Background: Valproic acid is considered as a promising anti-cancer therapeutic agent acting on unfolded protein response. SEL1L is an UPR-responsive gene.
Results: SEL1L interference synergy enhances VPA cytotoxic effects on glioma stem cells.
Conclusion: VPA treatment combined with SEL1L depletion may influence GSC pharmacological response.
Significance: Targeting SEL1L in association with valproic acid treatment may improve glioma treatment.
Keywords: Brain Tumors, Cancer Stem Cells, Cell Proliferation, Drug Resistance, Histone Deacetylase
Abstract
Valproic acid (VPA), an histone deacetylase inhibitor, is emerging as a promising therapeutic agent for the treatments of gliomas by virtue of its ability to reactivate the expression of epigenetically silenced genes. VPA induces the unfolded protein response (UPR), an adaptive pathway displaying a dichotomic yin yang characteristic; it initially contributes in safeguarding the malignant cell survival, whereas long-lasting activation favors a proapoptotic response. By triggering UPR, VPA might tip the balance between cellular adaptation and programmed cell death via the deregulation of protein homeostasis and induction of proteotoxicity. Here we aimed to investigate the impact of proteostasis on glioma stem cells (GSC) using VPA treatment combined with subversion of SEL1L, a crucial protein involved in homeostatic pathways, cancer aggressiveness, and stem cell state maintenance. We investigated the global expression of GSC lines untreated and treated with VPA, SEL1L interference, and GSC line response to VPA treatment by analyzing cell viability via MTT assay, neurosphere formation, and endoplasmic reticulum stress/UPR-responsive proteins. Moreover, SEL1L immunohistochemistry was performed on primary glial tumors. The results show that (i) VPA affects GSC lines viability and anchorage-dependent growth by inducing differentiative programs and cell cycle progression, (ii) SEL1L down-modulation synergy enhances VPA cytotoxic effects by influencing GSCs proliferation and self-renewal properties, and (iii) SEL1L expression is indicative of glioma proliferation rate, malignancy, and endoplasmic reticulum stress statuses. Targeting the proteostasis network in association to VPA treatment may provide an alternative approach to deplete GSC and improve glioma treatments.
Introduction
Although cancer has traditionally been considered as a disease originating from genetic and chromosomal alterations, epigenetic modifications or gene expression modulation through mechanisms other than changes in the underlying DNA sequence have emerged as contributing factors toward oncogenesis (1). Based on the central role of histone deacetylase inhibitors to influence chromatin structure, they represent a new class of anticancer drugs with the potential to revert aberrant epigenetic states associated with cancer (2). Histone deacetylase inhibitor, by increasing histone acetylation, give rise to a more permissive or open chromatin configuration leading to the potential reactivation of aberrantly suppressed genes, resulting in growth arrest, cell differentiation, and apoptosis of tumor cells (3–6). Among several histone deacetylase inhibitors, valproic acid (VPA),2 is currently being used as an anticancer agent in several clinical trials (7) either as a monotherapy (8) or in conjunction with other treatments such as chemotherapy or radiation (9). Moreover, VPA has a protective effect on cardiomyocite hypertrophy, the primary adaptive response to exogenous physiological or pathological signals (10).
The potential role of VPA in directly regulating the unfolded protein response (UPR) through acetylation of its central regulatory protein Bip was recently reported (11); however, the functional consequence of VPA-induced Bip acetylation as well as the translational potential in clinical settings has yet to be established. UPR is a conserved adaptive cellular response that aims at relieving proteostasis defects by (i) attenuating the rate of protein synthesis, (ii) up-regulating protein folding enzymes, and (iii) activating the endoplasmic reticulum-associated degradation machinery and secretory capacity (12). Finally, if homeostasis cannot be restored, death/senescence programs are activated (12). It represents a “yin-yang“ cancer process; early response is cell protective and supports chemoresistance (yang), whereas extensive activation turns on cell death program (yin) (13). These temporally distinct pro-survival/pro-apoptotic waves may provide therapeutic opportunities; indeed blocking adaptive and enhancing contra-adaptive signaling pathways could drastically contribute to cancer therapy (13).
UPR finely orchestrates the endoplasmic reticulum-associated degradation pathway, a temporally and spatially coordinated surveillance process charged with clearance of aberrant proteins in the ER by (i) substrate selection, (ii) dislocation across the ER membrane, and (iii) covalent conjugation with polyubiquitin and proteasomal degradation (14). SEL1L is an ER-embedded adaptor that links substrate recognition to the E3 ligase-coupled dislocation apparatus (15, 16). Considering its accurate timing and control in the proteolysis of key regulators as well as its position between two critical homeostatic pathways, SEL1L regulates several cellular functions and programs including cell fate developmental decision and homeostasis. It plays an essential role in maintaining ER homeostasis during vertebrate embryonic development (17) and in the maintenance of neural progenitor state and lineage determination (18). Based on its role in these critical cellular processes, subversion of SEL1L has been linked to important diseases including neurodegeneration and cancer. Interestingly, its expression is dependent on tumor context, being down-modulated in pancreatic and breast adenocarcinomas (19–21) and up-regulated in prostatic cancer (22, 23) and metastasis (24). Generally, it is up-regulated during the initial phases of neoplastic transformation, underlying a potential biomarker function in tumor progression (24–27). Moreover, the protein subcellular localization is able to distinguish lung cancer subtypes (27).
Considering its implication in neural stem cell and tumor biology as well as its potential link with UPR-dependent regulation by VPA, we explored the functional effect of SEL1L in GSC and its contribution in influencing stem cell drug sensitivity. Here we show the correlation between SEL1L, proliferation, and Ki-67 expression both in vitro and in vivo glioma model systems. Moreover, using silencing technology employed with short term survival and neurosphere assays, SEL1L emerges as a potential determining factor of GSC sensitivity to VPA treatment.
EXPERIMENTAL PROCEDURES
Cell Lines Growth Conditions, Nucleofection, and VPA Treatment
GSC lines (G166, G179, and GliNS2, kindly provided by Professor Austin Smith) and GBM2 and GBM7 (provided by Dr. Antonio Daga) were grown as described (28–30).
G179 cells (1 × 106) were transiently nucleofected with 100 pmol of two small interference RNAs (siRNAs) against the 5′ end of the SEL1L coding sequence and one non-targeting siRNA (NT siRNA) (Ambion, Monza, Italy) using the Nucleofector® and Amaxa nucleofector kit V (Lonza). GSC lines were treated with VPA (2 mm) (Sigma) for 96 h in all experiments. Two SEL1L-siRNAs were used to guarantee the minimal or no off-target activity and the reliability of the silenced phenotype.
qPCR Analysis
Total RNA was purified using TRI Reagent solution (Applied Biosystems) and reverse-transcribed with SuperScript TM II reverse transcriptase (Invitrogen).
RT-qPCR was performed in triplicate on Rotor-GeneQ (Qiagen) using SYBR Green (Fermentas) detection. Data were normalized to hypoxanthine-guanine phosphoribosyltransferase expression using the ΔΔCt method. Data are the averages of three independent experiments. See Table 1 for primer sequences.
TABLE 1.
Primers sequences
HPRT, hypoxanthine-guanine phosphoribosyltransferase; F, forward; R, reverse.
