Background: The mechanism of the transition from osteoprogenitor cell proliferation to differentiation is unclear.
Results: Panx3 inhibits osteoprogenitor proliferation by blocking canonical Wnt signaling and promoting p21 activation.
Conclusion: A Panx3 hemichannel induces multiple Panx3 signaling pathways critical for the cell cycle exit.
Significance: Our findings reveal that Panx3 is a new regulator to switch the stage from proliferation to differentiation in osteoprogenitor cells.
Keywords: ATP, Calcium Intracellular Release, Cell Proliferation, Pannexin, Wnt Signaling, Hemichannel and ER Ca2+ Channel, Intracellular ATP and Ca2+ levels, Pannexin 3, Wnt/β-Catenin Signaling, p21
Abstract
Canonical Wnt signaling and BMP promote the proliferation and differentiation of osteoprogenitors, respectively. However, the regulatory mechanism involved in the transition from proliferation to differentiation is unclear. Here, we show that Panx3 (pannexin 3) plays a key role in this transition by inhibiting the proliferation and promoting the cell cycle exit. Using primary calvarial cells and explants, C3H10T1/2 cells, and C2C12 cells, we found that Panx3 expression inhibited cell growth, whereas the inhibition of endogenous Panx3 expression increased it. We also found that the Panx3 hemichannel inhibited cell growth by promoting β-catenin degradation through GSK3β activation. Additionally, the Panx3 hemichannel inhibited cyclin D1 transcription and Rb phosphorylation through reduced cAMP/PKA/CREB signaling. Furthermore, the Panx3 endoplasmic reticulum Ca2+ channel induced the transcription and phosphorylation of p21, through the calmodulin/Smad pathway, and resulted in the cell cycle exit. Our results reveal that Panx3 is a new regulator that promotes the switch from proliferation to differentiation of osteoprogenitors via multiple Panx3 signaling pathways.
Introduction
Highly coordinated proliferation and differentiation programs regulate bone development and homeostasis. Canonical Wnt signaling plays an important role in osteoprogenitor proliferation and bone mass (see Refs. 15–22). Wnt proteins are secreted signaling molecules that regulate many biological processes, such as proliferation, differentiation, maintenance, and survival, through β-catenin-dependent (canonical) and -independent pathways (noncanonical) (1–3). Canonical Wnt signaling involves protein stabilization and nuclear translocation of the downstream β-catenin protein, from its multimeric protein complex consisting of glycogen synthase kinase-3β (GSK3β),3 APC, and Axin (4, 5). In the absence of Wnt, β-catenin is phosphorylated by GSK3β in the complex and degraded through ubiquitination. Upon Wnt binding to a frizzled receptor, and its co-receptors LRP5/6, Axin, and GSK3β are recruited to the plasma membrane with the scaffold protein disheveled, which disrupts the protein complexes (6, 7). This disruption of the protein complexes leads to the phosphorylation of GSK3β and inhibition of β-catenin phosphorylation, resulting in the stabilization of β-catenin and its translocation into the nucleus. β-Catenin in the nucleus binds to TCF/LEF transcription factors to activate Wnt/β-catenin-responsive genes, such as CDK1 and cyclin D1, which are required for cell cycle progression (1, 8, 9). In addition to the membrane association mechanism, Wnt signaling is regulated by PKA and PI3K/Akt, which phosphorylate and inactivate GSK3β, resulting in the stabilization of β-catenin (10–13).
Genetic studies in human patients with the osteoporosis-pseudoglioma syndrome (14) showed that loss and gain mutations in LRP5 or LRP6 result in low (14) and high bone mass (15, 16), respectively. Studies in mice also support the crucial role of canonical Wnt signaling in bone development. LRP5 KO mice display inhibition of bone formation and osteoblast proliferation (17). Mice with the mutation of Dkk1, which prevents Wnt signaling by binding LRP5/6, have high bone density and an increased number of osteoblasts (18). Conditional β-catenin KO mice show low bone mass (19–21). Although canonical Wnt signaling is required for osteoprogenitor cell proliferation, the mechanism of how Wnt signaling is regulated during osteogenesis is still not fully understood. Osterix (Osx) negatively regulates canonical Wnt signaling by promoting the expression of Dkk1 and inhibits osteoblast proliferation (22). However, Osx is expressed in differentiating osteoblasts, not in the transitional stage from osteoprogenitor proliferation to osteoblasts (23).
Pannexins (Panxs) were recently identified as a new gap junction protein family (24). The Panx family consists of three members, Panx1, 2, and 3. Panx1 is ubiquitously expressed with particularly strong expression in the central nervous system. Panx2 is expressed in the central nervous system and is shown to modulate Panx1 channel activities (24–27). Panx3 is the member that was most recently identified by genome bioinformatic analysis (24). Although Panx3 is expressed in certain soft tissues, such as skin and coronary arteries (28, 29), we found high levels of Panx3 expression in developing hard tissues, including cartilage and bone (23, 30).
Previously, we demonstrated that Panx3 is induced in the prehypertrophic zone and in the perichondrium of the growth plate. Panx3 inhibits PTH-mediated chondrocyte proliferation by its hemichannel activity and promotes the differentiation of chondrocytes (30). We also showed that Panx3 promotes osteoblast differentiation through its multiple pathways (23). In this study, we demonstrate that Panx3 inhibits osteoprogenitor proliferation by inhibiting Wnt/β-catenin and PKA/CREB signaling and promotes the cell cycle exit by increasing p21 activity. Our results demonstrate that Panx3 is a new regulator that promotes the switch from proliferation to differentiation in osteoblasts.
EXPERIMENTAL PROCEDURES
Reagents
The rabbit anti-Panx3 antibody, Panx3 expression vector (pEF1/Panx3), control vector (pEF1), shRNA vector for Panx3 (shPanx3), and control vector (sh control) were described previously (23, 30). The control adenovirus (AdCont) and Panx3 expression adenovirus (AdPanx3) were prepared and purified by Welgen, Inc. The Akt-CA and Akt-DN vectors and TCF-luciferase reporter plasmids (Topflash and Fopflash) were obtained from Addgene. The antibodies for P-β-catenin, P-GSK3β, GSK3β, PKA, P-PKA, CREB, P-CREB, cyclin D1, P-Rb, Smad1, and P-Smad1/5 were obtained from Cell Signaling Technology, Inc. P-p21 and Rb were obtained from Santa Cruz, p21 was from BD Biosciences, β-catenin and α-tubulin were from Sigma, and Ki67 was from Dako. Dkk1 was obtained from Invitrogen, and Wnt3a was from R&D Systems. The PPDA was obtained from Sigma, BMP2 was from Humanzyme, and iQ SYBR Green Supermix was from Bio-Rad. The HRP-conjugated goat anti-mouse and goat anti-rabbit IgG were obtained from United States Biological (Swampscott, MA). The inhibitory Panx3 peptide and its control scrambled peptide were described previously (23, 30).
Cell Culture
C2C12 cells were grown in DMEM (Invitrogen) containing 10% FBS (HyClone) at 37 °C under 5% CO2. For the proliferation assay, the cells (2.5 × 103/ml) stably transfected with either pEF1/Panx3 or control pEF1 were cultured in DMEM in 96-well plates for up to 5 days. C2C12 cells stably transfected with the Panx3 shRNA vector were incubated with BMP2 (300 ng/ml) in 96-well plates and cultured for up to 5 days. The C3H10T1/2 cells were grown in DMEM/F-12 (Invitrogen) containing 10% FBS. For the proliferation assay, C3H10T1/2 cells transiently transfected with either pEF1/Panx3 or pEF1 were cultured in normal media in 96-well plates for up to 4 days. The cells transiently transfected with the Panx3 shRNA vector were incubated with an osteogenic medium (DMEM/F-12, 10% FBS, 100 μg/ml ascorbic acid, 10 mm β-glycerol phosphate, and 100 ng/ml BMP2). The cell proliferation activity was evaluated using a cell counting kit (Dojindo). The absorbance was measured using a microplate reader. The primary calvarial cells were prepared from the calvaria of newborn mice and cultured in α-minimum essential medium (α-MEM; Invitrogen) with 10% FBS, 100 units/ml of penicillin, and 100 μg/ml of streptomycin as previously described (31). For the proliferation assay, primary calvarial cells transiently transfected with pEF1/Panx3 or pEF1 were cultured in α-MEM in 96-well plates for 2 days. For the TOPflash/FOPflash reporter assays, luciferase reporter plasmids were co-transfected with the pRLSV40 plasmid (32) as an internal control for transfection efficiency. Luciferase activities were assayed using the Dual-Luciferase ReporterTM assay system (Promega). Relative luciferase activities were expressed as ratios of luciferase activities of the experimental vectors to the internal control vector.
Ex Vivo Calvarial Organ Culture
Calvarial bones were isolated from either newborn C57BL/6 mice or heterozygous Axin2LacZ mice obtained from Jackson Labs (33) and were cultured in DMEM containing 5% FBS, 50 μg/ml ascorbic acid (Sigma), and 1 mm β-glycerol phosphate (Sigma) at 37 °C in a humidified atmosphere of 5% CO2 as previously described (34, 35). One day after starting the culture, the calvarial bones were infected with either recombinant adenovirus AdCont or AdPanx3 (1 × 109 pfu/ml) for 2 days. For the peptide inhibition assay, the Panx3 inhibitory peptide or scramble peptide (100 μg/ml) was added to the calvarial culture, and the culture was incubated for 2 days. All experimental procedures were approved by the Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research.
