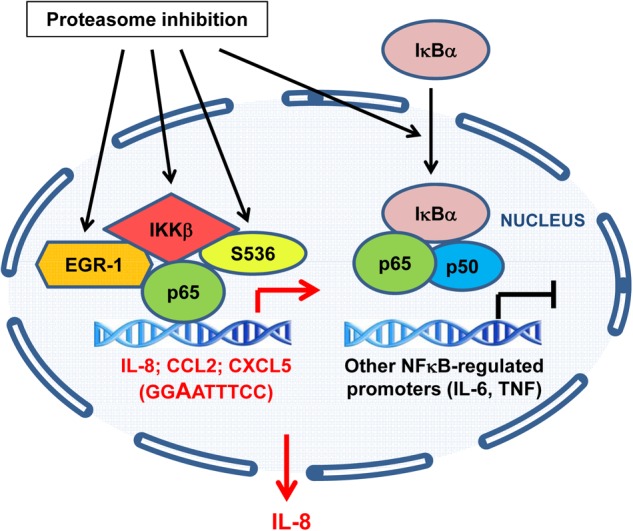
Model of the transcriptional regulation of NFκB-dependent genes by proteasome inhibition in ovarian cancer cells. In ovarian cancer cells, proteasome inhibition increases the nuclear accumulation of IKKβ, resulting in the increased recruitment of IKKβ, S536P-p65, and EGR-1 to the IL-8, CCL2, and CXCL5 promoters and increased IL-8, CCL2, and CXCL5 transcription. Both IKKβ activity and EGR-1 expression are required for the increased gene expression of IL-8, CCL2, and CXCL5 in ovarian cancer cells. However, only IL-8 is released from ovarian cancer cells in response to proteasome inhibition. Proteasome inhibition does not increase IKKβ, S536P-p65, and EGR-1 recruitment to TNFα and IL-6 promoters that are occupied by p50 NFκB, and does not increase transcription of these genes.
We have recently shown that bortezomib increases IL-8 expression in prostate cancer cells (33). But what are the differences between prostate and ovarian cancer cells in their responses to bortezomib? In prostate cancer cells, the increased expression of IL-8 is not mediated by IKKβ, but by IKKα. Furthermore, in prostate cancer cells, S536P-p65 is not recruited to the IL-8 promoter; instead, bortezomib increases IKKα-dependent recruitment of unphosphorylated p65 (33). In contrast, in ovarian cancer cells, the unphosphorylated p65 is not recruited to IL-8 promoter, whereas the S536P-p65 is recruited, and bortezomib further increases this recruitment (Fig. 5). Thus, even though proteasome inhibition induces IL-8 expression in both cancer types, the responsible mechanisms are clearly different.
Interestingly, we have shown that in contrast to ovarian and prostate cancer cells, BZ does not increase IL-8 release in multiple myeloma cells (Fig. 9). Because BZ has been very effective in the treatment of multiple myeloma, these data suggest that the BZ-induced IL-8 release in ovarian and prostate cancer cells and perhaps other solid tumors may represent one of the mechanisms responsible for the limited efficiency of BZ in solid tumors.
The initial rationale for BZ development and use in multiple myeloma was the inhibition of NFκB-dependent transcription by blocking the inducible proteasomal degradation of IκBα. In TNF-stimulated multiple myeloma (and other) cells, BZ prevented the inducible IκBα degradation, resulting in the inhibition of p65 and p50 nuclear translocation and suppressed transcription of NFκB-dependent genes (21–23). However, more recent studies have indicated that proteasome inhibition can induce the constitutive canonical NFκB pathway in unstimulated cells by increasing phosphorylation of IKK, resulting in the cytoplasmic degradation of IκBα and increased nuclear translocation of p65 and p50 subunits (24, 48). In this study we showed that p65 and p50 NFκB subunits are constitutively localized in the nucleus of ovarian cancer cells, and proteasome inhibition further increases p65 nuclear accumulation. However, in ovarian cancer cells, proteasome inhibition does not induce IκBα degradation; rather, it induces its translocation from the cytoplasm to the nucleus (Fig. 1). Thus, the “classical” canonical pathway does not seem to be involved in ovarian cancer cells. The mechanism responsible for the increased IL-8 expression in ovarian cancer cells involves the BZ-increased nuclear accumulation of IKKβ, p65 phosphorylation, and S536P-p65, EGR-1, and IKKβ recruitment to the IL-8 promoter.