| SEL1L-F | 5′-aagcacaggttggtcttgga-3′ |
| SEL1L-R | 5′-agctgtctcattactctgaggt-3′ |
| HRD1-F | 5′-caccgtgctggcctccttgg-3′ |
| HRD1-R | 5′-tcctggggttggggtcgtgg-3′ |
| GP78-F | 5′-tgtggtgcctctggtttgccg-3′ |
| GP78-R | 5′-aacagggacaggactcgaccgt-3′ |
| BIP-F | 5′-tgcagcaggacatcaagttc-3′ |
| BIP-R | 5′-cgctggtcaaagtcttctcc-3′ |
| ATF6-F | 5′-ctgatggctgttcaatacac-3′ |
| ATF6-R | 5′-aatgactcagggatggtgct-3′ |
| IRE1α-F | 5′-ttcagccgccgatctggggt-3′ |
| IRE1α-R | 5′-tgccaaaagggtggctgccc-3′ |
| XBP-1-F | 5′-ccttgtagttgagaaccagg-3′ |
| XBP-1-R | 5′-ggggcttggtatatatgtgg-3′ |
| CHOP-F | 5′-ggtggcagcgacagagccaa-3′ |
| CHOP-R | 5′-ccaggcttccagctcccagc-3′ |
| SOX2-F | 5′-gacagagcccattttctcca-3′ |
| SOX2-R | 5′-aaatcctgtcctcccattcc-3′ |
| TBB3-F | 5′-aatgaggcctcctctcacaag-3′ |
| TBB3-R | 5′-aggcctgaagagatgtccaa-3′ |
| GFAP-F | 5′-cgcacgcagtatgaggcaat-3′ |
| GFAP-R | 5′-cggtagtcgttggcttcgtg-3′ |
| NOTCH1-F | 5′-tgaaggcctcgctgctccct-3′ |
| NOTCH1-R | 5′-ccacgtcggtggcactcgg-3′ |
| GADD45β-F | 5′-ggaagagctcgtggcgtgcg-3′ |
| GADD45β-R | 5′-gtctcgggcctcggtggtgc-3′ |
| PTEN-F | 5′-cgaactggtgtaatgatatg-3′ |
| PTEN-R | 5′-catgaacttgtcttcccgtc-3′ |
| HPRT-F | 5′-ctggcgtcgtgattagtgatga-3′ |
| HPRT-R | 5′-gcacacagagggctacaatg-3′ |
Western Blotting
GSC lines were lysed in 10 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Nonidet P-40 containing protease inhibitors (Pierce). Samples were resolved on SDS-polyacrylamide gels (10%), blotted onto PVDF membranes, and probed with anti-SEL1L (32) and anti-vinculin (Sigma) in Xblot-100 as hybridization chamber. Filters were developed with ECL (Genespin). Densitometric analysis was determined using the Scion imaging program. Data are the average of two independent experiments.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 15 min, treated for 10 min with 0.1 m glycine, and incubated for 30 min at room temperature in blocking solution. Cells were immunostained overnight at 4 °C in blocking solution with mouse anti-SEL1L (5 μg/ml) (32), rabbit anti-SOX-2 (1:300, Millipore), mouse anti-Nestin (1:50, Millipore), rabbit anti-glial fibrillary acid protein (GFAP; 1:1000, Sigma), rabbit anti-TUBB3 (1:100, Sigma), mouse anti-O4 (1:50, Millipore), mouse anti-Ki-67 clone MIB (1:80, Dako), and rabbit anti-Cleaved CaspaseIII (1:500, Cell Signaling). Proteins were revealed with the appropriate secondary antibodies (Rhodamine Red antimouse IgM and anti-rabbit IgG, Alexa Fluor 488 anti-mouse IgG, Jackson ImmunoResearch). Nuclei were counterstained with Hoechst (Invitrogen). Images were acquired using a NIKON fluorescence microscope (NIKON Microsystems) evaluating at least 800 cells for each sample.
Whole Genome Gene Expression
Triplicate samples from independent experiments were used for whole-genome expression analysis. Briefly, 500 ng of total RNA was amplified and labeled using the Illumina TotalPrep RNA amplification kit (Ambion). 750 ng of labeled cRNA was hybridized on the BeadChip Array Human HT-12 v4.0 (Illumina) at 58 °C for 16 h. After hybridization, chips were washed, coupled with Cy3, and scanned in the Illumina BeadArray Reader. Data were processed using BRB-ArrayTools Version 4.2.1. Raw data were log-transformed, normalized by robust spline normalization, and filtered to exclude genes with a p value of the log-ratio variation >0.05 and with the percentage of data missing or filtered out exceeding 50% and annotated by Bioconductor annotation package lumiHumanAll.db (Version 1.14.0). Class comparison analysis was applied to the 17,548 genes that passed filtering criteria. Functional analysis was achieved using Gene Set Enrichment Analysis (GSEA) software (33). Data were collapsed using max_probe mode, and supervised analysis was performed on data from VPA-treated cells versus untreated samples. Permutation was executed on genesets 1000 times, weighted statistics were used for enrichment, and the metric for ranking genes was signal2noise. The genesets were from GSEA Molecular Signatures Database v3.0.
Cell Vitality, Colony Formation, and Neurosphere Assays
For the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay, GSC lines were plated onto 96-well plates at a density of 2000 cells/well. After 24 h cells were exposed to VPA and exposed to MTT (1 mg/ml) (Invitrogen) for 4 h at 37 °C. Formazan release was quantified at 560 nm using a Microplate Reader (Biotek). The data are the average of five measurements from three independent treatments.
For the colony-forming assay, GSC lines were seeded in triplicate in six-well plates at a density of 800 cells/well. After 24 h, cells were exposed to VPA; thereafter, the drug was removed, and fresh medium was added. Colonies were scored after 12 days, fixed in methanol, and stained with 10% Giemsa.
For the neurosphere assay, GSC lines were seeded in duplicate in low attachment six-well plates (Corning) at a density of 3000 cells/well in the absence or presence of VPA. Sphere diameter was calculated from the area of each sphere using ImageJ software, free access by the National Institutes of Health (rsbweb.nih.gov). The data are the averages of three independent experiments.
Immunohistochemistry
Thirty-four glial tumors were studied: 24 glioblastoma multiformes, 3 World Health Organization (WHO) grade II astrocytomas and 2 WHO grade III astrocytomas, 3 WHO grade II and 2 WHO grade III oligodendrogliomas following the (WHO) guidelines (31). The study was approved by the Ethic Committee of the Azienda Ospedaliera CTO-Maria Adelaide (n. 487/212) of Turin.
Immunohistochemistry was performed on 5-μm-thick sections from formalin-fixed paraffin-embedded tissue using anti-human SEL1L mouse monoclonal antibody (1:350) (32) and the anti-human Ki-67/MIB.1 mouse monoclonal antibody (M7240, 1:100, Dako) on a Ventana Full BenchMark® automatic immunostainer (Ventana). The UltraViewTM Universal DAB detection kit was used. Heat-induced epitope retrieval was performed in Tris-EDTA (pH 8; Ventana).
RESULTS
SEL1L Expression in GSC Lines
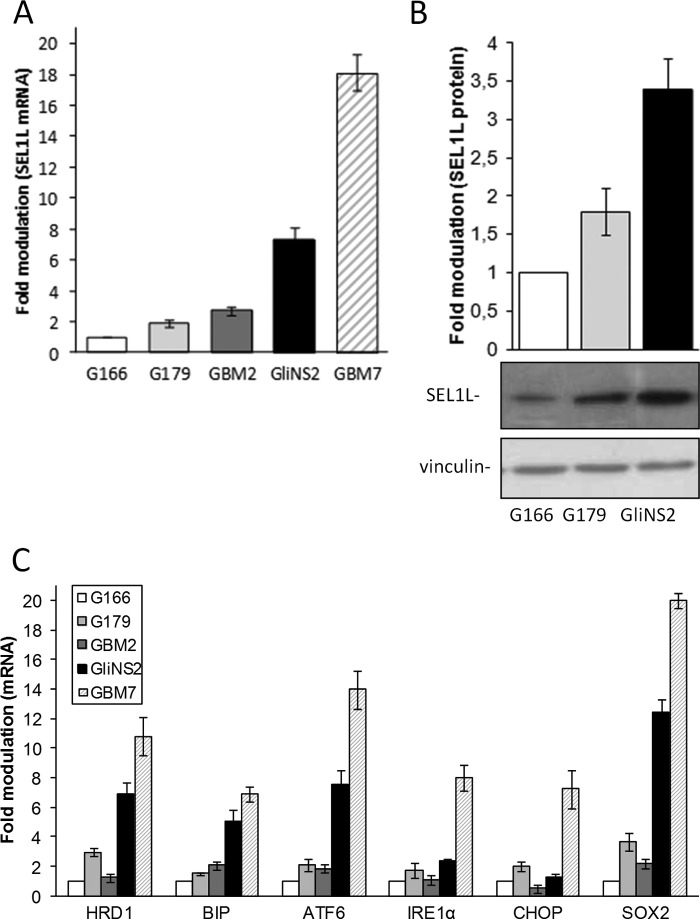
SEL1L mRNA and protein were evaluated in five GSC lines (G166, G179, GBM2, GliNS2, and GBM7) (28–30). The lowest levels were observed in G166, and the highest levels were observed in GliNS2 and GBM7 (Fig. 1, A and B). SEL1L followed the expression of SOX2 and that of several ER stress markers (Fig. 1C), suggesting a role in indicating ER stress conditions and GSC stemness conditions.