Immunostaining and X-Gal Staining
For X-gal staining, the calvarial samples were fixed with 4% paraformaldehyde overnight, then equilibrated in sucrose, and embedded in an O.C.T compound (Tissue-Tek) for cryosection. X-gal staining was performed as described previously. For immunostaining, after deparaffinization and rehydration, the sections were treated with heat-induced epitope retrieval in a pH 6.0 citrate buffer (Dako). The quantification of LacZ- and Ki67-positive cells was analyzed using ImageJ 1.40g. For the staining of cultured cells, the cells were blocked with Power block (Biocare Medical) and reacted for 2 h at room temperature with primary antibodies. Primary antibodies were detected by Alexa 488 (Invitrogen) or by Cy-3 (Jackson ImmunoResearch Laboratories)-conjugated secondary antibodies. Nuclear staining was performed using Hoechst dye (Sigma-Aldrich). The analysis was performed on an LSM 710 inverted confocal microscope (Carl Zeiss MicroImaging, Inc.), and co-localization was analyzed by MetaMorph (Molecular Devices).
RT-PCR
The total RNA was extracted using QG-810 and the QuickGene RNA cultured cell HC kit S (Fujifilm). The total RNA (1 μg) was used for reverse transcription to generate cDNA, which was used as a template for the PCRs with gene-specific primers (Table 1), as previously described (30). Real time PCR amplification was performed with iQ SYBR Green Supermix (Bio-Rad) and the Eco real time PCR system (Illumina). Real time PCR was performed for 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Gene expression was normalized to the housekeeping gene Hprt.
TABLE 1.
Primer sequences for quantitative RT-PCR
| Gene name | Sequence |
|---|---|
| β-Catenin forward | 5′-TTTTCACTCTGGTGGATACGGC-3′ |
| β-Catenin reverse | 5′-CCCATCAACTGGATAGTCAGCAC-3′ |
| E2F1 forward | 5′-TTTGACTGTGACTTTGGGGACC-3′ |
| E2F1 reverse | 5′-AATGAGGCAGGAAGGATGGC-3′ |
| p21 forward | 5′-CCCTCTATTTTGGAGGGTTAATCT-3′ |
| p21 reverse | 5′-GTACCCTGCATATACATTCCCTTC-3′ |
Flow Cytometry Analysis
Stably transfected C2C12 cells (1.0 × 104 cells) were seeded in a 60-mm dish and cultured with or without BMP2 (300 ng/ml) for 2 days. For the Panx3 hemichannel blocking experiment, the cells were cultured with the Panx3 antibody (1.5 μg/ml) for 2 days without BMP2. For the primary calvarial cells, 8.0 × 104 transiently transfected cells were cultured for 2 days with or without the Panx3 antibody (1.5 μg/ml). The cells were then collected by centrifugation at 120 × g for 5 min. DNA content was analyzed by propidium iodide staining (EMD Biosciences) with CellQuest software on FACSCalibur Station (Becton Dickinson).
Measurement of Intracellular cAMP
The cells were seeded at 1.0 × 104 cells/well in a 96-well plate and cultured for 1 day with either DMEM for the C2C12 cells or α-MEM for primary calvarial cells. The cells were then incubated with media containing 0.1% albumin medium for 12 h, followed by incubation in media containing 10% serum for 1 h. The level of cAMP was determined with a Bridge-It cAMP designer fluorescence assay kit (Mediomics) and measured as previously described (30).
Western Blot Analysis
The cell lysates were prepared as previously described (30). Ten μg of each protein was electrophoresed in 4–12% SDS-polyacrylamide gel (Invitrogen) and transferred onto a polyvinylidene difluoride membrane using iBlot (Invitrogen). The membranes were immunoblotted with antibodies.
Data Analysis
Each experiment was repeated several times, and the data were analyzed using Prism 5 software. Statistical differences between two groups of data were analyzed with the Student's t test. One-way analysis of variance was used for cell proliferation assays with Wnt3a and Dkk1 (see Fig. 3A). p < 0.05 was considered to be statistically significant.
FIGURE 3.
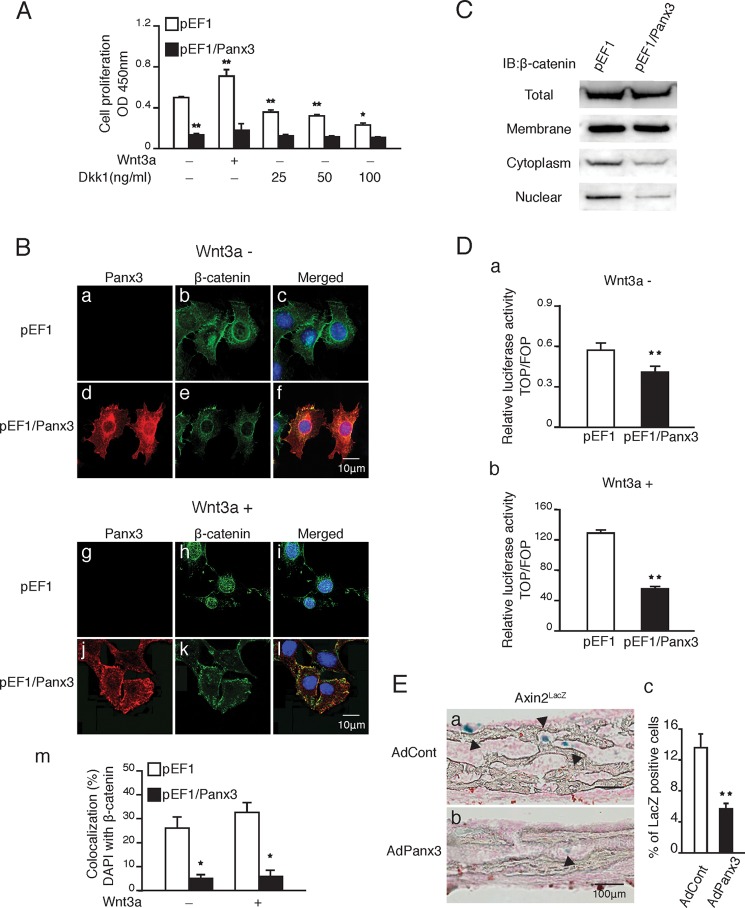
Panx3 inhibits Wnt/β-catenin signaling. A, proliferation of Panx3-overexpressing C2C12 cells with Wnt3a or Dkk1. pEF1 and pEF1/Panx3 transfected cells were cultured in DMEM with either Wnt3a (100 ng/ml) or several doses of Dkk1 for 2 days. B, panels a–l, cellular localization of β-catenin in Panx3-overexpressing cells with (panels g–l) or without (panels a–f) Wnt3a. Fluorescent confocal images showed Panx3 (red), β-catenin (green), and Hoechst nuclear staining (blue). Panel m, measurements show the percentage of β-catenin nuclear localization. C, β-catenin expression in cell membrane, cytoplasmic, and nuclear fractions. D, cells stably transfected with pEF1 and pEF1/Panx3 were co-transfected with either the TOPflash reporter construct or the FOPflash reporter construct as a negative control. The RLSV40 construct was co-transfected as an internal control. After transfection, cells were cultured in media with (panel b) or without (panel a) Wnt3a (100 ng/ml) for 1 day. The next day, the cell lysate was subjected to the dual luciferase reporter assay system. E, panels a and b, X-gal staining of ex vivo calvarial culture of Axin2LacZ mice infected with AdCont (panel a) or AdPanx3 (panel b). Panel c, quantification of LacZ-positive cells. Arrowheads show LacZ-positive cells. *, p < 0.05; **, p < 0.01. Error bars represent the means ± S.D., n = 3. IB, immunoblot.
RESULTS
Panx3 Inhibits Osteoprogenitor Cell Proliferation
Panx3 is induced during the transition from proliferation to differentiation in osteoprogenitor cells and promotes osteoblast differentiation (23, 30); therefore, we hypothesized that Panx3 regulates osteoprogenitor cell proliferation. We first examined the effects of Panx3 overexpression on the proliferation of primary calvarial cells from newborn mice. The neonatal calvarium contains progenitor cells for osteoblasts and adipocytes, of which the majority are osteoprogenitors (31, 36, 37). Transfection of a Panx3 expression vector (pEF1/Panx3) into primary calvarial cells cultured in proliferation media reduced proliferation compared with that of a control vector (pEF1) (Fig. 1A, panel a). We also tested the effect of Panx3 overexpression in multipotent C3H10T1/2 cells, which differentiate into osteoblasts with BMP2. Panx3 overexpression reduced the proliferation of C3H10T1/2 cells cultured in proliferation media (Fig. 1A, panel b). C2C12 cells are osteogenic and myogenic, depending on the culture conditions. C2C12 cells differentiate into osteoblasts with BMP2 in FBS-containing media, whereas they differentiate into myoblasts in horse serum containing media. As with primary calvarial cells and C3H10T1/2 cells, Panx3 overexpression reduced proliferation of the C2C12 cells cultured in FBS-containing media (Fig. 1A, panel c).
FIGURE 1.
