Background: The balance between mitochondrial biogenesis and autophagy controls cellular mitochondrial content.
Results: Mitochondrial deacetylation-induced mitophagy evokes concurrent and interdependent up-regulation of TFEB and PGC-1α to sustain cellular mitochondrial content.
Conclusion: Mitochondrial content is coordinately regulated by the mitochondrial and lysosome biogenesis programs under GCN5L1 control.
Significance: Counter-regulatory interdependent programs function to sustain mitochondrial content/homeostasis in a nutrient-sensing, acetylation-dependent manner.
Keywords: Acetyl Coenzyme A, Autophagy, Lysosomes, Mitochondria, Subcellular Organelles, GCN5L1, PGC1α, Acetylation, Mitophagy
Abstract
Cellular mitochondrial content is governed by the competing processes of organelle biogenesis and degradation. It is proposed that these programs are tightly regulated to ensure that the cell maintains sufficient organelles to meet its biosynthetic, energetic, and other homeostatic requirements. We recently reported that GCN5L1, a putative nutrient-sensing regulator, controls mitochondrial removal by autophagy. Here we show that genetic deletion of GCN5L1 has a direct positive effect on the expression and activity of Transcriptional Factor EB (TFEB), which acts as a master regulator of autophagy. Surprisingly, the induction of TFEB-mediated autophagy pathways does not diminish cellular mitochondrial content, as its activity is countered by induction of the mitochondrial biogenesis transcriptional co-activator PPARγ coactivator 1α (PGC-1α). Concurrent induction of the TFEB and PGC-1α pathways results in an increased mitochondrial turnover rate in GCN5L1−/− cells. Finally, we show that genetic knockdown of either TFEB or PGC-1α leads to a corresponding decrease in the expression of the other gene, indicating that these proteins act coordinately, and in opposition, to maintain cellular mitochondrial content in response to the modulation of nutrient-sensing signatures.
Introduction
Autophagy, an essential recycling process, is central to a number of cellular homeostatic mechanisms. Perhaps best known for its recycling of cellular components during periods of nutrient deprivation, autophagy is also necessary for the orderly removal of proteins, aggregates and organelles that have reached the end of their useful life (1–3). In the case of mitochondria, removal by autophagy (also known as “mitophagy”) is a key housekeeping process, which is used to safely remove damaged or redundant organelles from the cell (4). This mechanism is of particular importance to maintain the quality of the mitochondrial population, as aberrant mitophagy is implicated in numerous pathologies, such as the premature development of Parkinson Disease in young individuals (5).
The systems used to identify and remove particular mitochondrial organelles from the cell by mitophagy, and how these are regulated, is currently an area of intense focus. One of the first to be established was the Pink1-Parkin pathway, where the disruption of mitochondrial function through respiratory uncoupling, leads to the stabilization of Pink1 on the mitochondrial membrane (6). This acts as a trigger for the translocation of the ubiquitin ligase Parkin to the mitochondria, where it ubiquitylates mitochondrial outer membrane proteins and targets them for autophagic and proteosomal degradation (6, 7). In particular, Parkin ubiquitylates the mitofusin proteins involved in mitochondrial membrane fusion, thereby preventing uncoupled organelles from fusing to, and potentially damaging, other organelles (8). A second mechanism, utilizing members of the proapoptotic Bcl-2 family BNIP3 and Nix, is prevalent during periods of hypoxia, or during the maturation of erythrocytes (9, 10), respectively. When triggered, BNIP3 or Nix dimerize on the outer membrane of targeted mitochondria and bind to LC3, an ubiquitin-like protein involved in autophagosome maturation. With the aid of the linker protein p62/SQSTM1, these organelles are then transferred to the autophagosome for eventual degradation (11, 12). For an extensive review or these and other mechanisms involved in mitophagy, please see Refs. 4, 13.
To counter the effects of mitophagy and maintain sufficient organelles to meet their needs, the cell must stimulate the mechanisms that control mitochondrial biogenesis. Given the importance of mitochondria to the survival of the cell, it is expected that there is a high degree of regulation and coordination between these two opposing pathways. We therefore resolved to investigate these dual mechanisms in the context of General Control of Amino Acid Synthesis 5-like 1 (GCN5L1),2 a mitochondrial protein that we recently reported as controlling organelle clearance by mitophagy (14). Here we show that genetic deletion of GCN5L1 leads to an up-regulation of Transcription Factor EB (TFEB), a transcription factor which acts as a master regulator of autophagy (15). Despite an induction of TFEB-mediated autophagy, there is no net change in mitochondrial content, as there is a coordinated increase in mitochondrial biogenesis through the transcriptional co-activator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). Finally, we show that TFEB and PGC-1α are dynamically regulated in GCN5L1 cells, such that an increase in the expression of one gene is countered by an increase in the other, and vice versa. We therefore suggest that TFEB and PGC-1α act in opposition to each other, under the control of GCN5L1, to maintain cellular mitochondrial content.
EXPERIMENTAL PROCEDURES
Cells, Culture Conditions, and Transfection
Mouse embryonic fibroblasts (MEFs) were harvested at E11.5 and cultured in DMEM/15% FBS/Antibiotic-Antimycotic (Invitrogen) at 37 °C/5% CO2 for a maximum of 20 passages. The genotype of individual MEF lines was determined by multiplex PCR using the following primers: WT-F TGTTCACTCATCTGTGTGGGTGGG; WT-R GGGAAACAGCCTATAACAGTCGTGGG; KO-R CCAACAGCTTCCCCACAACGG. To knockdown TFEB and PGC-1α, MEFs were transduced with two independent lines of lentiviral shRNA particles (Sigma) under selection by puromycin. To reintroduce GCN5L1 into knock-out cells, we expressed GCN5L1-FLAG (16) using the Amaxa MEF 1 kit according to the manufacturer's instructions.