FIGURE 1.
SEL1L follows ER stress and stemness markers expression in GSC lines. A, SEL1L expression in GSC lines was analyzed by qPCR. The histogram indicates expression values normalized relative to housekeeping signals and expressed as -fold modulation relative to G166. B, Western blot analysis of SEL1L protein level in GSC lines. Lysates (50 μg) were resolved by SDS-PAGE and probed with SEL1L antibody. Vinculin was used as a loading control. The histogram shows expression values normalized relative to housekeeping signals and expressed as -fold modulation relative to G166. C, ER stress and stemness markers were analyzed by qPCR. The histogram shows expression values normalized relative to housekeeping gene and data expressed as -fold modulation relative to G166 cell line.
SEL1L Knockdown Reduces GSC Line Proliferation and Induces Neural Cell Differentiation
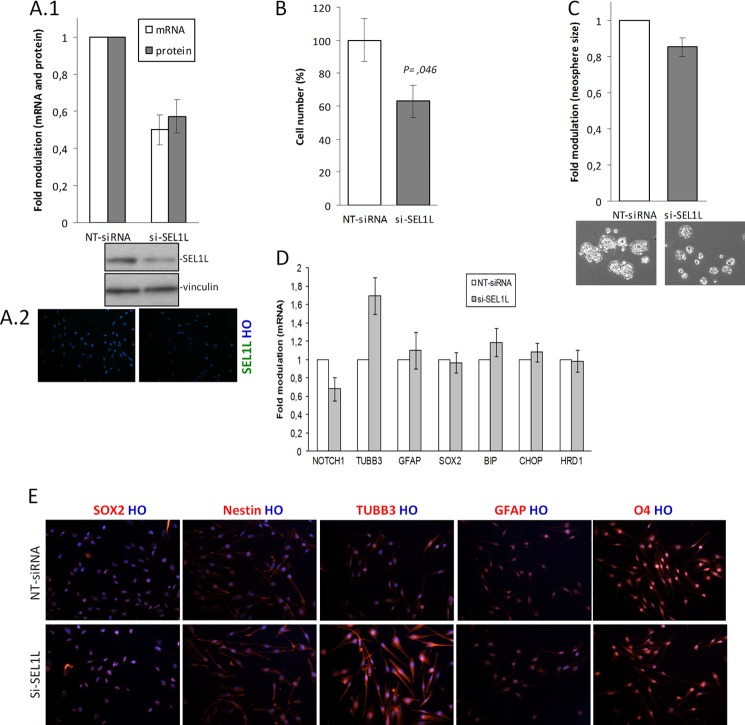
To explore the functional role of SEL1L in GSC lines, the G179 line was transiently nucleofected with two siRNAs against SEL1L (si-SEL1L) and one NT siRNA. Both si-SEL1L down-modulated SEL1L mRNA by at least 50% in the hard-to transfect G179 cell line and elicited the same silenced phenotype. Indeed, SEL1L silencing (50% of the mRNA and about 47% of the protein; Fig. 2, A1 and A2) impaired the cell proliferative capacity of 37% with respect to control si-NT siRNA cells (p = 0.046, Fig. 2B) in addition to reduction of neurosphere size (Fig. 2C).
FIGURE 2.
The effects of SEL1L silencing in G179 line. A, 1 and 2, SEL1L mRNA and protein silencing efficiency. G179 cell line (1 × 106) was nucleofected with 1 NT siRNA or 2 si-SEL1L for 48 h, and silencing efficiency was verified by mRNA using qPCR (1) and protein using Western blot (1) and immunofluorescence staining (2). A1, the histogram shows values normalized relative to housekeeping signals and expressed as-fold modulation relative to controls. The data reflect the average of two si-SEL1L and two independent knockdown experiments. B–E, G179 were treated with NT siRNA or two si-SEL1L for 48 h, harvested and analyzed for the following. B, trypan blue exclusion test. The histogram shows the averages of three independent experiments. Student's t test (p) was used to determine statistical significance. C, neurosphere assay. Cells were plated in six low attached wells (3000 cells/well) and maintained in neurosphere cell conditions for 10 days. The figure is a representative image of three independent experiments. D, qPCR analysis of si-SEL1L-mediated depletion determined a decrease in NOTCH1 expression but a parallel increase in TUBB3 levels. BIP, CHOP, and HRD1 as well as SOX2 and GFAP expression were unchanged after SEL1L depletion. The histogram shows expression values of treated cells normalized relative to housekeeping signals and expressed as -fold modulation relative to untreated samples. E, immunofluorescent analysis. A strong increase of TUBB3 immunoreactivity was observed in si-SEL1L cells with respect to controls. Nestin, SOX2, GFAP, O4, and Hoechst (HO) remained unchanged.
Interfered cells with the two si-SEL1L also showed: (i) a 32% decrease of Notch1 expression and (ii) a 1.7-fold increase of the neuronal differentiation marker βIII-tubulin (TUBB3) (Fig. 2D) (both genes have previously been described in SEL1L-linked signaling pathways (18, 34, 35)); (iii) neither SOX2 nor ER stress functional genes were affected (Fig. 2D), indicating no off-target effects. Immunofluorescence analysis confirmed TUBB3 increase and unvaried SOX2, Nestin, GFAP, and oligodendrocyte marker O4 (Fig. 2E). These data indicate that SEL1L may act as a supporter of GSC proliferation, and its depletion can instruct GSC toward neuronal fate.
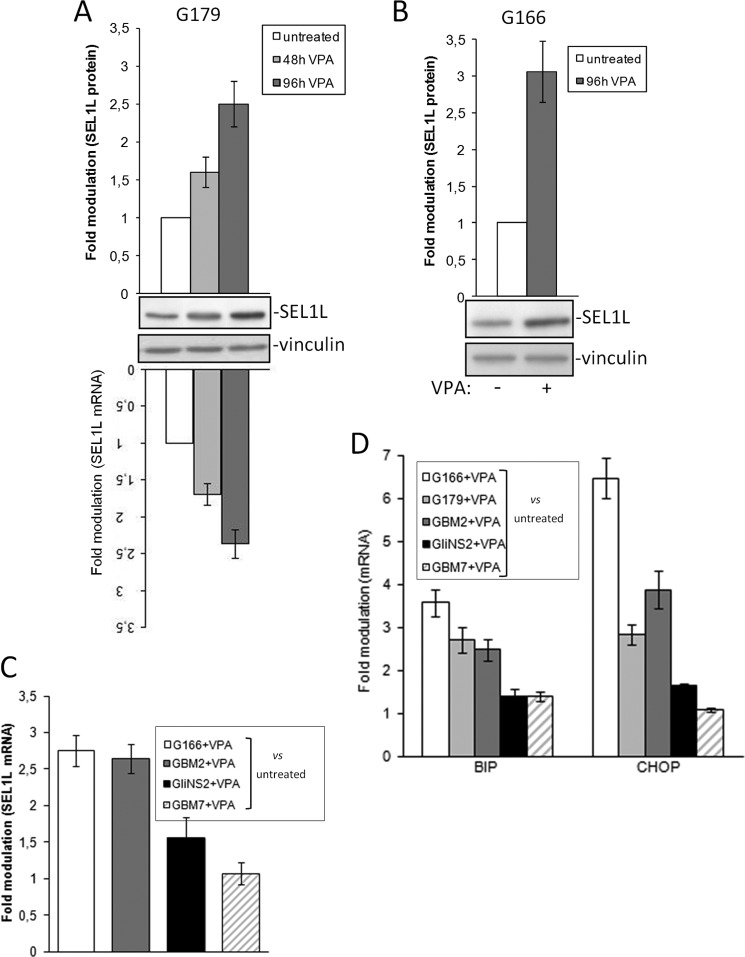
VPA Up-regulates SEL1L and UPR Gene Expression in GSC Lines
Histone deacetylase inhibitors, such as VPA, are used in several clinical trials including glioblastoma (8, 9, 36). Here we explored the effects of VPA on GSC by analyzing the levels of SEL1L and two central UPR-related genes (Bip and Chop) by a qPCR assay. G179 cells were exposed to clinically relevant VPA concentration of 2 mm (37) in a time course manner. The maximum effect was seen after 96 h post-treatment in the G179 line (Fig. 3A); hence, G166, GBM2, GliNS2, and GBM7 were assayed at the same time point. SEL1L levels strongly increased in G166 and GBM2 (Fig. 3, B and C); interestingly, these cells along with G179 showed the lowest endogenous levels of SEL1L (Fig. 1A). On the contrary, the GliNS2 and GBM7 with the highest initial expression (Fig. 1A) exhibited faint up-modulation of SEL1L after VPA addition (Fig. 3C).