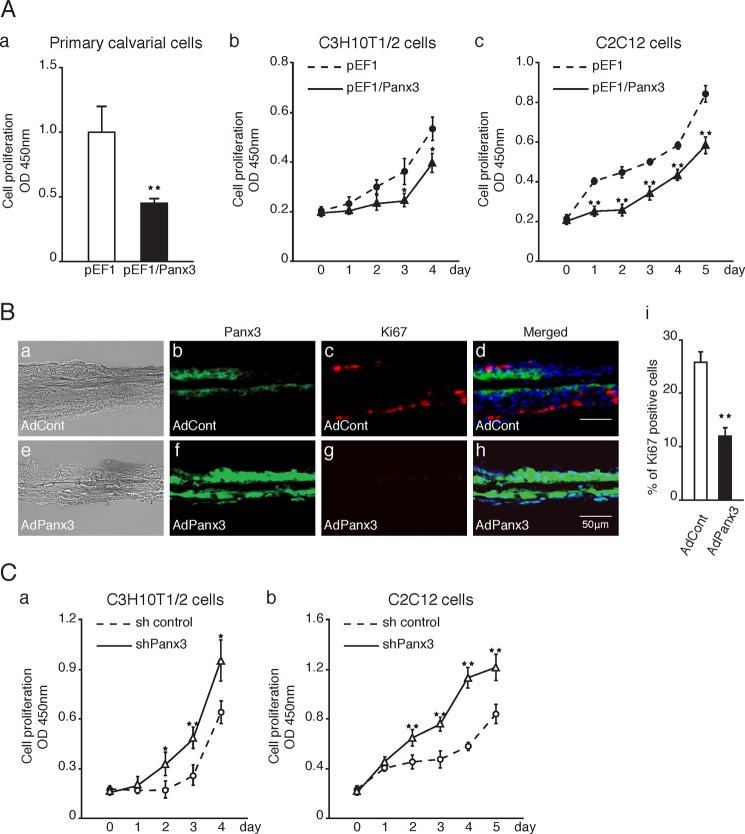
Panx3 inhibits cell proliferation. A, panel a, proliferation of Panx3-overexpressing primary calvarial cells. pEF1 or pEF1/Panx3 transiently transfected calvarial cells were cultured in α-MEM media for 2 days. Panels b and c, proliferation of Panx3-overexpressing or shPanx3 transfected C3H10T1/2 and C2C12 cells. Panel b, pEF1 or pEF1/Panx3 transiently transfected C3H10T1/2 cells were cultured in DMEM/F-12 media for the indicated days. Panel c, C2C12 cells were stably transfected with control vector (pEF1) or Panx3 expression vector (pEF1/Panx3). The transfected cells were cultured in undifferentiated media for the indicated days. B, newborn mouse calvarial bones were cultured and infected with AdPanx3 or AdCont for 2 days and were then immunostained. Panels a and e, images under light microscopy. Panels b and f, Panx3 antibody (green). Panels c and g, Ki67 antibody (red) and Hoechst nuclear staining (blue). Panels d and h, merged image. Panel i, quantification of Ki67-positive cells. C, proliferation of shPanx3 transfected osteogenic cell line. Panel a, sh control or shPanx3 transiently transfected C3H10T1/2 cells were cultured in osteogenic media. Panel b, sh control or shPanx3 stably transfected C2C12 cells were cultured with BMP2 (300 ng/ml). *, p < 0.05; **, p < 0.01. Error bars represent the means ± S.D., n = 7.
We next analyzed the inhibitory activity of Panx3 for proliferation in neonatal mouse calvarial organ culture using a recombinant adenovirus system (Fig. 1B). The control adenovirus infection (AdCont) showed staining of endogenous Panx3-expressing cells and Ki67-positive proliferating cells. The merged images revealed that these positive staining cells did not overlap (Fig. 1B, panels a–d). With a Panx3 adenovirus (AdPanx3) infection, the number of Panx3-expressing cells increased, whereas the number of Ki67-positive proliferating cells decreased (Fig. 1B, panels e–h). The Panx3-positive cells in either AdCont-infected (Fig. 1B, panels a–d) or AdPanx3-infected (Fig. 1B, panels e–h) calvaria did not overlap Ki67-positive cells, suggesting that the proliferation of Panx3-expressing cells is inhibited. The quantification also showed reduced numbers of Ki67-positive cells with the AdPanx3 infection (Fig. 1B, panel i). We next examined the effect of the suppression of endogenous Panx3 expression by Panx3 shRNA (shPanx3) on the proliferation of C3H10T1/2 and C2C12 cells cultured in the presence of BMP2, which induces endogenous Panx3 expression. The transfection of C3H10T1/2 and C2C12 cells with shPanx3 increased the proliferation of these cells compared with that in the control shRNA transfection cells (Fig. 1C, panels a and b). These results indicate that Panx3 inhibits osteoprogenitor proliferation.
Panx3 Promotes Cell Cycle Arrest at G0/G1 Phase
We examined the inhibitory activity of Panx3 in proliferation using the FACS analysis (Fig. 2). Calvarial cells overexpressing Panx3 accumulated in the G0/G1 phase and were reduced in both the S and G2/M phases (Fig. 2A). We also found a similar cell cycle arrest at the G0/G1 phase in C2C12 cells overexpressing Panx3 (Fig. 2B, panel a). Furthermore, shPanx3 transfected cells were reduced in the G0/G1 phases and increased in the G2/M phases (Fig. 2B, panel b). These results suggest that Panx3 expression in both primary calvarial cells and C2C12 cells inhibits proliferation by arresting the cell cycle at the G0/G1 phase.
FIGURE 2.
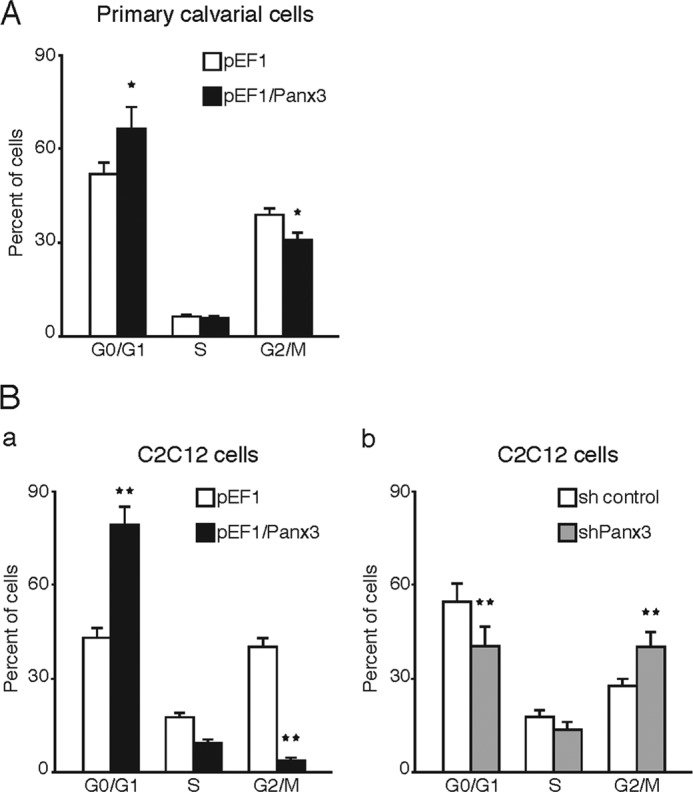
Panx3 arrests cell cycle at G0/G1 phase. FACS analysis of cell cycle was used. A, transiently transfected calvarial cells with pEF1 or pEF1/Panx3 were cultured in α-MEM for 2 days. B, 1 day after seeding, stably transfected C2C12 cells with pEF1, pEF1/Panx3, sh control, or shPanx3 vectors were incubated in serum-free 0.1% albumin containing DMEM for 12 h. pEF1- and Panx3-overexpressing cells were cultured in undifferentiated media for 2 days (panel a), whereas sh control and shPanx3 transfected cells were cultured with BMP2 (300 ng/ml) for 2 days (panel b). The cells were stained with propidium iodide, and cell cycle stages were measured by FACS analysis. The panels represent distribution of cells (%) in the G0/G1, S, and G2/M phases. *, p < 0.05; **, p < 0.01. Error bars represent means ± S.D. of three independent experiments.
Panx3 Inhibits Wnt/β-Catenin Signaling
Because canonical Wnt signaling promotes the proliferation of osteoprogenitor cells (17, 22, 38–41), Panx3 may block the Wnt/β-catenin pathway. To explore this possibility, we examined the effect of Wnt signaling on the proliferation of Panx3-overexpressing C2C12 cells (Fig. 3A). C2C12 cells produce canonical and noncanonical Wnts (data not shown) (42, 43). Without exogenous Wnt3a, the proliferation of Panx3-overexpressing cells was reduced when compared with that of the control cells similar to that shown in Fig. 1A. The addition of Wnt3a increased proliferation of the control cells but did not promote proliferation of the Panx3-overexpressing cells, which suggests that Panx3 inhibited Wnt signaling. Dkk-1, an antagonist of Wnt3a/LRP5 receptor interactions, inhibited proliferation even in the absence of exogenous Wnt3a. This occurred because the cells produce endogenous Wnts (43). Dkk-1 blocked the effect of Wnts in a dose-dependent manner. However, even at the highest dose (100 ng/ml) of Dkk-1, the inhibition level of the control cell proliferation by Dkk-1 was lower than that of the Panx3-overexpressing cells with or without Dkk-1 (last and first pairs of bars in Fig. 3A). This suggests that Panx3 inhibits cell proliferation through not only Wnt signaling, but also other pathways.