Very little is known about the nuclear functions of IKKβ, which does not contain the classical nuclear localization signal, and was originally thought to be localized only in the cytoplasm. Several studies have shown IKKβ also in the nucleus, where it seems to have both NFκB-dependent and NFκB-independent functions (49–55). One of the potential nuclear substrates of IKKβ is p65 NFκB, as it can be phosphorylated by IKKβ at Ser-536 (43, 56). Phosphorylation of p65 at Ser-536 regulates its interactions with other transcription factors as well as the specificity of NFκB promoter binding (56–58). Our data indicate that phosphorylation of p65 at Ser-536 increases its affinity for EGR-1 (Fig. 6), which may result in the increased recruitment of the S536P-p65·EGR-1 complex to the NFκB binding site in IL-8 promoter and increased IL-8 transcription. In addition, EGR-1 itself may be a potential IKKβ substrate, as its transcriptional activity is regulated by phosphorylation (59, 60).
EGR-1 has been shown to play a crucial role in the regulation of cell growth, differentiation, transformation, and apoptosis. In human ovarian carcinoma, EGR-1 mediates expression of the transmembrane collagenase membrane type 1 matrix metalloproteinase (MT1-MMP), resulting in the increased cellular invasion and potentiating metastasis (61). In addition, EGR-1 was shown to mediate expression of IL-8 in prostate cancer cells and human epidermal carcinoma cells (46, 47). However, because human IL-8 promoter does not contain the EGR-1 consensus binding sequence (GCG(G/T)GGGCG), it seems likely that EGR-1 and IKKβ associate with IL-8 promoter through the Ser-536-phosphorylated p65 NFκB.
Even though p65 as well as the other Rel-containing NFκB subunits can bind DNA through the Rel homology domain, several studies have shown that p65 phosphorylation on Ser-536 decreases its affinity for IκBα and increases its binding to DNA (56, 58, 62). Interestingly, recent studies have also indicated that a new non-Rel protein RPS3 can bind to p65-containing complexes, including p65 homodimers, and dramatically increase their affinity for specific NFκB binding sites (63, 64). Thus, it will be interesting in the future to determine whether RPS3 is a part of the IKKβ·EGR-1·S536P-p65 complex in ovarian cancer cells and whether proteasome inhibition increases its nuclear levels.
Previous studies have indicated that a single nucleotide can influence specificity of the recruitment of individual NFκB dimers and cofactors (65–67). Because our original results have shown that proteasome inhibition specifically increases IL-8, whereas it does not affect TNFα or IL-6 expression, we have compared the NFκB binding sites in human IL-8, TNFα, and IL-6 promoters and found that the proximal NFκB binding site in IL-8 promoter differs from TNFα and IL-6 promoters by having A instead of G in the third position (Table 1). We hypothesized that promoters having the same NFκB binding sequence as IL-8 would be also occupied by S536P-p65, IKKβ, and EGR-1, and this occupancy and gene expression would be increased by proteasome inhibition. We found two genes that have the same NFκB binding sequence as IL-8: CCL2 and CXCL5 (Table 1). Intriguingly, both CCL2 and CXCL5 are also pro-angiogenic chemokines that have similar functions as IL-8; IL-8 and CXCL5 (ENA-78) were originally discovered as the neutrophil chemoattractants (68, 69), and CCL2 (MCP-1) was discovered as a monocyte chemoattractant (70). All three chemokines promote inflammation, and their protein levels are increased in many tumors, including ovarian cancer ascites (3–6). Even though the ovarian carcinoma OVCAR3 and SKOV3 cell lines release only IL-8, the fact that proteasome inhibition specifically increases S536P-p65, IKKβ, and EGR-1 recruitment to all three promoters and IL-8, CCL2, and CXCL5 gene expression in ovarian cancer cells suggests that the GGAATTTCC NFκB binding site has an increased affinity for S536P-p65 binding, resulting in the increased transcription.
TABLE 1.