Subcellular Fractionation, Western Blotting, and qPCR
For subcellular fractionation, cells were harvested in ice-cold PBS, then disrupted in detergent-free sucrose lysis buffer using a 25-guage needle. Nuclear and cell debris were removed at 500 × g for 5 min, then heavy membranes were pelleted at 6000 × g for 15 min. The supernatant represents the cytosolic and light membrane fractions. For Western blots, cell lysates were prepared using 1% CHAPS buffer, separated by SDS-PAGE, and transferred to nitrocellulose membranes. Membranes were probed using the following antibodies: GCN5L1 (16), LC3 (Sigma), p62 (Progen Biotechnik) Tubulin, Beclin-1, Atg7, Atg3, Atg12–5, GAPDH, p-mTOR, mTOR, p-p70S6K, p70S6K, Raptor, Rictor, GβL, TFEB, LAMP1 (Cell Signaling), VDAC, GDH, ATP5a (Abcam), TOM20, and AIF (Santa Cruz Biotechnology). Images were captured using the Odyssey system (Li-Cor). For qPCR, mRNA was isolated using a total RNA extraction kit (Qiagen) and cDNA produced using a first-strand synthesis kit (Invitrogen). Transcript levels were measured using validated gene-specific primers (Qiagen). Each experiment was carried out at least three times, and a representative result is shown.
Bioenergetic Measurements and FACS Analysis
Measurements of mitochondrial respiration and glycolysis rates were performed using the Seahorse XF24 bioanalyzer following the manufacturer's protocols. After completion, cells were harvested and the rates adjusted relative to protein content. Cellular mitochondrial content was measured by FACS analysis, using Mitotracker Green (Invitrogen) fluorescence intensity as a proxy for mitochondrial volume. Each experiment was carried out at least three times, and a representative result is shown.
Protein Turnover Measurements
For SILAC experiments, wildtype and knock-out GCN5L1 MEF cells were cultured for six passages in “light” DMEM media, then switched to “heavy” DMEM containing 13C-Lys and 13C/15N-Arg for a chase period of 72 h. Proteins were harvested in 1% CHAPS lysis buffer, separated by SDS-PAGE, and stained with Gelcode Blue (Pierce). Twenty matching bands per lane were excised, and peptides were identified by LC-MS following trypsin digestion. The heavy:light ratio for proteins that were identified by at least two unique peptides was used to measure protein turnover rate between the two samples. Proteins were analyzed for cellular localization function using the GO component search (www. uniprot.com). For in vitro measurements of p62 and LAMP1 turnover, cells were treated with either 5 μg/ml cycloheximide or 250 nm Bafilomycin A1 for 4 or 20 h. Proteins were analyzed by Western blot, and the accumulation and degradation rates were calculated following densitometry measurements.
Electron Microscopy
WT and GCN5L1 KO MEFs were grown in Permanox dishes, fixed with 2.5% glutaraldehyde, 1% paraformaldehyde, 0.12 m sodium cacodylate buffer pH 7.3 at room temperature for 20 min, then on ice for 40 min. After buffer washes, cells were post-fixed for 1 h on ice in 1% OsO4, 0.12 m sodium cacodylate buffer pH 7.3. After water washes, samples were block stained in 1% uranyl acetate, dehydrated in graded ethanol solutions, transitioned with propylene oxide and embedded in EMbed-812 (Electron Microscopy Sciences). Thin sections were stained with uranyl acetate and lead citrate, then examined on a JEM 1400 electron microscope (JEOL USA) with an AMT XR-60 digital camera (Advanced Microscopy Techniques Corp.).
Animal Husbandry
Animals used in this study were handled in a manner consistent with the guidelines established by the NHLBI Animal Care and Use Committee. Heterozygous mice with a targeted insertion in GCN5L1 were obtained from the International Knock-out Mouse Consortium/EUCOMM.
RESULTS
GCN5L1−/− Mouse Embryonic Fibroblasts Display Hallmarks of Up-regulated Autophagy
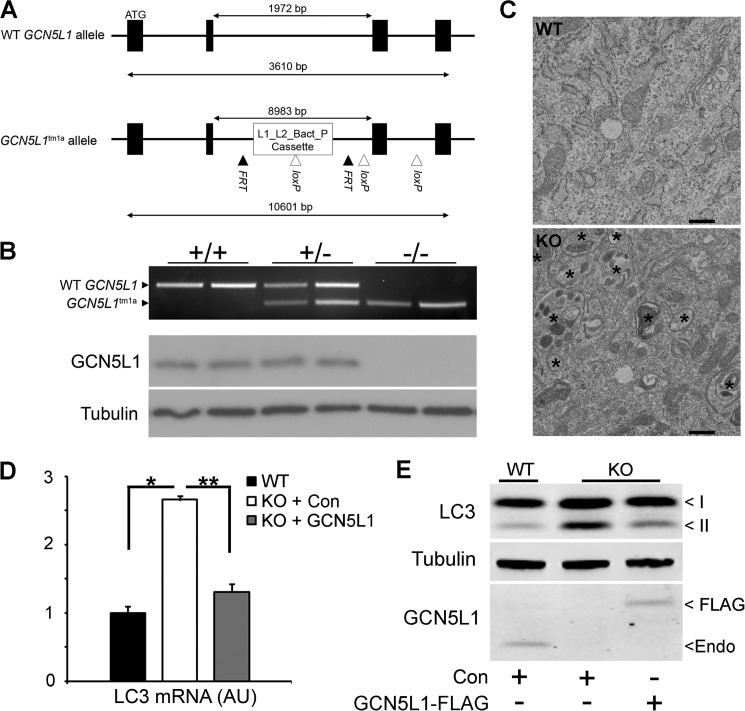
To further investigate the role of GCN5L1 in regulating mitochondrial clearance by autophagy, we attempted to produce a line of GCN5L1−/− mice using a targeted insertion construct (Fig. 1A). Unfortunately, numerous attempts failed to produce viable mice, and further investigation established that GCN5L1 knock-out embryos fail to develop beyond ∼E12.5. However, we were successful in isolating numerous wild type, hemizygous, and homozygous knock-out GCN5L1 MEF lines from the ∼E11.5 stage (Fig. 1B). As previously shown (14), GCN5L1 knock-out MEFs display a vastly increased number of autophagsomes relative to wild type cells (Fig. 1C). This correlates with increased transcript production of the autophagosome-related gene microtubule-associated protein 1A/1B-light chain 3 (LC3; Fig. 1D), and increased levels of the active lipidated form of the protein product (LC3-II) in knock-out MEFs (Fig. 1E). To ensure that these changes are related to the loss of GCN5L1, we reconstituted the protein in knock-out MEFs using a GCN5L1-FLAG construct. Expression of this construct led to a reduction in both LC3 gene expression and cellular LC3-II protein levels (Fig. 1, D and E).