β-Catenin is involved in downstream canonical Wnt signaling (1, 44, 45). We therefore examined the expression and localization of the β-catenin protein in Panx3-overexpressing C2C12 cells and in control cells (Fig. 3B). In the control cells, β-catenin was localized in the plasma membrane and in the nucleus, whereas in Panx3-overexpressing cells, the protein level and nuclear localization of β-catenin were reduced (Fig. 3B, panels a–f). The addition of Wnt3a to the control cells increased the β-catenin localization in the nucleus. In contrast, in Panx3-overexpressing cells, the nuclear localization of β-catenin was reduced (Fig. 3B, panels g–l). Quantitative analysis confirmed the reduced nuclear localization level of β-catenin in Panx3-overexpressing cells, compared with that in the control cells (Fig. 3B, panel m). Western blot analysis revealed that Panx3 reduced the protein level of β-catenin, especially in both the cytoplasm and nucleus (Fig. 3C). These results suggested that Panx3 inhibited canonical Wnt signaling by reducing β-catenin activity. Moreover, we analyzed β-catenin activity using a TOPflash reporter vector (Fig. 3D) and found that TOPflash reporter activity was reduced in the Panx3-overexpressing cells compared with that in the control cells (Fig. 3D, panel a). The reporter activity was strongly activated in the control cells by the addition of Wnt3a, whereas it was inhibited in the Panx3-overexpressing cells (Fig. 3D, panel b).
We also examined β-catenin activity in an ex vivo culture of calvarial bone from heterozygous Axin2LacZ mice containing an Axin2-lacZ knock-in allele, which is a target gene of β-catenin (33). Infection with AdPanx3 reduced the number of LacZ-positive cells compared with that of infection with AdCont (Fig. 3E, panel a and b). The quantification showed a lower percentage of LacZ-positive cells in the AdPanx3-infected cells (Fig. 3E, panel c). These results indicate that Panx3 inhibits Wnt signaling by reducing β-catenin activity.
Panx3 Promotes β-Catenin Degradation by GSK3β Activation
To identify the mechanism of inhibition of β-catenin activity by Panx3, we first analyzed the mRNA and protein levels of β-catenin as well as its activation level in Panx3-overexpressing C2C12 cells. We found that β-catenin mRNA levels did not change significantly between the control cells and Panx3-overexpressing cells (Fig. 4A). We also examined the effect of the inhibition of endogenous Panx3 expression on β-catenin mRNA expression by shPanx3 in C2C12 cells that had been treated with BMP2 for a short time to induce endogenous Panx3 expression. The shPanx3 did not change the β-catenin mRNA expression level when compared with that of the control shRNA (Fig. 4A).
FIGURE 4.
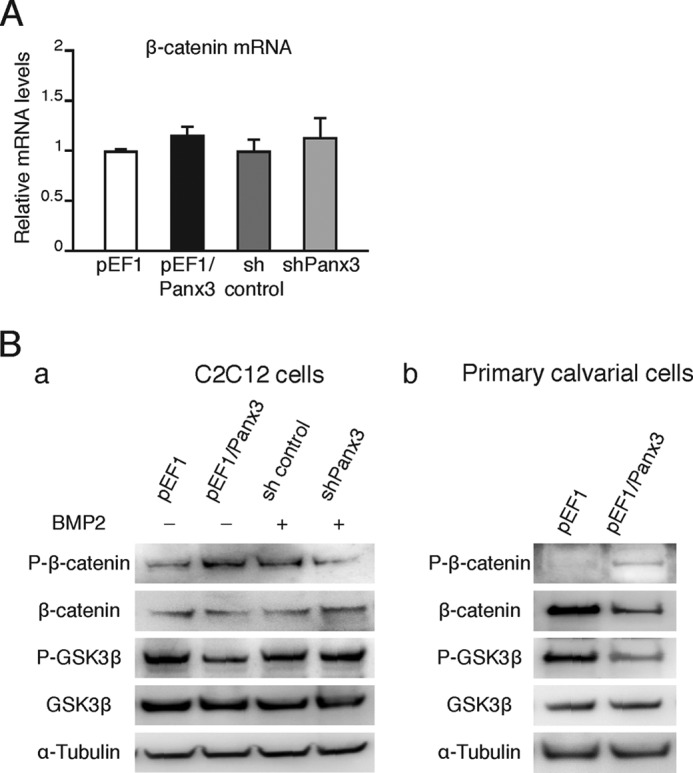
Panx3 promotes β-catenin degradation and GSK3β activity. A, quantitative RT-PCR for β-catenin mRNA expression in C2C12 cells stably transfected with either pEF1, pEF1/Panx3, sh control, or shPanx3. pEF1 and pEF1/Panx3 transfected cells were cultured in DMEM for 1 day, whereas sh control and shPanx3 transfected cells were cultured with BMP2 (300 ng/ml) in DMEM for 1 day. B, phosphorylation of β-catenin and GSK3β. Control and Panx3 overexpressed cells were cultured for 1 day in DMEM containing 10% FBS, whereas sh control and shPanx3 transfected cells were cultured with BMP2 for 1 day. Lysates were analyzed by Western blotting with antibodies to phospho-β-catenin (P-β-catenin), β-catenin, phospho-GSK3β (P-GSK3β), GSK3β, and α-tubulin (panel a). Calvarial cells transfected with pEF1 or pEF1/Panx3 were cultured in α-MEM for 2 days (panel b). Error bars represent the means ± S.D., n = 3.
Western blot analysis showed that, in contrast to the mRNA levels, β-catenin protein levels were reduced in Panx3-overexpressing cells and were higher in shPanx3 transfected cells (Fig. 4B, panel a). Furthermore, the phosphorylation of β-catenin was promoted in Panx3-overexpressing cells, whereas its phosphorylation levels were reduced by shPanx3 (Fig. 4B, panel a). We found that the phosphorylation of GSK3β was inhibited in Panx3-overexpressing cells, whereas shPanx3 produced the opposite effect (Fig. 4B, panel a). Additionally, we observed similar results in primary calvarial cells (Fig. 4B, panel b). These results suggest that that Panx3 induces β-catenin degradation via increasing GSK3β activity.
Panx3 Hemichannels Inhibit Osteoprogenitor Proliferation
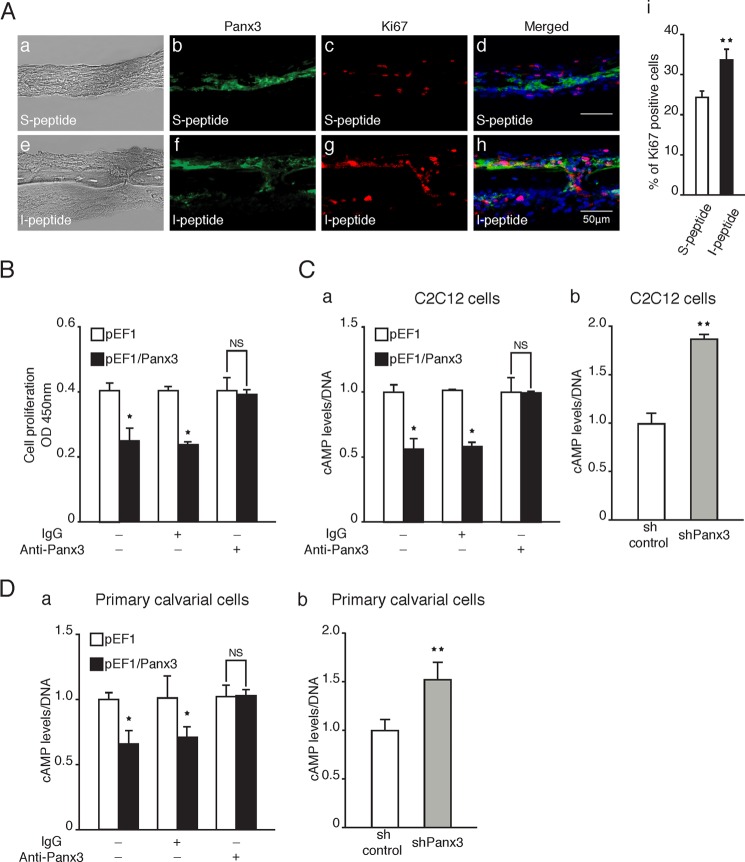
Hemichannels link the cytoplasm with the extracellular space and regulate cellular signaling through the release of small molecules such as ATP, and Panx3 functions as a hemichannel (23, 30). Therefore, the Panx3 hemichannel may be involved in the inhibition of osteoprogenitor proliferation. To address this possibility, we first examined whether I-peptide, which is an inhibitor specific to Panx3 hemichannel function (23, 30), abrogates the Panx3-mediated inhibition of cell proliferation in an ex vivo calvarial culture. The addition of I-peptide increased the number of Ki67-positive proliferating cells (Fig. 5A, panels e–h). We found that many Ki67-positive cells were also positive for Panx3. This is because the I-peptide inhibited the Panx3 hemichannel function but not the Panx3 expression and increased the proliferation of Panx3-positive cells. A control scramble peptide (S-peptide) containing the same amino acid contents of the I-peptide did not affect the proliferation state (Fig. 5A, panels a–d). Under this condition, the Panx3- and Ki67-positive cells did not overlap. The quantification confirmed the increased number of Ki67-positive cells as a result of the I-peptide addition (Fig. 5A, panel i). These results suggest that the Panx3 hemichannel plays an important role in the inhibition of osteoprogenitor proliferation.