NFκB binding sites in the NFκB-regulated promoters
| Gene | Function | NFκB site location | NFκB site sequence |
|---|---|---|---|
| IL-8 | Neutrophil chemoattractant; promotes survival and angiogenesis | −82 | GGAATTTCC |
| TNFα | Proinflammatory cytokine | −99 | GGGTATCCT |
| IL-6 | Proinflammatory cytokine | −73 | GGGATTTTC |
| CCL2 (MCP-1) | Monocyte chemoattractant; promotes survival and angiogenesis | −2600 | GGAATTTCC |
| CXCL5 (ENA-78) | Neutrophil chemoattractant; promotes survival and angiogenesis | −82 | GGAATTTCC |
Despite the limited effectiveness of BZ as a single agent in the treatment of ovarian carcinoma and other solid tumors, BZ has been considered in combination with cisplatin and other platinum drugs (27–30), as it prevents the proteasomal degradation of cisplatin influx transporter, resulting in the increased cisplatin uptake and tumor cell killing (31, 32). In addition, recent studies have suggested that anti-inflammatory therapy may enhance the cytotoxicity of platinum drugs and proteasome inhibitors in ovarian carcinoma (71–73). Findings in this study indicate that the increased expression and release of IL-8 induced by proteasome inhibition may represent one of the mechanisms responsible for the decreased effectiveness of BZ in ovarian cancer treatment and identify IKKβ and EGR-1 as potential new targets in ovarian cancer combination therapies.
This work was supported, in whole or in part, by National Institutes of Health Grants AI085497 and CA173452 (to I. V.).
- IKK
- IκB kinase
- BZ
- bortezomib
- EGR-1
- early growth response-1
- NE
- nuclear extract
- CE
- cytoplasmic extract
- CXCL8
- chemokine interleukin-8
- MCP-1
- monocyte chemoattractant protein-1.
REFERENCES
- 1. Xu L., Fidler I. J. (2000) Interleukin 8. An autocrine growth factor for human ovarian cancer. Oncol. Res. 12, 97–106 [DOI] [PubMed] [Google Scholar]
- 2. Waugh D. J., Wilson C. (2008) The interleukin-8 pathway in cancer. Clin. Cancer Res. 14, 6735–6741 [DOI] [PubMed] [Google Scholar]
- 3. Penson R. T., Kronish K., Duan Z., Feller A. J., Stark P., Cook S. E., Duska L. R., Fuller A. F., Goodman A. K., Nikrui N., MacNeill K. M., Matulonis U. A., Preffer F. I., Seiden M. V. (2000) Cytokines IL-1, IL-2, IL-6, IL-8, MCP-1, GM-CSF, and TNF in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int. J. Gynecol. Cancer 10, 33–41 [DOI] [PubMed] [Google Scholar]
- 4. Milliken D., Scotton C., Raju S., Balkwill F., Wilson J. (2002) Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin. Cancer Res. 8, 1108–1114 [PubMed] [Google Scholar]
- 5. Negus R. P., Stamp G. W., Hadley J., Balkwill F. R. (1997) Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am. J. Pathol. 150, 1723–1734 [PMC free article] [PubMed] [Google Scholar]
- 6. Furuya M., Suyama T., Usui H., Kasuya Y., Nishiyama M., Tanaka N., Ishiwata I., Nagai Y., Shozu M., Kimura S. (2007) Up-regulation of CXC chemokines and their receptors. Implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum. Pathol. 38, 1676–1687 [DOI] [PubMed] [Google Scholar]
- 7. Mukaida N., Mahe Y., Matsushima K. (1990) Cooperative interaction of NFκB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 265, 21128–21133 [PubMed] [Google Scholar]
- 8. Kunsch C., Rosen C. A. (1993) NFκB subunit-specific regulation of the IL-8 promoter. Mol. Cell. Biol. 13, 6137–6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang S., Robinson J. B., Deguzman A., Bucana C. D., Fidler I. J. (2000) Blockade of NFκB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of VEGF and IL-8. Cancer Res. 60, 5334–5339 [PubMed] [Google Scholar]
- 10. Mabuchi S., Ohmichi M., Nishio Y., Hayasaka T., Kimura A., Ohta T., Saito M., Kawagoe J., Takahashi K., Yada-Hashimoto N., Sakata M., Motoyama T., Kurachi H., Tasaka K., Murata Y. (2004) Inhibition of NFκB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J. Biol. Chem. 279, 23477–23485 [DOI] [PubMed] [Google Scholar]