FIGURE 3.
The effect of VPA on SEL1L and UPR expression. A, SEL1L expression in G179 exposed to VPA treatment was evaluated by qPCR and Western blot analysis in a time course experiment. The histograms shows expression values normalized relative to housekeeping signals and are expressed as -fold modulation relative to untreated G179. B, SEL1L protein levels in G166 exposed to VPA were evaluated by Western blot analysis. The histogram shows expression values normalized relative to housekeeping signals and are expressed as -fold modulation relative to untreated G166. C, SEL1L expression in GSC lines exposed to VPA was analyzed by qPCR. The histogram shows expression values normalized relative to housekeeping signals and are expressed as -fold modulation relative to untreated cells. D, UPR activation in GSC lines exposed to VPA was analyzed by qPCR. The histogram shows expression values normalized relative to housekeeping signals and are expressed as -fold modulation relative to untreated controls.
We next explored the ability of VPA to exacerbate the preexisting ER stress by monitoring the expression of BIP, the UPR master regulator, and CHOP, the executioner of the pro-apoptotic arm. Although G166, G179, and GBM2 displayed acute UPR activation upon VPA exposure, GliNS2 and GBM7 were moderately affected (Fig. 3D). The mechanism underlying the disparate UPR/SEL1L activation between cell lines still remains unclear, probably relying on the basal expression levels of the endogenous genes.
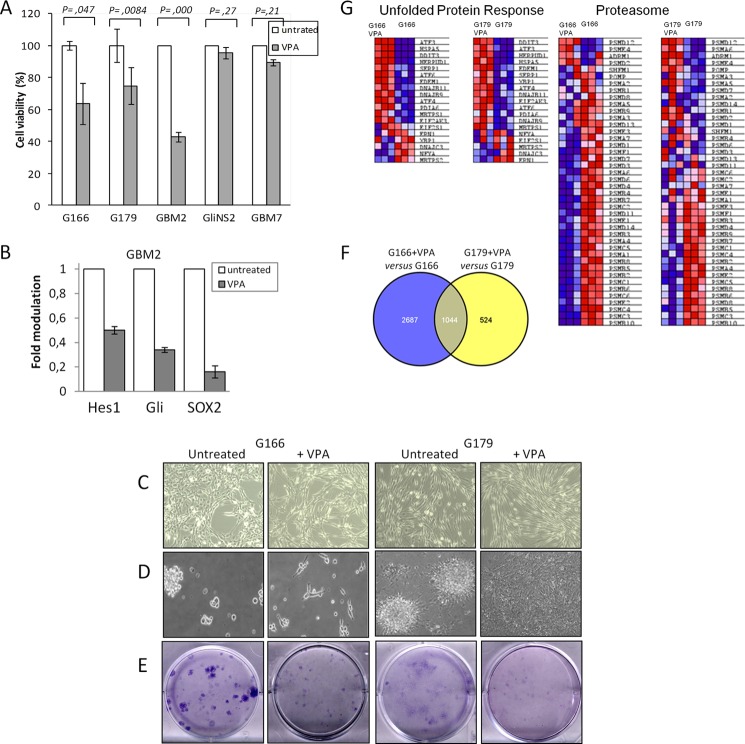
The biological relevance of VPA was evaluated using short and long term survival assays. MTT analysis revealed that VPA treatment significantly reduced cells viability by 36.5, 25.3, and 57.3% in G166, G179, and GBM2, respectively, whereas a modest effect was observed in GliNS2 and GBM7 (Fig. 4A). Among the GSC lines tested, GBM2 were the most responsive to the VPA cytotoxic activity, whereas GliNS2 and GBM7 were the more resistant (Fig. 4A). GBM2 exhibited, in addition to the aggravation of ER stress (Fig. 3D), a drastic decrease in SOX2, Notch, and Hedgehog signaling after VPA treatment (Fig. 4B), indicating that the mys-regulation of these networks may synergistically contribute in reducing protection against cytotoxicity mediated by VPA.
FIGURE 4.
VPA affects phenotypic and molecular characteristics of GSC lines. A, an MTT assay was applied to evaluate the viability of GSC lines after VPA treatment. The histogram shows cell viability values expressed as -fold modulation relative to untreated samples. p was used to determine statistical significance. B, pPCR of GBM2 VPA-treated. GBM2 were exposed to VPA treatment and analyzed for genes expression using qPCR. The histogram shows expression values of VPA-treated cells normalized relative to the housekeeping gene and are expressed as -fold modulation relative to untreated cell line. C and D, representative images of GSC lines plated on laminin-coated flasks (C) or maintained in neurosphere conditions (D) in the absence or presence of VPA. E, GSC lines (800 cells/well-6) were exposed to VPA and assayed for colony formation. The drug was replaced with fresh growth medium, and cells were allowed to form colonies for a further 12 days. Colonies were stained with Giemsa. The images are representative of three independent treatments. F, a Venn diagram shows the number of overlapping genes differentially expressed in VPA-treated GSC lines. G, UPR and a proteasome heat map in VPA-treated and untreated GSC lines.
Marked morphological changes were observed in both G166 and G179 after VPA exposure, both in adherent (Fig. 4C) and in neurosphere conditions (Fig. 4D). In adherent conditions, cells increased in volume and in length, whereas in neurosphere, cells grew from rolling spheres to adherent star-shaped cells with numerous cellular protrusions, indicating initial differentiation. A colony formation assay indicated drastic impairment of plating efficiency in both G166 and G179 after VPA exposure (Fig. 4E). These data indicate that VPA could be a pharmacologic ER stress aggravator able to affect aggressiveness and drug resistance.
Profiling GSC Lines Exposed to VPA
To gain insights into possible molecular mechanisms underlying sensitivity to VPA-induced cytotoxicity and the concomitant decrease in aggressiveness, whole G166 and G179 transcriptome exposed to VPA was profiled using Illumina beadchip arrays. A whole genome expression profile revealed that GSC lines exhibited extensive transcriptional changes as a consequence of VPA treatment. Expression profiles of VPA-treated versus untreated G166 cells revealed a total of 3731 differentially expressed genes at the nominal 0.0001 level of the univariate test, whereas 1568 genes were differentially expressed in VPA treated versus untreated G179 cells with an overlap of 1044 differentially expressed genes in both VPA-treated cells (30 and 67%, respectively) (Fig. 4F).
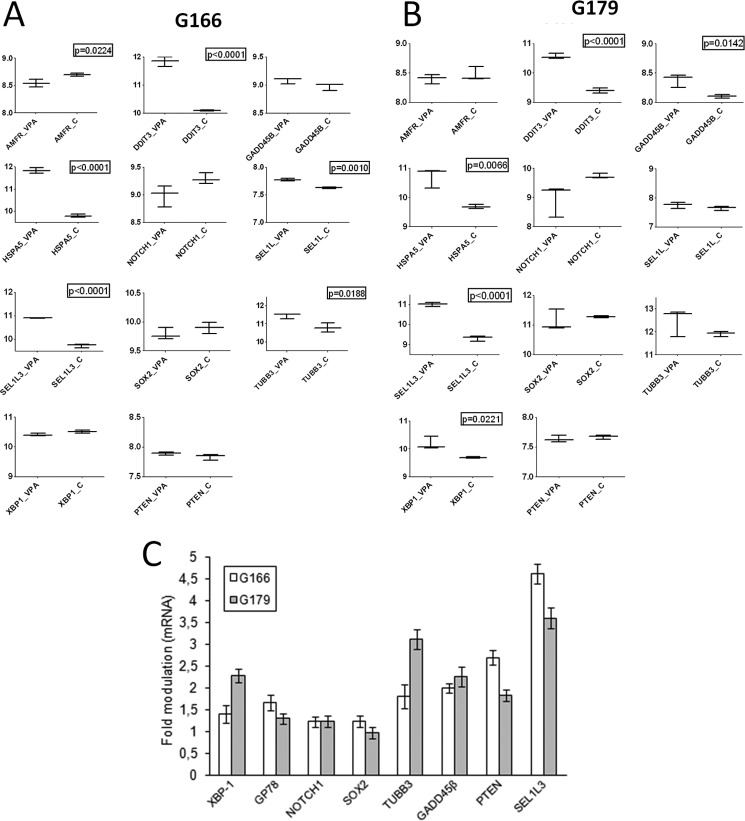
To better understand VPA effects on cell aggressiveness and proliferation, expression profiles from VPA-treated and untreated cells were subjected to functional analysis by GSEA using genesets from Canonical Pathways and Gene Ontology (GO)_Biological Process collections (supplemental Table S1). Although the proteasome protein-encoding genes were down-modulated, in both VPA-treated cells the UPR genes were significantly up-modulated (Fig. 4G, Table 2 and supplemental Table S1). Detailed analyses of UPR genes showed a greater increase of CHOP (DDIT3) and BIP (HSPA5) in VPA-treated cells (Fig. 5, A and B), confirming previous results.