FIGURE 5.
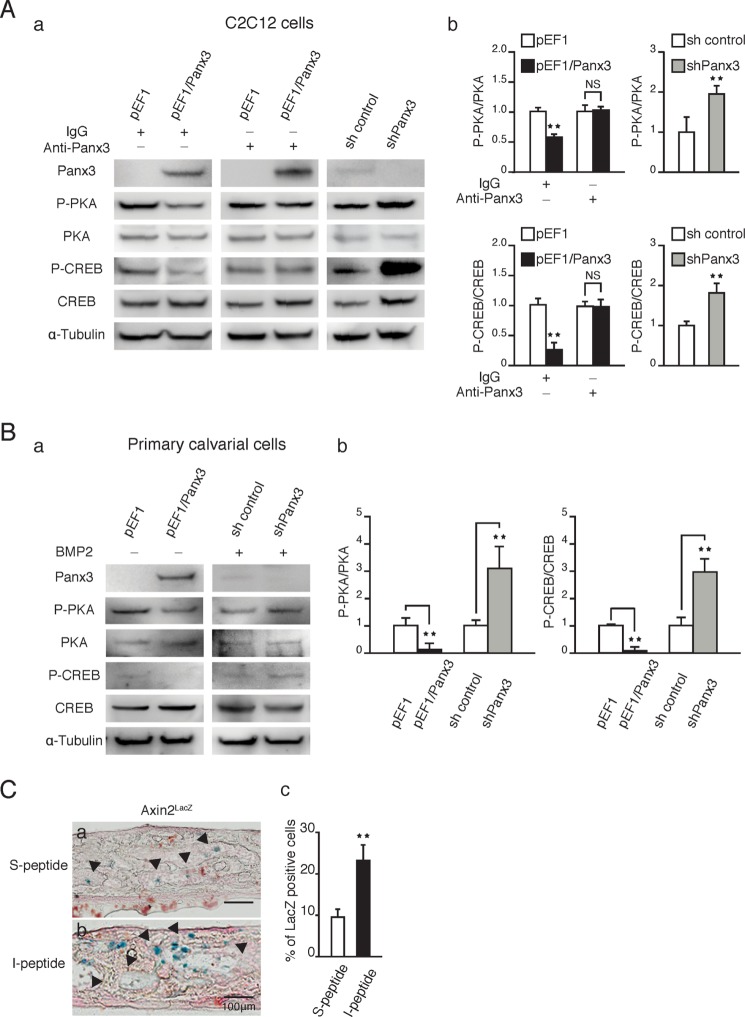
Panx3 reduces intracellular cAMP levels and the PKA/CREB signaling pathway. A, calvarial cultures were incubated with either I-peptide or S-peptide for 2 days followed by immunostaining. Panels a and e, image under light microscopy. Panels b and f, Panx3 antibody (green). Panels e and g, Ki67 antibody (red) and Hoechst nuclear staining (blue). Panels d and h, merged image. Panel i, quantification of Ki67-positive cells. B, cell proliferation in the presence of Panx3 antibody. pEF1 and pEF1/Panx3 transfected C2C12 cells were cultured with anti-Panx3 antibody for 2 days. C, the intracellular cAMP level. Panel a, pEF1 and pEF1/Panx3 transfected cells were incubated in serum-free DMEM with 0.1% albumin for 8 h and then incubated in 10% FBS-containing DMEM with or without Panx3 antibody for 1 day. Panel b, sh control and shPanx3 transfected cells were cultured with BMP2 (300 ng/ml) for 2 days. D, intracellular cAMP levels in Panx3-overexpressing or shPanx3 transfected calvarial cells. Panel a, At 1 day after transfection, the cells were cultured in 10% FBS-containing α-MEM with or without Panx3 antibody (1.5 μg/ml) for 1 day. Panel b, at 1 day after transfection, the cells were induced in 5% FBS-containing α-MEM with BMP2 (300 ng/ml) for 1 day. *, p < 0.05; **, p < 0.01. NS, nonsignificant. Error bars represent the means ± S.D., n = 3.
We confirmed this Panx3 hemichannel function using the Panx3 antibody, which reacts with the extracellular domain of Panx3 and inhibits the Panx3 hemichannel (23, 30). We showed that the addition of the Panx3 antibody to the culture abrogated the inhibition of Panx3-overexpressing C2C12 cell proliferation (Fig. 5B). Because intracellular ATP plays an important role in cell proliferation by regulating the intracellular cAMP levels (46, 47) (see Fig. 8), we examined the intracellular cAMP levels in C2C12 cells. Panx3 overexpression reduced the intracellular cAMP levels caused by the release of intracellular ATP via the Panx3 hemichannel (Fig. 5C, panel a), which is consistent with our previous findings (23, 30). We showed that the anti-Panx3 antibody abrogated the reduced cAMP levels by inhibiting the Panx3 hemichannel function (Fig. 5, C, panel a, and D, panel a), whereas shPanx3 increased the cAMP level in C2C12 cells and in primary calvarial cells (Fig. 5, C, panel b, and D, panel b). These cAMP levels correlate with the proliferation states of pEF1/Panx3 and Panx3 shRNA cells (Fig. 1), and our results suggest that Panx3 reduces cell proliferation in part through the ATP/cAMP pathway by the Panx3 hemichannel.
FIGURE 8.
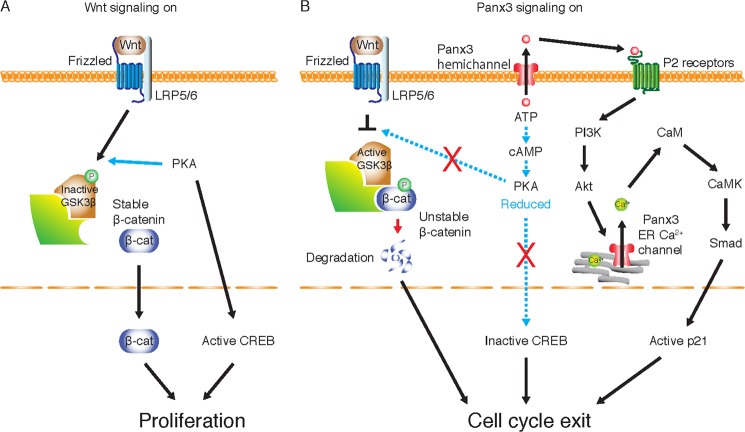
Panx3 signaling pathways inhibit osteoprogenitor cell proliferation and promote cell cycle exit. A, Wnt signaling in the proliferation stage of osteoprogenitor cells. Wnt binding to a frizzled receptor and its co-receptors LRP5/6 stabilizes the β-catenin protein. The inactivation of GSK-3β by phosphorylation through PKA is involved in this β-catenin stabilization process. The stable β-catenin is translocated to the nucleus and activates gene expression necessary for cell proliferation. PKA also activates CREB by phosphorylation, which induces expression of cell cycle genes for proliferation. B, Panx3 signaling in the transition from proliferation to differentiation of osteoprogenitor cells. Panx3 is induced by BMP and functions as a hemichannel. The Panx3 hemichannel releases intracellular ATP, resulting in a decrease in cAMP/PKA signaling, which in turn reduces proliferation. The reduced PKA activity promotes GSK3β activation, which phosphorylates β-catenin for its degradation, leading to the inhibition of cell growth. An increase in the inactive CREB by the reduced PKA activity results in reduced proliferation. In addition, ATP released from the Panx3 hemichannel binds to ATP receptors (P2Rs) in its own cell and/or in neighboring cells and activates PI3K and Akt signaling. Akt activation promotes the Panx3 ER Ca2+ channel to increase [Ca2+]i levels, which leads to activation of the CaM/calmodulin kinase (CaMK) pathway. The Panx3-mediated CaM kinase activation promotes Smad/p21 signaling for cell cycle exit. Dotted arrows in the ATP/cAMP/PKA pathways indicate reduced signaling by the Panx3 hemichannel. Red crossed bars indicate that the reduced PKA activity increases the active form of GSK3β and inactive form of CREB.
Panx3 Hemichannels Reduce PKA/CREB Signaling and β-Catenin Activity
Intracellular cAMP activates downstream PKA/CREB signaling, which induces the expression of genes involved in the progression of cell proliferation (48). To further delineate the Panx3 hemichannel pathway, which inhibits cell proliferation, we analyzed the downstream molecules of cAMP signaling in either pEF1/Panx3 or shPanx3 transfected C2C12 cells (Fig. 6A) and in primary calvarial cells (Fig. 6B). Panx3 overexpression reduced the phosphorylation of PKA and CREB (Fig. 6, A, panel a, and B, panel a, left panels; A, panel b, and B, panel b, left panels), whereas the addition of the Panx3 antibody to the Panx3-overexpressing C2C12 cells blocked the reduction in the PKA/CREB phosphorylation levels (Fig. 6A, panel a, middle panel, and panel b, left panels). The inhibition of endogenous Panx3 expression by Panx3 shRNA in C2C12 and primary calvarial cells increased the levels of both PKA and CREB phosphorylation, compared with that of the control shRNA transfected cells (Fig. 6, A, panel a, and B, panel a, right panels; A, panel b, and B, panel b, right panels). These results suggest that Panx3 inhibits proliferation of both C2C12 and primary calvarial cells through the PKA/CREB signaling pathway.