- 11. Annunziata C. M., Stavnes H. T., Kleinberg L., Berner A., Hernandez L. F., Birrer M. J., Steinberg S. M., Davidson B., Kohn E. C. (2010) NFκB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer 116, 3276–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leizer A. L., Alvero A. B., Fu H. H., Holmberg J. C., Cheng Y. C., Silasi D. A., Rutherford T., Mor G. (2011) Regulation of inflammation by the NFκB pathway in ovarian cancer stem cells. Am. J. Reprod. Immunol. 65, 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mabuchi S., Ohmichi M., Nishio Y., Hayasaka T., Kimura A., Ohta T., Kawagoe J., Takahashi K., Yada-Hashimoto N., Seino-Noda H., Sakata M., Motoyama T., Kurachi H., Testa J. R., Tasaka K., Murata Y. (2004) Inhibition of inhibitor of NFκB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Clin. Cancer Res. 10, 7645–7654 [DOI] [PubMed] [Google Scholar]
- 14. Chen R., Alvero A. B., Silasi D. A., Kelly M. G., Fest S., Visintin I., Leiser A., Schwartz P. E., Rutherford T., Mor G. (2008) Regulation of IKKβ by miR-199a affects NFκB activity in ovarian cancer cells. Oncogene 27, 4712–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernandez L., Hsu S. C., Davidson B., Birrer M. J., Kohn E. C., Annunziata C. M. (2010) Activation of NFκB signaling by inhibitor of NFκB kinase β increases aggressiveness of ovarian cancer. Cancer Res. 70, 4005–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu S., Kim M., Hernandez L., Grajales V., Noonan A., Anver M., Davidson B., Annunziata C. M. (2012) IKK-ϵ coordinates invasion and metastasis of ovarian cancer. Cancer Res. 72, 5494–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayden M. S., Ghosh S. (2008) Shared principles in NFκB signaling. Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 18. Liu F., Xia Y., Parker A. S., Verma I. M. (2012) IKK biology. Immunol. Rev. 246, 239–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smale S. T. (2012) Dimer-specific regulatory mechanisms within the NF-κB family of transcription factors. Immunol. Rev. 246, 193–204 [DOI] [PubMed] [Google Scholar]
- 20. Natoli G. (2012) NF-κB and chromatin. Ten years on the path from basic mechanisms to candidate drugs. Immunol. Rev. 246, 183–192 [DOI] [PubMed] [Google Scholar]
- 21. Hideshima T., Richardson P., Chauhan D., Palombella V. J., Elliott P. J., Adams J., Anderson K. C. (2001) The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 61, 3071–3076 [PubMed] [Google Scholar]
- 22. Richardson P. G., Mitsiades C., Hideshima T., Anderson K. C. (2005) Proteasome inhibition in the treatment of cancer. Cell Cycle 4, 290–296 [PubMed] [Google Scholar]
- 23. McConkey D. J., Zhu K. (2008) Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist. Updat. 11, 164–179 [DOI] [PubMed] [Google Scholar]
- 24. Hideshima T., Ikeda H., Chauhan D., Okawa Y., Raje N., Podar K., Mitsiades C., Munshi N. C., Richardson P. G., Carrasco R. D., Anderson K. C. (2009) Bortezomib induces canonical NFκB activation in multiple myeloma cells. Blood 114, 1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah J. J., Orlowski R. Z. (2009) Proteasome inhibitors in the treatment of multiple myeloma. Leukemia 23, 1964–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuhn D. J., Orlowski R. Z. (2012) The immunoproteasome as a target in hematologic malignancies. Semin. Hematol. 49, 258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frankel A., Man S., Elliott P., Adams J., Kerbel R. S. (2000) Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin. Cancer Res. 6, 3719–3728 [PubMed] [Google Scholar]
- 28. Aghajanian C., Dizon D. S., Sabbatini P., Raizer J. J., Dupont J., Spriggs D. R. (2005) Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J. Clin. Oncol. 23, 5943–5949 [DOI] [PubMed] [Google Scholar]