TABLE 2.
GSEA analysis of gene expression of two GSC lines exposed to VPA
Genesets enriched in VPA-treated and untreated samples. The Enrichement Score represents the degree to which each gene set is overrepresented at the top or bottom of the ranked list of genes in the expression dataset; Normalized Enrichement Score (NES) estimates the enrichment score after it has been normalized across analyzed genesets; NOM p-value estimates the statistical significance of the enrichment score for a single gene set; FDR q-value is adjusted for gene set size and multiple hypotheses testing.
| Genesets | Geneset collection | G166 |
G179 |
||||
|---|---|---|---|---|---|---|---|
| NES | NOM p-value | FDR q-value | NES | NOM p-value | FDR q-value | ||
| Genesets Enriched in VPA-treated cells | |||||||
| REACTOME_UNFOLDED_PROTEIN_RESPONSE | Canonical pathways | 1,91 | 0,0018 | 0,0300 | 1,80 | 0,0018 | 0,0408 |
| NERVOUS_SYSTEM_DEVELOPMENT | GO_Biological Process | 1,96 | <0.002 | 0,0210 | 1,66 | <0.001 | 0,1223 |
| Genesets Enriched in Untreated Cells | |||||||
| PROTEASOME | Canonical pathways | −2,41 | <0.002 | <0.00004 | −1,60 | 0,0096 | 0,0491 |
| REACTOME_CELL_CYCLE_MITOTIC | Canonical pathways | −2,89 | <0.002 | <0.00004 | −2,94 | <0.002 | <0.00002 |
| REACTOME_CELL_CYCLE_CHECKPOINTS | Canonical pathways | −2,55 | <0.002 | <0.00004 | −2,54 | <0.002 | <0.00002 |
FIGURE 5.
Array validation. Box plots of gene expression levels of selected genes in VPA-treated and untreated GSC line (A and B) and qPCR of the same genes (C).
Gene Ontology analysis highlighted a down-modulation of cell cycle and cell cycle control-encoding genes in VPA-treated samples that paralleled the impaired growth after drug exposure (Table 2, supplemental Table S1 and Fig. 4, A and E). In addition, enrichment of geneset collecting genes related to neuronal development and neuronal differentiation in VPA-treated cells supported that morphological changes of cells exposed to VPA are driven by a differentiated process (Table 1, supplemental Table S1 and Fig. 4, C and D).
Eleven genes were chosen for validation by a qPCR assay (Fig. 3, A, C, and D, and Fig. 5C). Overall, the two methods of analysis showed good concordance with the exception of few genes (e.g. PTEN and GP78 alias AMFR) that emerged to be slightly different between the transcriptome and gene-specific analysis (Fig. 5). This may be due to the endogenous low expression levels of these genes whose faint expression differences could only be detected through the high sensitivity and specificity of the real-time PCR but not through the stringent criteria used for array expression.
SEL1L Knockdown Affects GSC Line Proliferative and Self-renewal Potential after VPA Addition
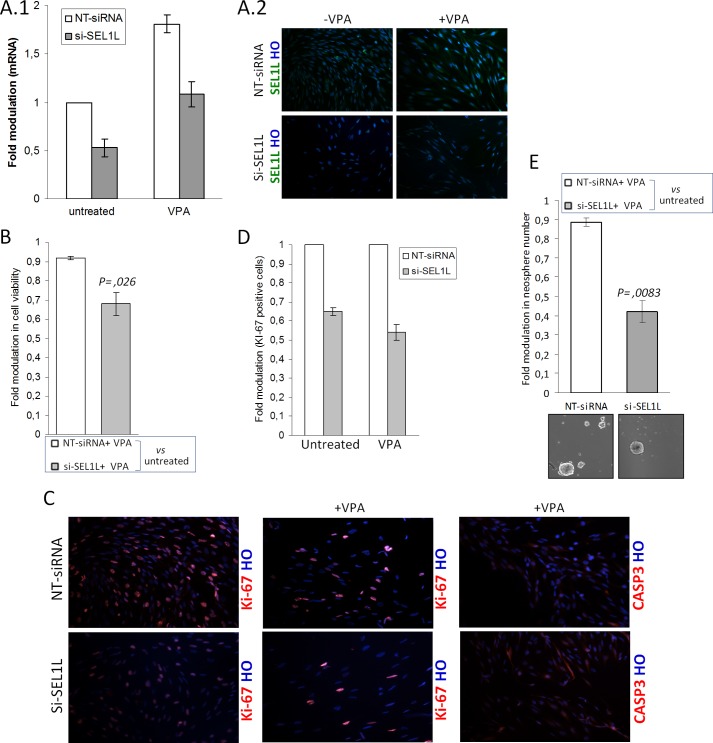
VPA treatment up-regulated SEL1L expression in almost all GSC lines under analysis (Fig. 3, A–C), suggesting the potential protection of SEL1L against VPA cytotoxicity. Here, we examined whether SEL1L down-modulation affected VPA toxicity on G179 line. Two si-SEL1L proteins were used to assess the minimal or no off-target activity and the reliability of the silenced phenotype.
Although VPA treatment induced SEL1L mRNA and protein in both mock and interfered cells (a 1.81-fold increase in VPA treated versus untreated NT-siRNA cells and a 2-fold increase in VPA-treated versus untreated si-SEL1L cells), inhibition of about 50 and 40% was preserved both in untreated and treated si-SEL1L cells, respectively, compared with controls (Fig. 6A, 1 and 2). An MTT assay shows that SEL1L depletion in combination with VPA treatment significantly decreased cell survival of about 24% (Fig. 6B) and concomitantly down-modulated the number of Ki-67 positive cells, as revealed by antigenic analysis (Fig. 6, C and D), suggesting that SEL1L abatement played a crucial role in improving drug efficiency specifically in acutely exacerbated stressful conditions.
FIGURE 6.
Effects of si-SEL1L and VPA combined treatments in G179. A, 1 and 2, SEL1L mRNA and protein silencing efficiency after VPA treatment. G179 cell line (1 × 106) was nucleofected with NT siRNA or two si-SEL1L for 48 h and maintained in the absence or presence of VPA for an additional 4 days. The data reflect the average of two si-SEL1L and two independent knockdown experiments. Silencing efficiency was verified by mRNA using qPCR (1) and protein using immunofluorescent staining (2). 1, the histogram shows values normalized relative to housekeeping signals and are expressed as -fold modulation relative to controls. HO, Hoechst. B–E, the G179 was treated with NT siRNA or si-SEL1L for 48 h and analyzed as follows. B, MTT cell viability assay. Nucleofected cells were seeded in 96-well plates at a density of 2000. After 24 h, cells were treated with VPA and analyzed for viability. The histogram shows cell viability values expressed as -fold modulation relative to drug-treated (VPA) versus untreated samples. p was used to determine statistical significance. C, Immunofluorescence analysis. Nucleofected cells were seeded on 12-well cover glass and maintained in the absence or presence of VPA for 4 days. A decrease in the number of positive Ki-67 cells was observed in si-SEL1L G179s with respect to control either in the absence or presence of VPA. Scattered areas with faint CASP3 immunoreactivity were observed in treated si-SEL1L G179 cells. D, Ki-67 was evaluated in terms of % of positively stained nuclei counted in at least 800 cells per group. E, Neurosphere assay. Nucleofected cells were seeded in 6 low attachment plates wells at density of 3000 and maintained in neurosphere cell conditions in the absence or presence of VPA for 4 days. Then the culture was allowed to recover from drug treatment by adding fresh medium in excess for an additional 7 days. The figure is a representative image of three independent experiment. p was is used to determine statistical significance.