FIGURE 6.
Panx3 hemichannels reduce PKA/CREB signaling and β-catenin activity. A, pEF1 and pEF1/Panx3 transfected C2C12 cells were cultured for 1 day in DMEM containing 10% FBS with or without Panx3 antibody, whereas stable sh control and shPanx3 transfected cells were cultured with BMP2 for 1 day. Panel a, Western blotting was performed with antibodies to Panx3, phospho-PKA (P-PKA), PKA, phospho-CREB (P-CREB), CREB, and α-tubulin. Panel b, quantification of the ratios of P-PKA/PKA (upper panel) and P-CREB/CREB (lower panel). B, panel a, pEF1 and pEF1/Panx3 transfected calvarial cells were cultured in α-MEM for 2 days, whereas sh control and shPanx3 transfected cells were cultured with BMP2 (300 ng/ml) for 2 days. Panel b, quantification of the ratios of P-PKA/PKA (left panel) and P-CREB/CREB (right panel). C, panels a and b, Axin2LacZ ex vivo calvarial cultures with either S-peptide (panel a) or I-peptide (panel b) were stained for X-gal. Panel c, quantification of LacZ-positive cells. **, p < 0.01. NS, nonsignificant. Error bars represent the means ± S.D., n = 3.
Because GSK3β kinase activity is inhibited through the phosphorylation of GSK3β by cAMP-dependent PKA (11, 12), Panx3-mediated β-catenin degradation may be regulated through decreased cAMP/PKA signaling by the Panx3 hemichannel, which promotes GSK3β kinase activity resulting in an increase in β-catenin phosphorylation for the degradation. We examined β-catenin activity in an ex vivo calvarial culture using AxinLacZ mice with I-peptide to inhibit the Panx3 hemichannel. We found that an addition of I-peptide increased the number of the LacZ-positive cells, indicating increased β-catenin activity in the ex vivo calvarial culture (Fig. 6C, panels a and b). The quantification confirmed the increased number of LacZ-positive cells by the addition of I-peptide (Fig. 6C, panel c). This result indicates that the Panx3 hemichannel inhibits β-catenin signaling through the cAMP/PKA pathway.
Panx3 Inhibits the Activity of Cell Cycle Regulators
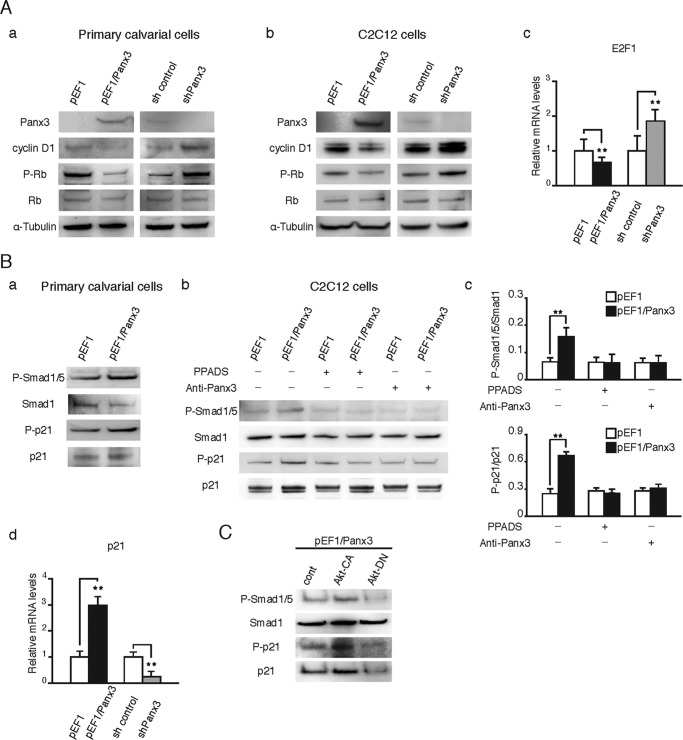
We further analyzed the activity of cell cycle regulators downstream of the PKA/CREB pathway (Fig. 7). PKA/CREB signaling induces the expression of E2F1, cyclin D1, and CDK4, which promote cell cycle progression (49). The cyclin D1-CDK4 complex induces the phosphorylation of Rb, which dissociates Rb from the Rb-E2F1 complex. Free E2F1 induces gene expression for cell cycle progression (50). Wnt/β-catenin signaling activates genes for cell cycle molecules, such as c-Myc and cyclin D1, and promotes cell growth (8, 22). In both the Panx3-overexpressing primary calvarial cells and C2C12 cells, cyclin D1 protein levels and Rb phosphorylation levels were reduced, whereas Panx3 shRNA cells showed the opposite effects (Fig. 7A, panels a and b). Panx3 overexpression also reduced E2F1 mRNA levels (Fig. 7A, panel c). These results suggest that Panx3 promotes cell cycle arrest at the G0/G1 phase by inhibiting the activity of the cell cycle molecules involved in cell cycle progression from the G1 to the S phase.
FIGURE 7.
Panx3 reduces cell cycle signaling pathways and increases p21 activation. A, Western blotting with antibodies to Panx3, cyclin D1, phospho-Rb (P-Rb), Rb, and α-tubulin. Panels a and b, pEF1 and pEF1/Panx3 transfected cells were cultured for 2 days in α-MEM for primary calvarial cells (panel a) or for 1 day in DMEM for C2C12 cells (panel b). sh control and shPanx3 transfected cells were cultured with BMP2 (300 ng/ml) for 2 days for calvarial cells (panel a) or 1 day for C2C12 cells (panel b). pEF1 and pEF1/Panx3 transfected C2C12 cells were cultured in DMEM for 2 days. sh control and shPanx3 transfected cells were cultured with BMP2 (300 ng/ml) for 2 days. Panel c, E2F1 mRNA levels were then observed by quantitative PCR. B, Panels a–c, phosphorylation of Smad1/5 and p21 cultured in α-MEM for pEF1 and pEF1/Panx3 transfected calvarial cells (panel a) and DMEM with PPADS or anti-Panx3 antibody in pEF1 and pEF1/Panx3 transfected C2C12 cells (panels b and c). Panel c, quantification of protein levels for P-Smad1/5 (upper panel) and P-p21 (lower panel). Panel d, quantitative PCR for p21 mRNA levels on the same experimental condition as E2F1. C, Panx3-promoted Smad1/5 and p21 activation via Akt signaling pathways. Panx3 overexpressed C2C12 cells transfected with Akt CA or Akt DN or mock vector were cultured in DMEM for 1 day. **, p < 0.01. Error bars represent the means ± S.D., n = 3.
Panx3 ER Ca2+ Channel Promotes the Smad1/5 and p21 Pathway for Cell Cycle Exit
The Panx3 ER Ca2+ channel is activated by PI3K/Akt signaling and increases intracellular Ca2+ levels, ([Ca2+]i), following the activation of calmodulin (CaM) signaling (23). In these processes, the external ATP released from the cells through the Panx3 hemichannel binds to purinergic P2 receptors, which activate PI3K/Akt signaling. It is known that the CaM pathway activates Smad1/5 signaling, which regulates p21, a cell cycle inhibitor (51, 52). Therefore, we examined whether Panx3 promotes p21 activation via Smad signaling in primary calvarial cells and in C2C12 cells. The Panx3 overexpression resulted in the activation of Smad1/5 and p21 (Fig. 7B, panels a–c). The activation of these proteins was blocked by PPADS, an inhibitor of the P2 receptors, and by the Panx3 antibody, which inhibited the Panx3 hemichannel (Fig. 7B, panels b and c). Panx3 overexpression also promoted the expression of p21 mRNA (Fig. 7B, panel d) and protein levels (Fig. 7B, panels a–c). Because the Panx3 ER Ca2+ channel promotes [Ca2+]i by Akt signaling, we examined the effects of Akt activation on the phosphorylation levels of the Smad1/5 and p21 pathway by transfecting the Akt constitutive active (Akt-CA) and dominant negative (Akt-DN) vectors to Panx3-overexpressing C2C12 cells. The Akt-CA promoted increased phosphorylation of the Smad1/5 and p21 over that of the mock vector transfection. In contrast, Akt-DN inhibited the activation of the Smad1/5 and p21 by Panx3 overexpression (Fig. 7C). These results suggest that Panx3 promotes the cell cycle exit by the activation of Smad1/5 and p21 through the Panx3 function as an ER Ca2+ channel.