- 29. Ramirez P. T., Landen C. N., Jr., Coleman R. L., Milam M. R., Levenback C., Johnston T. A., Gershenson D. M. (2008) Phase I trial of the proteasome inhibitor bortezomib in combination with carboplatin in patients with platinum- and taxane-resistant ovarian cancer. Gynecol. Oncol. 108, 68–71 [DOI] [PubMed] [Google Scholar]
- 30. Aghajanian C., Blessing J. A., Darcy K. M., Reid G., DeGeest K., Rubin S. C., Mannel R. S., Rotmensch J., Schilder R. J., Riordan W., and Gynecologic Oncology Group (2009) A phase II evaluation of bortezomib in the treatment of recurrent platinum-sensitive ovarian or primary peritoneal cancer. A Gynecologic Oncology Group study. Gynecol. Oncol. 115, 215–220 [DOI] [PubMed] [Google Scholar]
- 31. Jandial D. D., Farshchi-Heydari S., Larson C. A., Elliott G. I., Wrasidlo W. J., Howell S. B. (2009) Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin. Cancer Res. 15, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Howell S. B., Safaei R., Larson C. A., Sailor M. J. (2010) Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol. 77, 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manna S., Singha B., Phyo S. A., Gatla H. R., Chang T. P., Sanacora S., Ramaswami S., Vancurova I. (2013) Proteasome inhibition by bortezomib increases IL-8 expression in androgen-independent prostate cancer cells. The role of IKKα. J. Immunol. 191, 2837–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vu H. Y., Juvekar A., Ghosh C., Ramaswami S., Le D. H., Vancurova I. (2008) Proteasome inhibitors induce apoptosis of prostate cancer cells by inducing nuclear translocation of IκBα. Arch. Biochem. Biophys. 475, 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Juvekar A., Manna S., Ramaswami S., Chang T. P., Vu H. Y., Ghosh C. C., Celiker M. Y., Vancurova I. (2011) Bortezomib induces nuclear translocation of IκBα resulting in gene-specific suppression of NFκB-dependent transcription and induction of apoptosis in CTCL. Mol. Cancer Res. 9, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghosh C. C., Ramaswami S., Juvekar A., Vu H. Y., Galdieri L., Davidson D., Vancurova I. (2010) Gene-specific repression of proinflammatory cytokines in stimulated human macrophages by nuclear IκBα. J. Immunol. 185, 3685–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramaswami S., Manna S., Juvekar A., Kennedy S., Vancura A., Vancurova I. (2012) Chromatin immunoprecipitation analysis of NFκB transcriptional regulation by nuclear IκBα in human macrophages. Methods Mol. Biol. 809, 121–134 [DOI] [PubMed] [Google Scholar]
- 38. Saccani S., Marazzi I., Beg A. A., Natoli G. (2004) Degradation of promoter-bound p65/RelA is essential for the prompt termination of the NFκB response. J. Exp. Med. 200, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levêque D., Carvalho M. C., Maloisel F. (2007) Clinical pharmacokinetics of bortezomib. In Vivo 21, 273–278 [PubMed] [Google Scholar]
- 40. Lee C. M., Kumar V., Riley R. I., Morgan E. T. (2010) Metabolism and action of proteasome inhibitors in primary human hepatocytes. Drug Metab. Dispos. 38, 2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Powers G. L., Ellison-Zelski S. J., Casa A. J., Lee A. V., Alarid E. T. (2010) Proteasome inhibition represses ERα gene expression in ER+ cells. A new link between proteasome activity and estrogen signaling in breast cancer. Oncogene 29, 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gasparian A. V., Guryanova O. A., Chebotaev D. V., Shishkin A. A., Yemelyanov A. Y., Budunova I. V. (2009) Targeting transcription factor NFκB. Comparative analysis of proteasome and IKK inhibitors. Cell Cycle 8, 1559–1566 [DOI] [PubMed] [Google Scholar]
- 43. Sakurai H., Chiba H., Miyoshi H., Sugita T., Toriumi W. (1999) IκB kinases phosphorylate NFκB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274, 30353–30356 [DOI] [PubMed] [Google Scholar]