We examined cell death in these cells by assaying for caspase 3 cleavage (data not shown) and by immunoreactivity, which did not indicate pronounced apoptosis (Fig. 6C). We also examined the effect of SEL1L knockdown combined with VPA treatment on neurosphere formation. As shown in Fig. 6E, the number of neo-formed neurospheres was much lower in G179 cells treated with both si-SEL1L and VPA than that observed in control. This indicates that SEL1L knockdown affects the self-renewal process in aggravated ER stress conditions. These results indicate SEL1L as a protector against VPA cytotoxic effect, acting in the maintenance of proliferative and self-renewal potential.
Immunohistochemistry of SEL1L Expression in Human Primary Gliomas
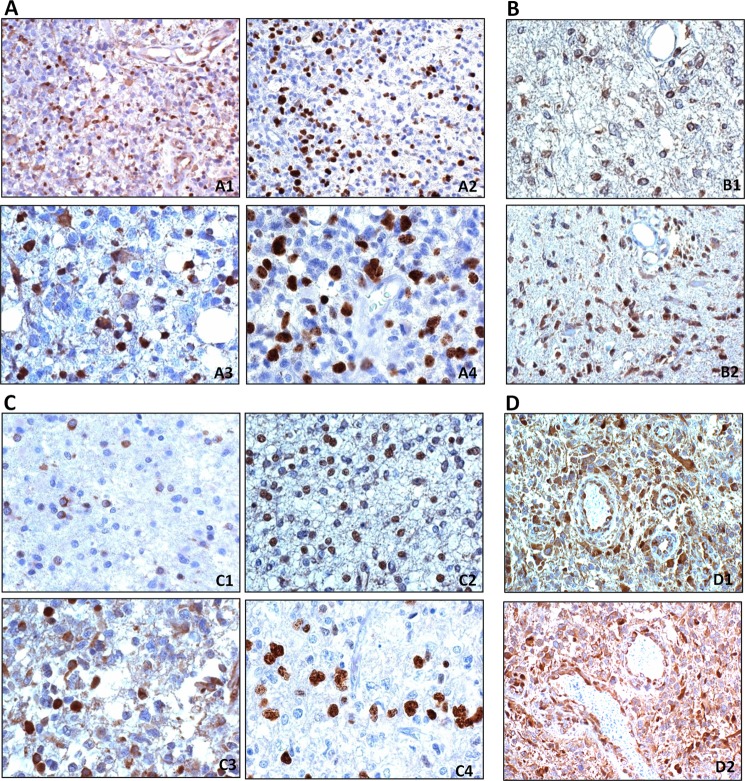
SEL1L expression was evaluated in a small series of primary gliomas. Immunohistochemistry analysis was performed on a total of 34 formalin-fixed paraffin sections of glial tumors (24 glioblastomas, 5 WHO grade II-III astrocytomas, and 5 WHO grade II-III oligodendrogliomas). In glioblastomas, a diffuse and variable staining of SEL1L was observed in tumor cells both in the nuclei and cytoplasms. More intense SEL1L immunoreactivity was observed in scattered nuclei (Fig. 7A, 1 and 3) and cytoplasms. which show the same distribution as those positive for Ki-67/MIB.1 (Fig. 7A, 2 and 4).
FIGURE 7.
Immunohistochemistry of SEL1L in gliomas. A, glioblastoma. A1, SEL1L-positive cells are mainly seen in the nucleus of proliferating area. A2, the parallel section was stained for Ki-67/MIB.1, DAB, 200×; A3, cells with SEL1L nuclear and cytoplasmic expression distributed as Ki-67/MIB.1-positive nuclei (A4). DAB, 400×. B, astrocytoma. B1, in diffuse WHO grade II astrocytoma, SEL1L cytoplasmic and perinuclear expression is very low; B2, WHO grade III astrocytoma show higher numbers of nuclei-positive SEL1L cells. DAB, 400×. C, oligodendroglioma. C1, WHO grade II oligodendroglioma, variable SEL1L nuclear, perinuclear, and cytoplasmic expression; the distribution of the most intense nuclei parallels (C2) that of Ki-67/MIB.1-positive nuclei; C3, WHO grade III oligodendroglioma, high frequency of SEL1L-positive nuclei show the same distribution as those positive for Ki-67/MIB.1 (C4). DAB, 400×. D, tumor vessels. D1 and D2, SEL1L-positive staining in the cytoplasm of hypertrophic endothelial cells. DAB, 200×.
Low grade astrocytomas displayed weak SEL1L cytoplasmic and perinuclear expression (Fig. 7B1), but in WHO grade III areas its expression became also nuclear (Fig. 7B2). The limited number of cases does not allow for a clear correlation with Ki-67/MIB.1 labeling index. In WHO grade II oligodendrogliomas, not more than 5–10% of cells showed a variable positive nuclear, perinuclear, and cytoplasmic staining (Fig. 7C1). The most intensely stained nuclei correspond to the Ki-67/MIB.1 distribution (Fig. 7C2). This is more evident in WHO grade III oligodendroglioma (Fig. 7C, 3 and 4). Tumor vessel cells and endothelia are positive (Fig. 7D, 1 and 2). Nonetheless, further work on a higher number of cases is necessary to draw any significant conclusion on the role of SEL1L in glioblastomas. Taken together, the data indicate that SEL1L expression is associated with the histological malignancy grade and that the expression intensity seems to parallel the Ki-67/MIB.1 distribution.
DISCUSSION
High grade gliomas, especially glioblastoma, are the most aggressive and vascularized brain tumors characterized by high resistance to intensive combined therapies and low median survival rate (38). Ongoing studies are focusing on overcoming the main therapeutic resistance determinants represented by (i) methylation status of O6-methylguanine-DNA methyltransferase, an important mechanism contributing in decreasing the resistance to alkylating agents, (ii) mis-regulation of several complex signaling pathways, (iii) existence of glioma stem-like cells, a small population of brain tumor exhibiting stem cell features and implicated in radio and chemoresistance, (iv) the blood-brain barrier, and (v) angiogenesis (38).
VPA emerges as a promising therapeutic agent by virtue of its anticancer and antiangiogenesis properties and the capacity to cross the blood-brain barrier with minimal toxicity over long term administration (39–42). Being a UPR pathway activator in glioblastoma (11), VPA may also deregulate protein homeostasis and induce proteotoxic stress, favoring the dominance of the proapoptotic over the adaptive response. Although adaptive and contra-adaptive modulation of UPR may represent a promising strategy for glioblastoma, the impact of this signaling, as well as the downstream targets, in GCS is still neglected.
Here we explore the potential therapeutic advantages of VPA treatment combined with perturbation of the proteostasis network on GSC by targeting SEL1L, a crucial modulator of homeostasis (15–17, 43) and linked to neural stemness maintenance and lineage determination (18) as well as cancer aggressiveness (19, 21, 43).
VPA treatment combined with SEL1L interference synergy enhanced the GSC drug sensitivity likely through affecting proliferative and self-renewal properties implicating that SEL1L protein sustain GSC to remain in an undifferentiative state and preserve their stem cell-like features. We hypothesize that SEL1L may be a defensive mechanism adopted by GSC to maintain stemness homeostasis under stressful conditions and highlight the necessity of its abatement to overwhelm the stem cell drug resistance.
Impairment of the GSC proliferative rate and neurosphere size occurred when the GSC line was subjected to SEL1L down-modulation. Indeed, SEL1L depletion (i) instructed GSC toward a neuronal rather than astrocytic or oligodendrocyte differentiation and (ii) affected Notch1 signaling, a well known pathway that supports GSC propagation (44). A direct link between SEL1L and Notch has already been shown in Caenorhabditis elegans whereas it acts as a negative regulator of Lin-12/Notch activity (34, 45); in mammalian cells indirect evidence underscored that SEL1L may regulate pancreatic epithelial growth and differentiation by suppressing Notch-mediated signaling (35) and influence the self-renewal and lineage commitment of the murine neural stem cells likely by mis-regulation of the Notch pathway (18). Studies are in progress to define whether SEL1L depletion is a direct determinant of the GSC phenotypic reversion or indirect through Notch deregulation. The neuronal commitment of the GSC line in response to SEL1L knockdown seems to be specific in tumor context as neural stem cell depleted for SEL1L exhibited a preferential astrocytic differentiation (18).