DISCUSSION
In this study, we demonstrate that Panx3 plays an important role in the transition from proliferation to differentiation in osteoprogenitor cells. We found that Panx3 inhibits osteoprogenitor proliferation and promotes the cell cycle exit. Fig. 8 presents a schematic diagram of the mechanism of Panx3 actions that negatively regulate the proliferation state of osteoprogenitor cells. At the proliferation stage, canonical Wnt signaling stabilizes and translocates β-catenin to the nucleus, where it activates genes for cell cycle progression (Fig. 8A). When Panx3 is induced by BMP at the early transition stage, Panx3 functions as a hemichannel to release intracellular ATP into the extracellular space. This Panx3 hemichannel activity induces all subsequent Panx3 signaling pathways involved in the inhibition of proliferation. It reduces cAMP/PKA signaling, resulting in β-catenin degradation via GSK3β activation, which leads to a reduction in the β-catenin/TCF/Lef-dependent transcription of the genes necessary for proliferation. The Panx3 hemichannel also reduces activity of CREB, a DNA-binding protein factor downstream of cAMP/PKA, resulting in the reduced transcription of the CREB target genes required for cell cycle progression. In addition, the binding of ATP released from the Panx3 hemichannel to ATP receptors activates the Panx3 ER Ca2+channel/CaM/CaM kinase/Smad1/5 pathway via PI3K/Akt signaling, following an increase in the transcription and activation of p21. All of these signaling pathways are abrogated by the inhibition of the Panx3 hemichannel. Thus, the Panx3 hemichannel is the most critical first step to switch proliferation to the cell cycle exit.
Wnt signaling plays an important role in many aspects of tissue development, including osteogenesis (45). In bone formation, Wnt signaling promotes osteoprogenitor cell proliferation and mineralization while inhibiting osteoclastogenesis (40, 53). Wnt3a enhances the cell proliferation of the osteoblastic cell lines C2C12, MC3T3-E1, and C3H10T1/2 (39, 54, 55). Wnt1, 2, and 3a also induce alkaline phosphatase activity in osteogenic cells (55). Thus, Wnt signaling regulates osteoprogenitor cell proliferation and differentiation. However, the mechanisms of Wnt signaling regulation in the cell proliferation stage and in the late differentiation stage remain unclear. BMPs and Wnt signaling interactions regulate cell proliferation and differentiation during development (56). BMPs antagonize the Wnt-mediated proliferation of osteoprogenitor cells and promote osteoblast differentiation by inducing Runx2 (57, 58). Subsequently, Runx2 induces Osx that inhibits Wnt signaling by inducing Dkk1 (22). In this paper, our data show a different pathway for Wnt signaling inhibition by Panx3. We previously demonstrated that BMP2 induces Panx3 during osteoblast differentiation (23). The suppression and overexpression of Panx3 inhibits and promotes the expression of Osx and other osteoblast markers, respectively (23). These results indicate that Panx3 is an upstream molecule of Osx. Thus, Panx3 inhibits Wnt signaling first, followed by Osx-mediated inhibition during osteoblast differentiation to ensure the differentiation process.
[Ca2+]i plays a critical role in cell proliferation and differentiation (59). The ER serves as the major Ca2+ storage space in the cell. Panx3 functions as an ER Ca2+ channel, which promotes the osteoprogenitor cell cycle exit (Fig. 7). CaM is the major transducer of Ca2+ signaling in many cell types. In osteoblasts, upon binding to Ca2+, CaM interacts with and activates protein factors such as calmodulin kinase II and calcineurin and induces differentiation (60–62). We found that Panx3 stimulated the phosphorylation of Smad1/5 and increased both the protein levels and phosphorylation levels of p21 (Fig. 7B). Our results indicate that the Panx3 ER Ca2+ channel regulates the CaM/Smads/p21 signaling pathway (Fig. 8). The anti-Panx3 antibody and PPADS inhibited the Panx3-promoted Smad1/5 activation and p21 expression, suggesting that the Panx3 hemichannels and P2 receptors are involved in these processes. The increase in p21 contributes to the Panx3-promoted cell cycle exit (Fig. 2). These results suggest that Panx3-promoted Ca2+ signaling activates Smad/p21 signaling, which promotes the cell cycle exit.
IP3 receptors (IP3Rs), which consist of three members (IP3R1, 2, and 3), are ubiquitous ER Ca2+ channels, and they release Ca2+ from the ER upon its binding to IP3 (63). Three types of IP3Rs are expressed in osteogenic C2C12 cells (data not shown). The expression levels of IP3Rs did not change during the osteoblast differentiation (data not shown). Ryanodine receptors, which are another ER Ca2+ channel, are not expressed in C2C12 cells and are not induced during osteoblast differentiation (23). We previously showed that PPADS strongly blocks the Panx3 ER Ca2+ channel, but not IP3Rs, in C2C12 cells. The reduction of Smad1/5 and p21 activity by PPADS indicates that the Panx3 ER Ca2+ channel primarily regulates p21 activation to inhibit osteoprogenitor cell proliferation (Fig. 7B). Mice lacking either IP3R2 or IP3R3 are viable and show no obvious abnormalities in skeletal development. Mice lacking both IP3R2 and IP3R3 are born with a normal appearance but begin losing body weight after weaning because of defects in exocrine secretion (64). Thus, the Panx3 ER Ca2+ channel likely plays a major role in osteogenic proliferation as well as in osteogenic differentiation.
In summary, we have shown that Panx3 inhibits osteoprogenitor proliferation through the multiple signaling pathways induced by the Panx3 hemichannel. Our results reveal that Panx3 promotes the switch from osteoprogenitor proliferation to osteoblast differentiation.
This work was supported, in whole or in part, by National Institutes of Health Intramural Research Program at the NIDCR. This work was also supported by Grant-in-Aid 20679006 from the Ministry of Education, Science, and Culture of Japan (to S. F.) and NEXT program Grant LS010 (to S. F.).
- GSK
- glycogen synthase kinase
- Panx3
- pannexin 3
- Osx
- Osterix
- ER
- endoplasmic reticulum
- α-MEM
- α-minimum essential medium
- CaM
- calmodulin.
REFERENCES
- 1. Clevers H., Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 2. Moon R. T., Bowerman B., Boutros M., Perrimon N. (2002) The promise and perils of Wnt signaling through β-catenin. Science 296, 1644–1646 [DOI] [PubMed] [Google Scholar]
- 3. Veeman M. T., Axelrod J. D., Moon R. T. (2003) A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 5, 367–377 [DOI] [PubMed] [Google Scholar]
- 4. Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 5. Li V. S., Ng S. S., Boersema P. J., Low T. Y., Karthaus W. R., Gerlach J. P., Mohammed S., Heck A. J., Maurice M. M., Mahmoudi T., Clevers H. (2012) Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149, 1245–1256 [DOI] [PubMed] [Google Scholar]
- 6. Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilic J., Huang Y.-L., Davidson G., Zimmermann T., Cruciat C.-M., Bienz M., Niehrs C. (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619–1622 [DOI] [PubMed] [Google Scholar]
- 8. Behrens J., von Kries J. P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382, 638–642 [DOI] [PubMed] [Google Scholar]
- 9. Huber O., Korn R., McLaughlin J., Ohsugi M., Herrmann B. G., Kemler R. (1996) Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech. Dev. 59, 3–10 [DOI] [PubMed] [Google Scholar]
- 10. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 11. Fang X., Yu S. X., Lu Y., Bast R. C., Jr., Woodgett J. R., Mills G. B. (2000) Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. U.S.A. 97, 11960–11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki A., Ozono K., Kubota T., Kondou H., Tachikawa K., Michigami T. (2008) PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3β in osteoblastic Saos-2 cells. J. Cell. Biochem. 104, 304–317 [DOI] [PubMed] [Google Scholar]
- 13. Hino S., Tanji C., Nakayama K. I., Kikuchi A. (2005) Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase stabilizes β-catenin through inhibition of its ubiquitination. Mol. Cell Biol. 25, 9063–9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong Y., Slee R. B., Fukai N., Rawadi G., Roman-Roman S., Reginato A. M., Wang H., Cundy T., Glorieux F. H., Lev D., Zacharin M., Oexle K., Marcelino J., Suwairi W., Heeger S., Sabatakos G., Apte S., Adkins W. N., Allgrove J., Arslan-Kirchner M., Batch J. A., Beighton P., Black G. C., Boles R. G., Boon L. M., Borrone C., Brunner H. G., Carle G. F., Dallapiccola B., De Paepe A., Floege B., Halfhide M. L., Hall B., Hennekam R. C., Hirose T., Jans A., Juppner H., Kim C. A., Keppler-Noreuil K., Kohlschuetter A., LaCombe D., Lambert M., Lemyre E., Letteboer T., Peltonen L., Ramesar R. S., Romanengo M., Somer H., Steichen-Gersdorf E., Steinmann B., Sullivan B., Superti-Furga A., Swoboda W., van den Boogaard M. J., Van Hul W., Vikkula M., Votruba M., Zabel B., Garcia T., Baron R., Olsen B. R., Warman M. L., and Osteoporosis-Pseudoglioma Syndrome Collaborative Group (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 [DOI] [PubMed] [Google Scholar]
- 15. Boyden L. M., Mao J., Belsky J., Mitzner L., Farhi A., Mitnick M. A., Wu D., Insogna K., Lifton R. P. (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346, 1513–1521 [DOI] [PubMed] [Google Scholar]