FIGURE 1.
Characterization of autophagy hallmarks in GCN5L1 MEFs. A, schematic representation of alleles produced by GCN5L1 gene-targeting construct. B, genotype and protein expression in wild type, hemizygous, and homozygous knock-out GCN5L1 MEF cells. C, electron micrograph of GCN5L1 wild type and knock-out MEF cells. Autophagosomes are marked by an asterisk. D and E, transcript and active lipidated protein form (II) of LC3 are increased in GCN5L1 knock-out MEFs. This can be reversed by the reconstitution of GCN5L1 expression.
Loss of GCN5L1 Induces the Expression of TFEB and Its Downstream Targets
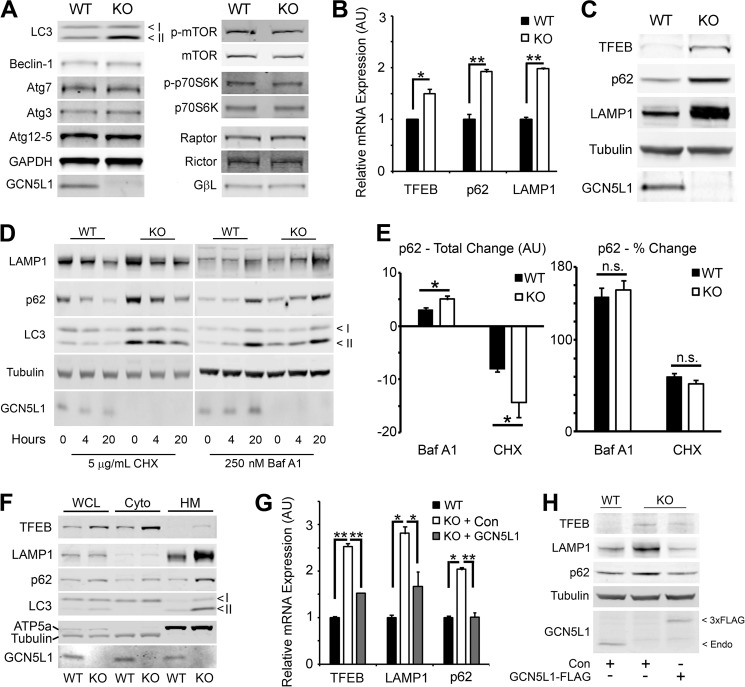
We next investigated whether GCN5L1−/− MEFs exhibited other alterations in their cellular autophagy programs. Proteins in the autophagy-regulated (ATG) pathway are involved in the conjugation and delivery of substrates to the autophagosome (17). On examination, we found that there was no difference in the cellular protein levels of Beclin-1, Atg7, Atg3, or Atg12–5 between wild type and knock-out cells, indicating that these pathways are intact in GCN5L1−/− MEFs (Fig. 2A). The mammalian target of rapamycin (mTOR) pathway acts a sensing mechanism to regulate autophagy induction in response to nutrient and growth stress (17). Again, we found that there was no significant difference in the expression or phosphorylation of mTOR, or its complex members Raptor, Rictor and GβL, in wild type and knock-out cells (Fig. 2A). Additionally, there was equivalent phosphorylation of the mTOR substrate p70S6K (Fig. 2A), indicating that this pathway was functioning normally. We next examined the expression of TFEB, a transcription factor that acts as a master regulator of autophagic and lysosomal pathways. Here we found that there was a significant increase in both TFEB transcript and protein levels in GCN5L1 knock-out cells (Fig. 2, B and C). Additionally, the transcript and protein levels of lysosomal membrane protein 1 (LAMP1), and the autophagy-substrate conjugation protein p62, both targets of TFEB-mediated transcription, were greatly elevated in GCN5L1−/− MEFs (Fig. 2, B and C).
FIGURE 2.