- 44. Yemelyanov A., Gasparian A., Lindholm P., Dang L., Pierce J. W., Kisseljov F., Karseladze A., Budunova I. (2006) Effects of IKK inhibitor PS1145 on NFκB function, proliferation, apoptosis, and invasion activity in prostate carcinoma cells. Oncogene 25, 387–398 [DOI] [PubMed] [Google Scholar]
- 45. Domingo-Domenech J., Oliva C., Rovira A., Codony-Servat J., Bosch M., Filella X., Montagut C., Tapia M., Campás C., Dang L., Rolfe M., Ross J. S., Gascon P., Albanell J., Mellado B. (2006) Interleukin 6, a NFκB target, predicts resistance to docetaxel in hormone-independent prostate cancer and NFκB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin. Cancer Res. 12, 5578–5586 [DOI] [PubMed] [Google Scholar]
- 46. Ma J., Ren Z., Ma Y., Xu L., Zhao Y., Zheng C., Fang Y., Xue T., Sun B., Xiao W. (2009) Targeted knockdown of EGR1 inhibits IL-8 production and IL-8-mediated invasion of prostate cancer cells through suppressing EGR1/NFκB synergy. J. Biol. Chem. 284, 34600–34606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoffmann E., Ashouri J., Wolter S., Doerrie A., Dittrich-Breiholz O., Schneider H., Wagner E. F., Troppmair J., Mackman N., Kracht M. (2008) Transcriptional regulation of EGR-1 by the interleukin-1-JNK-MKK7-c-Jun pathway. J. Biol. Chem. 283, 12120–12128 [DOI] [PubMed] [Google Scholar]
- 48. Li C., Chen S., Yue P., Deng X., Lonial S., Khuri F. R., Sun S. Y. (2010) Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IκBα degradation. J. Biol. Chem. 285, 16096–16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anest V., Hanson J. L., Cogswell P. C., Steinbrecher K. A., Strahl B. D., Baldwin A. S. (2003) A nucleosomal function for IκB kinase-α in NFκB-dependent gene expression. Nature 423, 659–663 [DOI] [PubMed] [Google Scholar]
- 50. Birbach A., Gold P., Binder B. R., Hofer E., de Martin R., Schmid J. A. (2002) Signaling molecules of the NFκB pathway shuttle constitutively between cytoplasm and nucleus. J. Biol. Chem. 277, 10842–10851 [DOI] [PubMed] [Google Scholar]
- 51. Aguilera C., Hoya-Arias R., Haegeman G., Espinosa L., Bigas A. (2004) Recruitment of IKKα to the hes1 promoter is associated with transcriptional repression. Proc. Natl. Acad. Sci. 101, 16537–16542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ear T., Cloutier A., McDonald P. P. (2005) Constitutive nuclear expression of the IκB kinase complex and its activation in human neutrophils. J. Immunol. 175, 1834–1842 [DOI] [PubMed] [Google Scholar]
- 53. Tsuchiya Y., Asano T., Nakayama K., Kato T., Jr., Karin M., Kamata H. (2010) Nuclear IKKβ is an adaptor protein for IκBα ubiquitination and degradation in UV-induced NFκB activation. Mol. Cell. 39, 570–582 [DOI] [PubMed] [Google Scholar]
- 54. Espinosa L., Bigas A., Mulero M. C. (2011) Alternative nuclear functions for NFκB family members. Am. J. Cancer Res. 1, 446–459 [PMC free article] [PubMed] [Google Scholar]
- 55. Sakamoto K., Hikiba Y., Nakagawa H., Hirata Y., Hayakawa Y., Kinoshita H., Nakata W., Sakitani K., Takahashi R., Akanuma M., Kamata H., Maeda S. (2013) Promotion of DNA repair by nuclear IKKβ phosphorylation of ATM in response to genotoxic stimuli. Oncogene 32, 1854–1862 [DOI] [PubMed] [Google Scholar]
- 56. Buss H., Dörrie A., Schmitz M. L., Hoffmann E., Resch K., Kracht M. (2004) Constitutive and IL-1-inducible phosphorylation of p65 NFκB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKϵ, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated IL-8 transcription. J. Biol. Chem. 279, 55633–55643 [DOI] [PubMed] [Google Scholar]
- 57. Moreno R., Sobotzik J. M., Schultz C., Schmitz M. L. (2010) Specification of the NFκB transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKK epsilon. Nucleic Acids Res. 38, 6029–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sasaki C. Y., Barberi T. J., Ghosh P., Longo D. L. (2005) Phosphorylation of RelA/p65 on serine 536 defines an IκBα-independent NFκB pathway. J. Biol. Chem. 280, 34538–345347 [DOI] [PubMed] [Google Scholar]