- 16. Kokubu C., Heinzmann U., Kokubu T., Sakai N., Kubota T., Kawai M., Wahl M. B., Galceran J., Grosschedl R., Ozono K., Imai K. (2004) Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development 131, 5469–5480 [DOI] [PubMed] [Google Scholar]
- 17. Kato M., Patel M. S., Levasseur R., Lobov I., Chang B. H., Glass D. A., 2nd, Hartmann C., Li L., Hwang T.-H., Brayton C. F., Lang R. A., Karsenty G., Chan L. (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morvan F., Boulukos K., Clément-Lacroix P., Roman Roman S., Suc-Royer I., Vayssière B., Ammann P., Martin P., Pinho S., Pognonec P., Mollat P., Niehrs C., Baron R., Rawadi G. (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 21, 934–945 [DOI] [PubMed] [Google Scholar]
- 19. Akiyama H., Kim J. E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T., de Crombrugghe B. (2005) Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. U.S.A. 102, 14665–14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glass D. A., 2nd, Bialek P., Ahn J. D., Starbuck M., Patel M. S., Clevers H., Taketo M. M., Long F., McMahon A. P., Lang R. A., Karsenty G. (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 [DOI] [PubMed] [Google Scholar]
- 21. Mak K. K., Chen M.-H., Day T. F., Chuang P.-T., Yang Y. (2006) Wnt/β-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development 133, 3695–3707 [DOI] [PubMed] [Google Scholar]
- 22. Zhang C., Cho K., Huang Y., Lyons J. P., Zhou X., Sinha K., McCrea P. D., de Crombrugghe B. (2008) Inhibition of Wnt signaling by the osteoblast-specific transcription factor Osterix. Proc. Natl. Acad. Sci. U.S.A. 105, 6936–6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishikawa M., Iwamoto T., Nakamura T., Doyle A., Fukumoto S., Yamada Y. (2011) Pannexin 3 functions as an ER Ca2+ channel, hemichannel, and gap junction to promote osteoblast differentiation. J. Cell Biol. 193, 1257–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., Tiunova A., Born T. L., Usman N., Staroverov D., Lukyanov S., Panchin Y. (2004) The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 83, 706–716 [DOI] [PubMed] [Google Scholar]
- 25. Bruzzone R., Hormuzdi S. G., Barbe M. T., Herb A., Monyer H. (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. U.S.A. 100, 13644–13649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ray A., Zoidl G., Wahle P., Dermietzel R. (2006) Pannexin expression in the cerebellum. Cerebellum 5, 189–192 [DOI] [PubMed] [Google Scholar]
- 27. Vogt A., Hormuzdi S. G., Monyer H. (2005) Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res. Mol. Brain Res. 141, 113–120 [DOI] [PubMed] [Google Scholar]
- 28. Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., Laird D. W. (2007) Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120, 3772–3783 [DOI] [PubMed] [Google Scholar]
- 29. Lohman A. W., Billaud M., Straub A. C., Johnstone S. R., Best A. K., Lee M., Barr K., Penuela S., Laird D. W., Isakson B. E. (2012) Expression of pannexin isoforms in the systemic murine arterial network. J. Vasc. Res. 49, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwamoto T., Nakamura T., Doyle A., Ishikawa M., de Vega S., Fukumoto S., Yamada Y. (2010) Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J. Biol. Chem. 285, 18948–18958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsunobu T., Torigoe K., Ishikawa M., de Vega S., Kulkarni A. B., Iwamoto Y., Yamada Y. (2009) Critical roles of the TGF-β type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev. Biol. 332, 325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farr A., Roman A. (1992) A pitfall of using a second plasmid to determine transfection efficiency. Nucleic Acids Res. 20, 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P. M., Birchmeier W., Behrens J. (2002) Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell Biol. 22, 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohammad K. S., Chirgwin J. M., Guise T. A. (2008) Assessing new bone formation in neonatal calvarial organ cultures. Methods Mol. Biol. 455, 37–50 [DOI] [PubMed] [Google Scholar]
- 35. Wu X., Downes S., Watts D. C. (2010) Evaluation of critical size defects of mouse calvarial bone. An organ culture study. Microsc. Res. Tech. 73, 540–547 [DOI] [PubMed] [Google Scholar]
- 36. Bellows C. G., Heersche J. N. (2001) The frequency of common progenitors for adipocytes and osteoblasts and of committed and restricted adipocyte and osteoblast progenitors in fetal rat calvaria cell populations. J. Bone Miner. Res. 16, 1983–1993 [DOI] [PubMed] [Google Scholar]
- 37. Ecarot-Charrier B., Glorieux F. H., van der Rest M., Pereira G. (1983) Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J. Cell Biol. 96, 639–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raucci A., Bellosta P., Grassi R., Basilico C., Mansukhani A. (2008) Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J. Cell Physiol. 215, 442–451 [DOI] [PubMed] [Google Scholar]
- 39. Haÿ E., Nouraud A., Marie P. J. (2009) N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signalling. PLoS One 4, e8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kubota T., Michigami T., Ozono K. (2009) Wnt signaling in bone metabolism. J. Bone Miner. Metab. 27, 265–271 [DOI] [PubMed] [Google Scholar]
- 41. Urano T. (2007) [Regulation of bone metabolism by pathogenic mutations and polymorphism in the LRP5-Wnt signaling genes]. Nihon Rinsho 65, (Suppl. 9) 95–100 [PubMed] [Google Scholar]
- 42. Bernardi H., Gay S., Fedon Y., Vernus B., Bonnieu A., Bacou F. (2011) Wnt4 activates the canonical β-catenin pathway and regulates negatively myostatin. Functional implication in myogenesis. Am. J. Physiol. Cell Physiol. 300, C1122–C1138 [DOI] [PubMed] [Google Scholar]
- 43. Nakashima A., Katagiri T., Tamura M. (2005) Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J. Biol. Chem. 280, 37660–37668 [DOI] [PubMed] [Google Scholar]
- 44. van Amerongen R., Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 [DOI] [PubMed] [Google Scholar]
- 45. Westendorf J. J., Kahler R. A., Schroeder T. M. (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341, 19–39 [DOI] [PubMed] [Google Scholar]
- 46. Cooper D. M. (2003) Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 375, 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ryten M., Dunn P. M., Neary J. T., Burnstock G. (2002) ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J. Cell Biol. 158, 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dumont J. E., Jauniaux J. C., Roger P. P. (1989) The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem. Sci. 14, 67–71 [DOI] [PubMed] [Google Scholar]
- 49. Beier F., LuValle P. (2002) The cyclin D1 and cyclin A genes are targets of activated PTH/PTHrP receptors in Jansen's metaphyseal chondrodysplasia. Mol. Endocrinol. 16, 2163–2173 [DOI] [PubMed] [Google Scholar]
- 50. Wang A., Schneider-Broussard R., Kumar A. P., MacLeod M. C., Johnson D. G. (2000) Regulation of BRCA1 expression by the Rb-E2F pathway. J. Biol. Chem. 275, 4532–4536 [DOI] [PubMed] [Google Scholar]
- 51. Bellosta P., Masramon L., Mansukhani A., Basilico C. (2003) p21(WAF1/CIP1) acts as a brake in osteoblast differentiation. J. Bone Miner. Res. 18, 818–826 [DOI] [PubMed] [Google Scholar]
- 52. Pardali K., Kowanetz M., Heldin C.-H., Moustakas A. (2005) Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1). J. Cell Physiol. 204, 260–272 [DOI] [PubMed] [Google Scholar]
- 53. Almeida M., Han L., Bellido T., Manolagas S. C., Kousteni S. (2005) Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 280, 41342–41351 [DOI] [PubMed] [Google Scholar]
- 54. Zhang L., Shi S., Zhang J., Zhou F., ten Dijke P. (2012) Wnt/β-catenin signaling changes C2C12 myoblast proliferation and differentiation by inducing Id3 expression. Biochem. Biophys. Res. Commun. 419, 83–88 [DOI] [PubMed] [Google Scholar]
- 55. Rawadi G., Vayssière B., Dunn F., Baron R., Roman-Roman S. (2003) BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 18, 1842–1853 [DOI] [PubMed] [Google Scholar]
- 56. Huelsken J., Birchmeier W. (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 11, 547–553 [DOI] [PubMed] [Google Scholar]
- 57. Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 [DOI] [PubMed] [Google Scholar]
- 58. Otto F., Thornell A. P., Crompton T., Denzel A., Gilmour K. C., Rosewell I. R., Stamp G. W., Beddington R. S., Mundlos S., Olsen B. R., Selby P. B., Owen M. J. (1997) Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 [DOI] [PubMed] [Google Scholar]
- 59. Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 60. Seo J. H., Jin Y.-H., Jeong H. M., Kim Y.-J., Jeong H. G., Yeo C.-Y., Lee K.-Y. (2009) Calmodulin-dependent kinase II regulates Dlx5 during osteoblast differentiation. Biochem. Biophys. Res. Commun. 384, 100–104 [DOI] [PubMed] [Google Scholar]
- 61. Sun L., Blair H. C., Peng Y., Zaidi N., Adebanjo O. A., Wu X. B., Wu X. Y., Iqbal J., Epstein S., Abe E., Moonga B. S., Zaidi M. (2005) Calcineurin regulates bone formation by the osteoblast. Proc. Natl. Acad. Sci. U.S.A. 102, 17130–17135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zayzafoon M. (2006) Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell Biochem. 97, 56–70 [DOI] [PubMed] [Google Scholar]
- 63. Mikoshiba K. (2007) IP3 receptor/Ca2+ channel. From discovery to new signaling concepts. J. Neurochem. 102, 1426–1446 [DOI] [PubMed] [Google Scholar]
- 64. Futatsugi A., Nakamura T., Yamada M. K., Ebisui E., Nakamura K., Uchida K., Kitaguchi T., Takahashi-Iwanaga H., Noda T., Aruga J., Mikoshiba K. (2005) IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309, 2232–2234 [DOI] [PubMed] [Google Scholar]