Expression of autophagy pathway mediators in GCN5L1 MEFs. A, GCN5L1 knock-out MEFs show no protein expression defects in either the ATG or mTOR autophagy pathways. B and C, expression of the autophagy-related transcription factor TFEB, along with its targets p62 and LAMP1, is increased in GCN5L1 knock-out MEFs. D, degradation and accumulation of LAMP1, p62, and LC3 in cells treated with cycloheximide (CHX) and Bafilomycin A1 (Baf A1) at the noted concentrations for 4 or 20 h. E, absolute and relative turnover of p62 following treatment with Bafilomycin A1 and cycloheximide for 6 h. F, proteins involved in autophagy are present in the correct cellular compartment in both wild type and knock-out GCN5L1 MEFs. WCL, whole cell lysate; Cyto, cytoplasm; HM, heavy membrane. G and H, reconstitution of GCN5L1 in knock-out MEF cells reverses the increased expression of TFEB and its targets.
The presence of elevated p62 levels have variously been ascribed to either an increase in autophagic activity (14) or a block in autophagic flux (18). While the increased transcript levels (Fig. 2B) would argue for the former, we wished to establish whether the autophagic flux rate was impaired. Both wild type and knock-out cells were susceptible to similar changes in p62, LAMP1, and LC3 protein levels caused by the protein synthesis inhibitor cycloheximide, and the lysosomal inhibitor Bafilomycin A1 (Fig. 2D). While the higher initial protein levels in the GCN5L1−/− MEFs meant that the absolute amount of p62 protein turnover was higher, there was no significant difference in relative percentage protein turnover between wild type and knock-out cells (Fig. 2E). As such, it appears that the autophagic flux is maintained in knock-out cells, and that the increase in autophagy-related proteins in GCN5L1−/− cells is a consequence of up-regulated TFEB activity.
We then examined whether the accumulation of TFEB pathway proteins in knock-out MEFs was caused by their mislocalization, and subsequent reduced activity, within the cell. We separated wild type and knock-out MEF cells into fractions using differential centrifugation. While there was an increase in TFEB, p62, LAMP1, and LC3-II protein levels in knock-out cells, there was no disruption in the localization of these proteins to the autophagosome/lysosome membrane fraction (Fig. 2F).
Finally, we asked whether the increase in TFEB pathway protein expression was a direct effect of GCN5L1 ablation. As such, we reconstituted GCN5L1 expression in knock-out MEFs using transient transfection. As seen previously, loss of GCN5L1 led to an increase in the levels of TFEB and its downstream targets, p62 and LAMP1 (Fig. 2, G and H). However, expression of a GCN5L1-FLAG construct for 48 h negated this induction, bringing both the transcript and protein expression levels back down to those found in wild type cells (Fig. 2, G and H). In summary, we conclude that loss of GCN5L1 leads to a direct increase in the expression and activity of TFEB and its downstream targets.
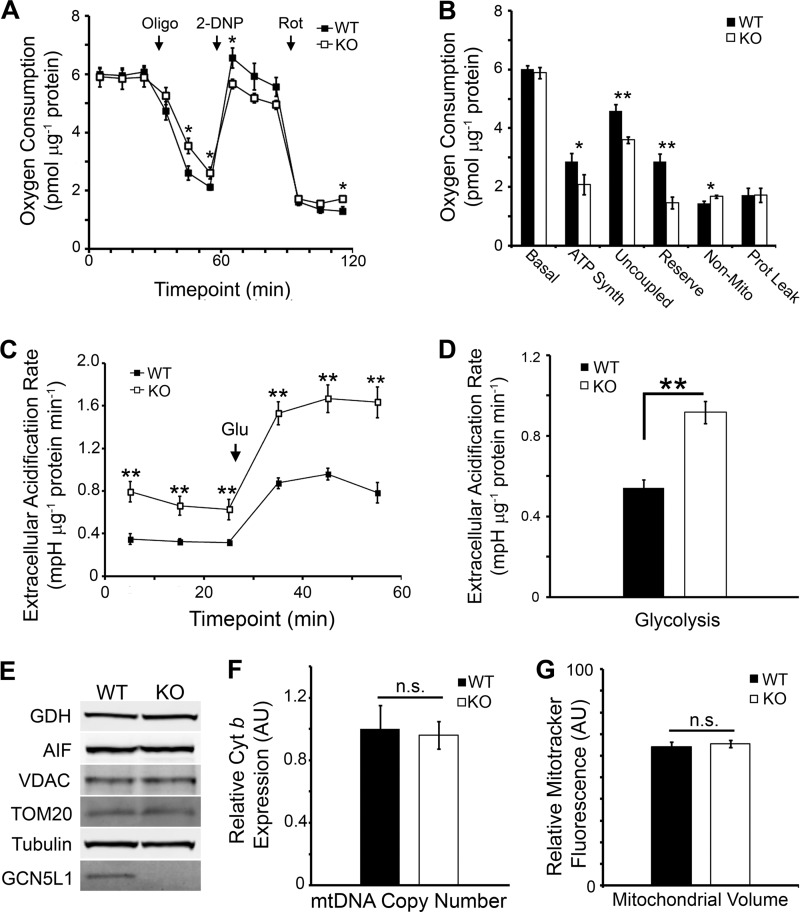
Despite Reduced Respiration Capacity, GCN5L1−/− MEFs Are Not Deficient in Mitochondrial Content
We previously demonstrated that HepG2 liver cells with shRNA-depleted GCN5L1 levels were deficient in mitochondrial content, and that this led to a reduction in their oxidative respiration capacity (14). We therefore examined whether MEF KO cells that are completely deficient in GCN5L1 exhibited a similar phenotype. While there was no significant difference in the baseline levels of oxidative phosphorylation between wild type and knock-out cells, GCN5L1−/− MEFs were deficient in their maximal uncoupled respiration rate, reserve capacity and use of oxygen for ATP synthesis (Fig. 3, A and B). To counter this defect, GCN5L1 knock-out MEFs displayed an elevated level of glycolysis, both at baseline and when stimulated with glucose (Fig. 3, C and D).