VPA alone affects GSC viability and anchorage-dependent growth likely by inducing the differentiation programs and by affecting the cell cycle progression, as underscored by whole genome analysis. VPA-modulated genes mainly aggregated into the functional categories of “cell cycle mitotic,” “cell cycle checkpoints,” and “nervous system development.” In addition, VPA exacerbated the pre-existing ER stress conditions in GSC, implying it might act as a well established ER stress aggravator of pharmacological compounds. These pharmacological agents have become particularly appealing for potential cancer therapy as, by exacerbating the ER stress, they might promote the switch of the engaged pro-survival response versus the pro-apoptotic wave of UPR, thus favoring tumor cell death (13). Consistently, a whole genome expression profile revealed the down-modulation of several genes coding for proteasome components in response to VPA treatment, implying that VPA might drastically compromise the degradation and secretion efficiency with a consequent overload of misfolded protein in the ER lumen and promotion of cell death, similar to the proteasome inhibitors (47). Among several ER stress aggravators, Gamitrinib-TPP and nelfinavir are inducers of SEL1L protein expression in glioblastoma cell line (48).3
Interestingly, VPA cytotoxicity testing on GSC shows different drug responses, stratifying GSC in responders and non-responders. Among responders, GBM2 displays a strong down-modulation in Notch and Hedgehog pathways, a signaling cascade responsible for cell stemness and survival maintenance as well as chemo/radio-resistance in glioma tumor initiating cell (44, 49, 50). Although the molecular mechanisms behind VPA action on these networks are currently unclear, our findings indicate a potential link between abatement of these pathways and reduced protection against VPA cytotoxicity. It can be speculated that the VPA effect on GSC viability could partially be a consequence of the interference on Notch/Hedgehog cascades, with important implications in glioma therapy. In support to our findings, several reports underlined the mis-regulation of Notch signaling cascade mediated by VPA as a novel strategy for cancer therapy (46, 51, 52).
Notably, the SEL1L link with cell proliferation was also observed in primary gliomas where SEL1L distribution and intensity paralleled those of Ki-67 and with tumor grade and proliferation. Interestingly, the SEL1L subcellular localization (nucleus or cytoplasm) may result from SEL1L variant activation in response to genotoxic insults, which is a previously described phenomenon (26, 27).
In summary, we reported on the synergic effect of SEL1L down-modulation combined with VPA treatment on GSC drug response; although the current study focused on the histone deacetylase inhibitor, SEL1L subversion might have relevance also in association with radiation treatment. Targeting SEL1L and its interacting partners might provide an innovative approach to deplete GSC and improve glioma treatment.
This work was supported by grants from Ministero della Salute (RF-MUL-2008-1248034) and Compagnia di San Paolo, Turin (2011.0438).

This article contains supplemental Table 1.
I. Biunno, personal communication.
- VPA
- valproic acid
- UPR
- unfolded protein response
- GSC
- glioma stem cell
- ER
- endoplasmic reticulum
- NT
- non-targeting si-RNA
- qPCR
- quantitative PCR
- GSEA
- Gene Set Enrichment Analysis
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- GFAP
- glial fibrillary acid protein.
REFERENCES
- 1. Baylin S. B., Ohm J. E. (2006) Epigenetic gene silencing in cancer. A mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer 6, 107–116 [DOI] [PubMed] [Google Scholar]
- 2. Walkinshaw D. R., Yang X. J. (2008) Histone deacetylase inhibitors as novel anticancer therapeutics. Curr. Oncol. 15, 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun P., Xia S., Lal B., Eberhart C. G., Quinones-Hinojosa A., Maciaczyk J., Matsui W., Dimeco F., Piccirillo S. M., Vescovi A. L., Laterra J. (2009) DNER, an epigenetically modulated gene, regulates glioblastoma. Derived neurosphere cell differentiation and tumor propagation. Stem Cells 27, 1473–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Das C. M., Aguilera D., Vasquez H, Prasad P, Zhang M, Wolff JE, Gopalakrishnan V. (2007) Valproic acid induces p21 and topoisomerase-II (α/β) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J. Neurooncol. 85, 159–170 [DOI] [PubMed] [Google Scholar]
- 5. Mottet D., Castronovo V. (2008) Histone deacetylases. Target enzymes for cancer therapy. Clin. Exp. Metastasis 25, 183–189 [DOI] [PubMed] [Google Scholar]
- 6. Shankar S., Srivastava R. K. (2008) Histone deacetylase inhibitors. Mechanisms and clinical significance in cancer. HDAC inhibitor-induced apoptosis. Adv. Exp. Med. Biol. 615, 261–298 [DOI] [PubMed] [Google Scholar]
- 7. Masoudi A., Elopre M., Amini E., Nagel M. E., Ater J. L., Gopalakrishnan V., Wolff J. E. (2008) Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res. 28, 2437–2442 [PubMed] [Google Scholar]
- 8. Su J. M., Li X. N., Thompson P., Ou C. N., Ingle A. M., Russell H., Lau C. C., Adamson P. C., Blaney S. M. (2011) Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors. A children's oncology group report. Clin. Cancer Res. 17, 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shabason J. E., Tofilon P. J., Camphausen K. (2011) Grand Rounds at the National Institutes of Health. HDAC inhibitors as radiation modifiers, from bench to clinic. J. Cell Mol. Med. 15, 2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu Y., Yang S. (2009) Angiotensin II induces cardiomyocyte hypertrophy probably through histone deacetylases. Tohoku J. Exp. Med. 219, 17–23 [DOI] [PubMed] [Google Scholar]
- 11. Kahali S., Sarcar B., Fang B., Williams E. S., Koomen J. M., Tofilon P. J., Chinnaiyan P. (2010) Activation of the unfolded protein response contributes toward the antitumor activity of vorinostat. Neoplasia 12, 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malhotra J. D., Kaufman R. J. (2007) The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 18, 716–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schönthal A. H. (2013) Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem. Pharmacol. 85, 653–666 [DOI] [PubMed] [Google Scholar]
- 14. Olzmann J. A., Kopito R. R., Christianson J. C. (2013) The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb. Perspect. Biol. 5, a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cattaneo M., Otsu M., Fagioli C., Martino S., Lotti L. V., Sitia R., Biunno I. (2008) SEL1L and HRD1 are involved in the degradation of unassembled secretory Ig-μ chains. J. Cell Physiol. 215, 794–802 [DOI] [PubMed] [Google Scholar]
- 16. Christianson J. C., Shaler T. A., Tyler R. E., Kopito R. R. (2008) OS-9 and GRP94 deliver mutant α1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat. Cell Biol. 10, 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Francisco A. B., Singh R., Li S., Vani A. K., Yang L., Munroe R. J., Diaferia G., Cardano M., Biunno I., Qi L., Schimenti J. C., Long Q. (2010) Deficiency of suppressor enhancer Lin12 1 like (SEL1L) in mice leads to systemic endoplasmic reticulum stress and embryonic lethality. J. Biol. Chem. 285, 13694–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cardano M., Diaferia G. R., Cattaneo M., Dessì S. S., Long Q., Conti L., Deblasio P., Cattaneo E., Biunno I. (2011) mSEL-1L (suppressor/enhancer Lin12-like) protein levels influence murine neural stem cell self-renewal and lineage commitment. J. Biol. Chem. 286, 18708–18719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cattaneo M., Orlandini S., Beghelli S., Moore P. S., Sorio C., Bonora A., Bassi C., Talamini G., Zamboni G., Orlandi R., Ménard S., Bernardi L. R., Biunno I., Scarpa A. (2003) SEL1L expression in pancreatic adenocarcinoma parallels SMAD4 expression and delays tumor growth in vitro and in vivo. Oncogene 22, 6359–6368 [DOI] [PubMed] [Google Scholar]