- 59. Jain N., Mahendran R., Philp R., Guy G. R., Tan Y. H., Cao X. (1996) Casein kinase II associates with Egr-1 and acts as a negative modulator of its DNA binding and transcription activities in NIH 3T3 cells. J. Biol. Chem. 271, 13530–13536 [DOI] [PubMed] [Google Scholar]
- 60. Huang R. P., Fan Y., deBelle I., Ni Z., Matheny W., Adamson E. D. (1998) Egr-1 inhibits apoptosis during the UV response. Correlation of cell survival with Egr-1 phosphorylation. Cell Death Differ. 5, 96–106 [DOI] [PubMed] [Google Scholar]
- 61. Barbolina M. V., Adley B. P., Ariztia E. V., Liu Y., Stack M. S. (2007) Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J. Biol. Chem. 282, 4924–4931 [DOI] [PubMed] [Google Scholar]
- 62. Bohuslav J., Chen L. F., Kwon H., Mu Y., Greene W. C. (2004) p53 induces NFκB activation by an IκB kinase independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 279, 26115–26125 [DOI] [PubMed] [Google Scholar]
- 63. Wan F., Anderson D. E., Barnitz R. A., Snow A., Bidere N., Zheng L., Hegde V., Lam L. T., Staudt L. M., Levens D., Deutsch W. A., Lenardo M. J. (2007) Ribosomal protein S3. A KH domain subunit in NFκB complexes that mediates selective gene regulation. Cell 131, 927–939 [DOI] [PubMed] [Google Scholar]
- 64. Wier E. M., Neighoff J., Sun X., Fu K., Wan F. (2012) Identification of an N-terminal truncation of the NFκB p65 subunit that specifically modulates ribosomal protein S3-dependent NFκB gene expression. J. Biol. Chem. 287, 43019–43029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leung T. H., Hoffmann A., Baltimore D. (2004) One nucleotide in a κB site can determine cofactor specificity for NFκB dimers. Cell 118, 453–464 [DOI] [PubMed] [Google Scholar]
- 66. Natoli G. (2006) Tuning up inflammation. How DNA sequence and chromatin organization control the induction of inflammatory genes by NFκB. FEBS Lett. 580, 2843–2849 [DOI] [PubMed] [Google Scholar]
- 67. Hochrainer K., Racchumi G., Anrather J. (2013) Site-specific phosphorylation of the p65 protein subunit mediates selective gene expression by differential NFκB and RNA polymerase II promoter recruitment. J. Biol. Chem. 288, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kunkel S. L., Strieter R. M., Chensue S. W., Basha M., Standiford T., Ham J., Remick D. G. (1990) Tumor necrosis factor-α, interleukin-8, and chemotactic cytokines. Prog. Clin. Biol. Res. 349, 433–444 [PubMed] [Google Scholar]
- 69. Walz A., Burgener R., Car B., Baggiolini M., Kunkel S. L., Strieter R. M. (1991) Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J. Exp. Med. 174, 1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leonard E. J., Yoshimura T. (1990) Human monocyte chemoattractant protein-1 (MCP-1). Immunol. Today 11, 97–101 [DOI] [PubMed] [Google Scholar]
- 71. Zerbini L. F., Tamura R. E., Correa R. G., Czibere A., Cordeiro J., Bhasin M., Simabuco F. M., Wang Y., Gu X., Li L., Sarkar D., Zhou J. R., Fisher P. B., Libermann T. A. (2011) Combinatorial effect of non-steroidal anti-inflammatory drugs and NFκB inhibitors in ovarian cancer therapy. PLoS ONE 6, e24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Murphy M. A., Trabert B., Yang H. P., Park Y., Brinton L. A., Hartge P., Sherman M. E., Hollenbeck A., Wentzensen N. (2012) Non-steroidal anti-inflammatory drug use and ovarian cancer risk. Findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control 23, 1839–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Valle B. L., D'Souza T., Becker K. G., Wood W. H., 3rd, Zhang Y., Wersto R. P., Morin P. J. (2013) Non-steroidal anti-inflammatory drugs decrease E2F1 expression and inhibit cell growth in ovarian cancer cells. PLoS One 8, e61836. [DOI] [PMC free article] [PubMed] [Google Scholar]