FIGURE 3.
Bioenergetics and mitochondrial content of GCN5L1 MEFs. A and B, mitochondrial bioenergetic profile of GCN5L1 MEFs. Knock-out cells are defective in maximal respiration capacity and ATP synthesis. C and D, mitochondrial energetic defects in GCN5L1 knock-out cells are complemented by an increase in glycolysis. E–G, there is no significant difference in mitochondrial protein levels, mtDNA copy number or mitochondrial volume between wild type and knock-out cells.
In concert, these data suggest that GCN5L1 knock-out MEF cells are depleted in mitochondrial bioenergetic output. Given that the TFEB autophagy pathway is elevated in these cells, we speculated that this may be due to an increased rate of mitophagy, which would result in a depleted mitochondrial cellular content. Surprisingly, we found that there was no significant difference between wild type and knock-out cells in the expression of numerous mitochondrial proteins (Fig. 3E), in mitochondrial DNA content (Fig. 3F), or in total mitochondrial Mitotracker Green fluorescence (Fig. 3G). Therefore, we conclude that despite the increased levels of autophagy proteins, the mitochondrial defects observed in the GCN5L1−/− cells are not related to any reduction in mitochondrial content.
GCN5L1−/− MEFs Maintain Mitochondrial Content by Up-regulation of PGC-1α-mediated Biogenesis Pathways
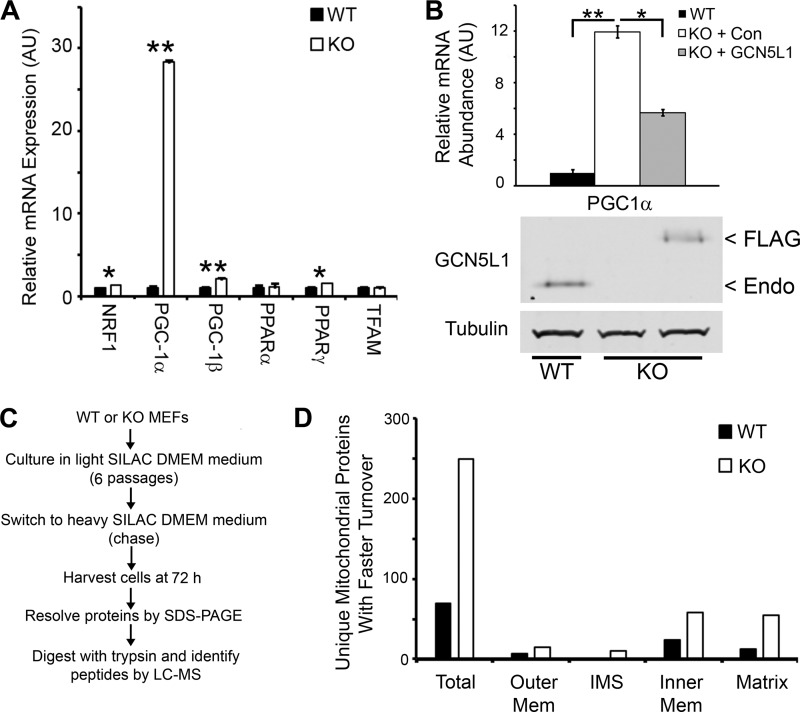
As there was no difference observed in the mitochondrial content of wild type and knock-out GCN5L1 MEFs, we speculated that there may be a mechanism by which the cell counters the increased autophagic activity in GCN5L1−/− cells. We therefore examined the expression levels of genes involved in mitochondrial biogenesis. Several genes (including NRF1, PGC-1β, and PPARγ) showed modest, yet significant increases in knock-out cells relative to wild type (Fig. 4A). However, more significantly, there was a vast increase in the expression levels of the mitochondrial biogenesis transcriptional co-activator, PGC-1α (Fig. 4A). To ensure that this increase was directly related to the loss of GCN5L1, we reconstituted expression of the protein in knock-out cells using the GCN5L1-FLAG construct. Transient transfection of this plasmid significantly reduced PGC-1α expression, suggesting that this increase was triggered by GCN5L1 loss (Fig. 4B).
FIGURE 4.
Mitochondrial biogenesis and turnover in GCN5L1 MEFs. A, mitochondrial biogenesis genes, particularly PGC-1α, show a large induction in expression in GCN5L1 knock-out MEFs. B, reconstitution of GCN5L1 ablates the induction of PGC-1α expression in knock-out MEFs. C, schematic of SILAC procedures used to measure mitochondrial turnover. D, mitochondrial turnover is greatly increased in GCN5L1 knock-out MEFs. A similar rate of increase is found in all mitochondrial compartments.
We reasoned that this increase in PGC-1α-mediated mitochondrial biogenesis in GCN5L1−/− cells would reverse the loss of mitochondrial content promoted by the TFEB pathway induction. As this would be difficult to measure by looking at steady-state mitochondrial levels (as evidenced by the data shown in Fig. 3), we decided to look at the mitochondrial turnover in wild type and knock-out cells, using stable isotope labeling of amino acids in cell culture (SILAC)-based proteomics (Fig. 4C). Following a 72 h chase period with heavy SILAC media, we used LC-MS to identify over 4000 unique proteins, 1600 of which were identifiable by at least two unique peptides. Of these, 318 were identified as being localized to mitochondria using bioinformatic searches, which represents roughly one-third of the mitochondrial proteome (19). The heavy:light SILAC incorporation ratio was used as a proxy for protein turnover, and the values were compared between wild type and knock-out cells. Of the mitochondrial proteins identified, over 78% displayed higher turnover levels in GCN5L1−/− cells, with an equal distribution of protein turnover ratios found in all compartments of the organelle (Fig. 4D, supplemental Table S1). From these data, we conclude that GCN5L1 knock-out MEFs up-regulate their mitochondrial biogenesis mechanisms to counteract the increased TFEB-mediated mitophagy rate. This results in an increased rate of mitochondrial turnover in GCN5L1−/− cells, without differences in the overall steady-state mitochondrial content between wild type and knock-out lines.