- 20. Liu Q., Chen J., Wang J., Amos C., Killary A. M., Sen S., Wei C., Frazier M. L. P. (2013) Putative tumor suppressor gene SEL1L was down-regulated by aberrantly up-regulated hsa-mir-155 in human pancreatic ductal adenocarcinoma. Mol. Carcinog. 10.1002/mc.22023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orlandi R., Cattaneo M., Troglio F., Casalini P., Ronchini C., Ménard S., Biunno I. (2002) SEL1L expression decreases breast tumor cell aggressiveness in vivo and in vitro. Cancer Res. 62, 567–574 [PubMed] [Google Scholar]
- 22. Barberis M. C., Roz E., Biunno I. (2006) SEL1L expression in prostatic intraepithelial neoplasia and adenocarcinoma. An immunohistochemical study. Histopathology 48, 614–616 [DOI] [PubMed] [Google Scholar]
- 23. Liu P., Ramachandran S., Ali Seyed M., Scharer C. D., Laycock N., Dalton W. B., Williams H., Karanam S., Datta M. W., Jaye D. L., Moreno C. S. (2006) Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 66, 4011–4019 [DOI] [PubMed] [Google Scholar]
- 24. Chandran U. R., Ma C., Dhir R., Bisceglia M., Lyons-Weiler M., Liang W., Michalopoulos G., Becich M., Monzon F. A. (2007) Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Granelli P., Cattaneo M., Ferrero S., Bottiglieri L., Bosari S., Fichera G., Biunno I. (2004) SEL1L and squamous cell carcinoma of the esophagus. Clin. Cancer Res. 10, 5857–5861 [DOI] [PubMed] [Google Scholar]
- 26. Ashktorab H., Green W., Finzi G., Sessa F., Nouraie M., Lee E. L., Morgano A., Moschetta A., Cattaneo M., Mariani-Costantini R., Brim H., Biunno I. (2012) SEL1L, an UPR response protein, a potential marker of colonic cell transformation. Dig. Dis. Sci. 57, 905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrero S., Falleni M., Cattaneo M., Malferrari G., Canton C., Biagiotti L., Maggioni M., Nosotti M., Coggi G., Bosari S., Biunno I. (2006) SEL1L expression in non-small cell lung cancer. Hum. Pathol. 37, 505–512 [DOI] [PubMed] [Google Scholar]
- 28. Pollard S. M., Yoshikawa K., Clarke I. D., Danovi D., Stricker S., Russell R., Bayani J., Head R., Lee M., Bernstein M., Squire J. A., Smith A., Dirks P. (2009) Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 4, 568–580 [DOI] [PubMed] [Google Scholar]
- 29. Griffero F., Daga A., Marubbi D., Capra M. C., Melotti A., Pattarozzi A., Gatti M., Bajetto A., Porcile C., Barbieri F., Favoni R. E., Lo Casto M., Zona G., Spaziante R., Florio T., Corte G. (2009) Different response of human glioma tumor-initiating cells to epidermal growth factor receptor kinase inhibitors. J. Biol. Chem. 284, 7138–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baronchelli S., Bentivegna A., Redaelli S., Riva G., Butta V., Paoletta L., Isimbaldi G., Miozzo M., Tabano S., Daga A., Marubbi D., Cattaneo M., Biunno I., Dalprà L. (2013) Delineating the cytogenomic and epigenomic landscapes of glioma stem cell lines. PLoS ONE 8, e57462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Louis D. N., Ohgaki H., Wiestler O. D., Cavenee W. K. (2007) WHO Classification of Tumors of the Central Nervous Systems, 4th Ed, International Agency for Research on Cancer (IARC), Lyon, France [Google Scholar]
- 32. Orlandi R., Cattaneo M., Troglio F., Campiglio M., Biunno I., Ménard S. (2002) Production of a monoclonal antibody directed against the recombinant SEL1L protein. Int. J. Biol. Markers 17, 104–111 [DOI] [PubMed] [Google Scholar]
- 33. Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P. (2005) Gene set enrichment analysis. A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grant B., Greenwald I. (1996) The Caenorhabditis elegans Sel-1 gene, a negative regulator of Lin-12 and Glp-1, encodes a predicted extracellular protein. Genetics 143, 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li S., Francisco A. B., Munroe R. J., Schimenti J. C., Long Q. (2010) SEL1L deficiency impairs growth and differentiation of pancreatic epithelial cells. BMC Dev. Biol. 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galanis E., Jaeckle K. A., Maurer M. J., Reid J. M., Ames M. M., Hardwick J. S., Reilly J. F., Loboda A., Nebozhyn M., Fantin V. R., Richon V. M., Scheithauer B., Giannini C., Flynn P. J., Moore D. F., Jr., Zwiebel J., Buckner J. C. (2009) Phase II trial of vorinostat in recurrent glioblastoma multiforme. A north central cancer treatment group study. J. Clin. Oncol. 27, 2052–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bowden C. L., Brugger A. M., Swann A. C., Calabrese J. R., Janicak P. G., Petty F., Dilsaver S. C., Davis J. M., Rush A. J., Small J. G. (1994) Efficacy of divalproex vs. lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA 271, 918–924 [PubMed] [Google Scholar]
- 38. Thomas R. P., Recht L., Nagpal S. (2013) Advances in the management of glioblastoma. The role of temozolomide and MGMT testing. Clin. Pharmacol. 5, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Y., Tsai Y. H., Tseng S. H. (2012) Valproic acid affected the survival and invasiveness of human glioma cells through diverse mechanisms. J. Neurooncol. 109, 23–33 [DOI] [PubMed] [Google Scholar]
- 40. Shao C. J., Wu M. W., Chen F. R., Li C., Xia Y. F., Chen Z. P. (2012) Histone deacetylase inhibitor, 2-propylpentanoic acid, increases the chemosensitivity and radiosensitivity of human glioma cell lines in vitro. Chin. Med. J. 125, 4338–4343 [PubMed] [Google Scholar]
- 41. Ryu C. H., Yoon W. S., Park K. Y., Kim S. M., Lim J. Y., Woo J. S., Jeong C. H., Hou Y., Jeun S. S. (2012) Valproic acid down-regulates the expression of MGMT and sensitizes temozolomide-resistant Glioma Cells. J. Biomed Biotechnol. 2012, 987495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osuka S., Takano S., Watanabe S., Ishikawa E., Yamamoto T., Matsumura A. (2012) Valproic acid inhibits angiogenesis in vitro and glioma angiogenesis in vivo in the brain. Neurol. Med. Chir. (Tokyo) 52, 186–193 [DOI] [PubMed] [Google Scholar]
- 43. Biunno I., Cattaneo M., Orlandi R., Canton C., Biagiotti L., Ferrero S., Barberis M., Pupa S. M., Scarpa A., Ménard S. (2006) SEL1L a multifaceted protein playing a role in tumor progression. J. Cell Physiol. 208, 23–38 [DOI] [PubMed] [Google Scholar]
- 44. Fan X., Khaki L., Zhu T. S., Soules M. E., Talsma C. E., Gul N., Koh C., Zhang J., Li Y. M., Maciaczyk J., Nikkhah G., Dimeco F., Piccirillo S., Vescovi A. L., Eberhart C. G. (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28, 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi M. S., Yoo A. S., Greenwald I. (2010) sel-11 and cdc-42, two negative modulators of LIN-12/Notch activity in C. elegans. PloS ONE 5, e11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adler J. T., Hottinger D. G., Kunnimalaiyaan M., Chen H. (2008) Histone deacetylase inhibitors up-regulate Notch-1 and inhibit growth in pheochromocytoma cells. Surgery. 144, 956–961; discussion 961-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Obeng E. A., Carlson L. M., Gutman D. M., Harrington W. J., Jr., Lee K. P., Boise L. H. (2006) Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107, 4907–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siegelin M. D., Dohi T., Raskett C. M., Orlowski G. M., Powers C. M., Gilbert C. A., Ross A. H., Plescia J., Altieri D. C. (2011) Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. J. Clin. Invest. 121, 1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Natsume A., Kinjo S., Yuki K., Kato T., Ohno M., Motomura K., Iwami K., Wakabayashi T. (2011) Glioma-initiating cells and molecular pathology. Implications for therapy. Brain Tumor Pathol. 28, 1–12 [DOI] [PubMed] [Google Scholar]
- 50. Takezaki T., Hide T., Takanaga H., Nakamura H., Kuratsu J., Kondo T. (2011) Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci. 102, 1306–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Machado M. C., Bellodi-Privato M., Kubrusly M. S., Molan N. A., Tharcisio T., Jr., de Oliveira E. R., D'Albuquerque L. A. (2011) Valproic acid inhibits human hepatocellular cancer cells growth in vitro and in vivo. J. Exp. Ther. Oncol. 9, 85–92 [PubMed] [Google Scholar]
- 52. Stockhausen M. T., Sjölund J., Manetopoulos C., Axelson H. (2005) Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br. J. Cancer 92, 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]