TFEB and PGC-1α Expression Levels Are Dynamically Regulated to Maintain Mitochondrial Content in GCN5L1−/− MEFs
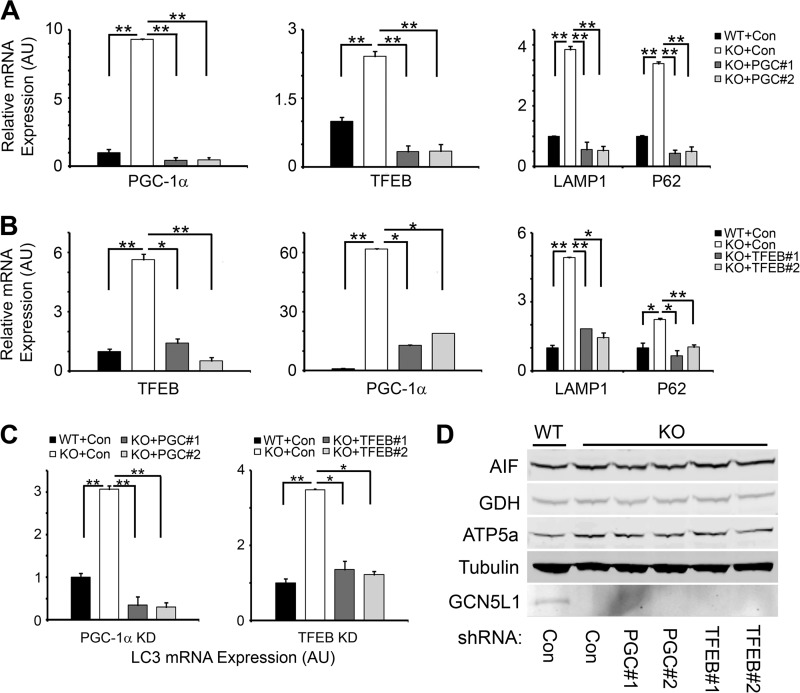
Contrasting previous studies have shown that PGC-1α acts as a transcriptional co-activator of TFEB expression (20), and that TFEB acts as a transcription factor for PGC-1α expression (21). We therefore sought to determine whether PGC-1α or TFEB act in a linear pathway as suggested by these studies, or whether they act in opposition to each other at the same level, under the control of GCN5L1, to regulate mitochondrial content. We first used two independent lentivrial shRNA constructs to knockdown the levels of PGC-1α in GCN5L1−/− MEFs. Expression of these constructs reduced the transcription of PGC-1α in knock-out MEFs to levels similar as those found in wild type cells (Fig. 5A). We then examined how these changes affected the expression of TFEB and its downstream targets, p62 and LAMP1. In GCN5L1−/− cells, PGC-1α shRNA knockdown led to a corresponding decrease in TFEB expression, with a subsequent reduction in p62 and LAMP1 transcript levels (Fig. 5A).
FIGURE 5.
Counter-regulation of TFEB and PGC-1α expression in GCN5L1 MEFs. A, knockdown of PGC-1α, using two independent shRNA lentiviral vectors, in GCN5L1 knock-out MEFs. Ablation of the PGC-1α expression increase in knock-out MEFs leads to a similar decrease in TFEB expression and activity. B, knockdown of TFEB, using two independent shRNA lentiviral vectors, in GCN5L1 knock-out MEFs. Reducing TFEB levels in GCN5L1 knock-out cells has a corresponding effect on PGC-1α expression. C, expression of LC3 in GCN5L1 MEFs following knockdown of PGC-1α or TFEB. D, mitochondrial protein levels in wild type and knock-out MEFs following knockdown of PGC-1α or TFEB.
We next examined how knockdown of TFEB would affect the expression of PGC-1α. As above, expression of two independent lentiviral shRNA vectors reduced the transcription levels of TFEB to wild type levels, with a corresponding effect on the transcription of p62 and LAMP1 (Fig. 5B). This was mirrored by a similar reduction in the expression levels of PGC-1α in GCN5L1 knock-out MEFs treated with TFEB shRNA (Fig. 5B). Additionally, knockdown of both PGC-1α and TFEB reduced the transcript levels of LC3, indicating that both have an effect on autophagy pathways (Fig. 5C). At the mitochondrial level, the net result of these coordinated changes is maintenance in the size of the total mitochondrial pool in both TFEB and PGC-1α knockdown cells (Fig. 5D). We therefore conclude that PGC-1α and TFEB expression levels are dynamically- and coordinately-regulated via a negative feedback loop, and that this occurs downstream of GCN5L1 (see model, Fig. 6). In this manner, the cell can closely regulate the opposing mechanisms of mitochondrial biogenesis (via PGC-1α) and lysosomal biogenesis (via TFEB), to maintain a functional population of healthy mitochondria.
FIGURE 6.

Proposed model of mitochondrial content regulation, mediated by GCN5L1 control of PGC-1a and TFEB expression.
DISCUSSION
The first identification of a role for GCN5L1 in the regulation of mitochondrial function came through a search for proteins involved in mitochondrial lysine residue acetylation. Here it was shown that the expression of GCN5L1 led to an increase in lysine acetylation in the mitochondria, and that this worked in opposition to the mitochondrial deacetylase SIRT3 (16). We then found that a decrease in acetylation caused by GCN5L1 shRNA knockdown in HepG2 cells acted as a molecular marker of mitochondrial nutrient depletion, and that this resulted in an increase in mitophagy (14). These findings were in agreement with previous studies that suggested GCN5L1 (also known as BLOC1S1) may be involved in the formation of lysosome-related organelles (22), and that its loss was associated with an increase in mitophagy in a unbiased genetic siRNA library screen (23). We now show that genetic deletion of GCN5L1 stimulates the cellular pathways that target mitochondria for degradation via autophagy, and that this is countered by an increase in the systems that bolster mitochondrial volume within the cell. This indicates that one of the functions performed by GCN5L1 is to act as a negative regulator of both mitochondrial biogenesis and degradation systems.
The up-regulated expression of both mitophagy- and biogenesis-related genes in GCN5L1−/− MEFs led to an increase in the mitochondrial turnover rate in these cells. However, the coordination of these two pathways led to no net difference being observed in mitochondrial content between wild type and knock-out cells. It is interesting to note that despite the parity in organelle content and baseline respiration, there is a deficit in GCN5L1−/− mitochondrial output that leads to an increased reliance on glycolysis (Fig. 3). It is tempting to speculate that the increased turnover rate in knock-out MEF cells results in a preponderance of immature or poorly formed mitochondria that may be limited in their bioenergetic capabilities. It will be of interest in future studies to determine whether the increased turnover rate seen in GCN5L1−/− MEFs results in some dysregulation of the oxidative phosphorylation architecture of these organelles.
The transcriptional regulation of the autophagosome-lysosome degradation system has recently come under a great deal of scrutiny. The main player in this field is TFEB, which has been shown to induce the transcription of over 400 genes, many of which are involved in autophagosome and lysosome biogenesis, including p62/SQSTM1, LAMP1, and the LC3-related protein GABARAP (24, 25). TFEB recognizes a CLEAR box sequence (5′-GTCACGTGAC-3′) in the promoter region of lysosomal genes, and its activity leads to the coordinated up-regulation of degradation pathways. TFEB normally resides in the cytosol, where it is sequestered by 14-3-3 proteins following phosphorylation of either mTOR or ERK1/2 (25). Upon activation, TFEB is dephosphorylated, which induces it release and translocation to the nucleus to effect transcription of genes in the “CLEAR network” (25). Interestingly, one of the downstream targets of TFEB - p62, has also been shown to have roles in the transcriptional response to autophagy. While generally identified as an adaptor protein for autophagic substrates, accumulation of p62 can also lead to the up-regulation of antioxidant and detoxifying enzymes, via an interaction with the complex regulating the Nrf2 transcription factor (26). As such, p62 appears to have multiple roles in the regulation of autophagy, and its increased abundance in GCN5L1−/− MEFs may have additional cytoprotective functions outside the trafficking of autophagic cargo.
The first link between the expression of TFEB and PGC-1α, the transcriptional co-activator responsible for the induction of numerous mitochondrial biogenesis systems (27), was discovered in a model of Huntington Disease (HD). HD causes neurodegeneration by the accumulation of polyglutamine-rich proteins that are resistant to degradation (20). Overexpression of PGC-1α, whose signaling is abrogated in HD, led to the clearance of protein aggregates in a mouse HD model. Further examination showed that PGC-1α induced the expression of TFEB, whose expression alone could clear polyglutamine aggregates through lysosomal degradation, which placed PGC-1α upstream of TFEB in this pathway. Conversely, it was recently shown that in mouse and Caenorhabditis elegans models of nutrient deprivation, TFEB could up-regulate lysosome-mediated lipid hydrolysis via the activation of PGC-1α and its downstream target PPARα. Here the authors discovered the presence of multiple CLEAR motifs in the PGC-1α promoter region, and deletion of these abrogated the response of this TFEB>PGC-1α pathway in response to reduced food availability (21).
In this study, we demonstrate that the loss of GCN5L1 leads to an increase in the expression of both TFEB and PGC-1α (Figs. 2 and 4), and that the depletion of either transcriptional activator leads to a coordinated decrease in the other in GCN5L1−/− cells (Fig. 5). We therefore postulate that there is a tightly regulated coordination of TFEB and PGC-1α expression, and that this mechanism has evolved in order for the cell to maintain its mitochondrial content. For example, in response to an increase in mitophagy (enacted through increased TFEB expression), the cell may up-regulate the activity of PGC-1α to replenish their mitochondrial content. Given that both proteins have been shown to induce the expression of the other, it is conceivable that this may be a direct consequence of their transcription factor activity. As such, we suggest that instead of a linear pathway relationship, both TFEB and PGC-1α (under the control of GCN5L1) function in a feedback loop to control cellular mitochondrial content (Fig. 6).
This study demonstrates that GCN5L1 occupies a pinnacle role in the regulation of mitochondrial turnover, acting as a brake on the opposing processes of mitophagy and biogenesis. Future work will be required to determine the molecular mechanisms behind this regulation: for example, whether TFEB/PGC-1α interacts directly with some portion of the GCN5L1 pool, or whether there is some retrograde signaling pathway which controls these factors emanating from mitochondria. It will also be necessary to determine how the dysregulation of this pathway, caused by GCN5L1 loss, will affect physiological processes that rely on the correct removal of mitochondria at the end of their usable life.
Acknowledgments
We thank members of the Puertollano Laboratory (NHLBI) for helpful discussions. We thank the Electron Microscopy and Proteomics core facilities of the National Heart, Lung, and Blood Intramural Research Program.
This work was funded by the NHLBI Intramural Research Program (HL006047-01).

This article contains supplemental Table S1.
- GCN5L1
- general control of amino acid synthesis 5-like protein 1
- TFEB
- transcription factor EB
- ATG
- autophagy-regulated
- mTOR
- mammalian target of rapamycin
- LAMP
- lysosomal membrane protein
- SILAC
- stable isotope labeling of amino acids in cell culture
- HD
- Huntington Disease.
REFERENCES
- 1. Klionsky D. J. (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937 [DOI] [PubMed] [Google Scholar]
- 2. Huang J., Klionsky D. J. (2007) Autophagy and human disease. Cell Cycle 6, 1837–1849 [DOI] [PubMed] [Google Scholar]
- 3. Hubbard V. M., Valdor R., Macian F., Cuervo A. M. (2012) Selective autophagy in the maintenance of cellular homeostasis in aging organisms. Biogerontology 13, 21–35 [DOI] [PubMed] [Google Scholar]
- 4. Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wild P., Dikic I. (2010) Mitochondria get a Parkin' ticket. Nat Cell Biol. 12, 104–106 [DOI] [PubMed] [Google Scholar]
- 6. Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin S. M., Lazarou M., Wang C., Kane L. A., Narendra D. P., Youle R. J. (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y., Dorn G. W., 2nd., (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H., Bosch-Marce M., Shimoda L. A., Tan Y. S., Baek J. H., Wesley J. B., Gonzalez F. J., Semenza G. L. (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892–10903 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Schweers R. L., Zhang J., Randall M. S., Loyd M. R., Li W., Dorsey F. C., Kundu M., Opferman J. T., Cleveland J. L., Miller J. L., Ney P. A. (2007) NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. U.S.A. 104, 19500–19505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glick D., Zhang W., Beaton M., Marsboom G., Gruber M., Simon M. C., Hart J., Dorn G. W., 2nd, Brady M. J., Macleod K. F. (2012) BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol. Cell. Biol. 32, 2570–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Novak I., Kirkin V., McEwan D. G., Zhang J., Wild P., Rozenknop A., Rogov V., Löhr F., Popovic D., Occhipinti A., Reichert A. S., Terzic J., Dötsch V., Ney P. A., Dikic I. (2010) Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kubli D. A., Gustafsson Å. B. (2012) Mitochondria and mitophagy: the yin and yang of cell death control. Circ. Res. 111, 1208–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webster B. R., Scott I., Han K., Li J. H., Lu Z., Stevens M. V., Malide D., Chen Y., Samsel L., Connelly P. S., Daniels M. P., McCoy J. P., Jr., Combs C. A., Gucek M., Sack M. N. (2013) Restricted mitochondrial protein acetylation initiates mitochondrial autophagy. J. Cell Sci. 126, 4843–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Settembre C., Di Malta C., Polito V. A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S. U., Huynh T., Medina D., Colella P., Sardiello M., Rubinsztein D. C., Ballabio A. (2011) TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott I., Webster B. R., Li J. H., Sack M. N. (2012) Identification of a molecular component of the mitochondrial acetyl transferase program; a novel role for GCN5L1. Biochem. J. 443, 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizushima N., Komatsu M. (2011) Autophagy: renovation of cells and tissues. Cell 147, 728–741 [DOI] [PubMed] [Google Scholar]
- 18. Mizushima N., Yoshimori T., Levine B. (2010) Methods in mammalian autophagy research. Cell 140, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S. E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., Mootha V. K. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsunemi T., Ashe T. D., Morrison B. E., Soriano K. R., Au J., Roque R. A., Lazarowski E. R., Damian V. A., Masliah E., La Spada A. R. (2012) PGC-1α rescues Huntington's disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci. Transl. Med. 4, 142ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Settembre C., De Cegli R., Mansueto G., Saha P. K., Vetrini F., Visvikis O., Huynh T., Carissimo A., Palmer D., Klisch T. J., Wollenberg A. C., Di Bernardo D., Chan L., Irazoqui J. E., Ballabio A. (2013) TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15, 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Starcevic M., Dell'Angelica E. C. (2004) Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J. Biol. Chem. 279, 28393–28401 [DOI] [PubMed] [Google Scholar]
- 23. Orvedahl A., Sumpter R., Jr., Xiao G., Ng A., Zou Z., Tang Y., Narimatsu M., Gilpin C., Sun Q., Roth M., Forst C. V., Wrana J. L., Zhang Y. E., Luby-Phelps K., Xavier R. J., Xie Y., Levine B. (2011) Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature 480, 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roczniak-Ferguson A., Petit C. S., Froehlich F., Qian S., Ky J., Angarola B., Walther T. C., Ferguson S. M. (2012) The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal 5, ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmieri M., Impey S., Kang H., di Ronza A., Pelz C., Sardiello M., Ballabio A. (2011) Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 [DOI] [PubMed] [Google Scholar]
- 26. Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y. S., Ueno I., Sakamoto A., Tong K. I., Kim M., Nishito Y., Iemura S., Natsume T., Ueno T., Kominami E., Motohashi H., Tanaka K., Yamamoto M. (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12, 213–223 [DOI] [PubMed] [Google Scholar]
- 27. McLeod C. J., Pagel I., Sack M. N. (2005) The mitochondrial biogenesis regulatory program in cardiac adaptation to ischemia–a putative target for therapeutic intervention. Trends Cardiovasc. Med. 15, 118–123 [DOI] [PubMed] [Google Scholar]