Background: Biohydrogen production from formate may aid in efficient chemical and fuel production using H2 as the energy carrier.
Results: The hyperthermophile Pyrococcus furiosus was engineered to convert formate to H2.
Conclusion: An 18-gene cluster can be inserted into the P. furiosus chromosome for active production of a membrane-bound system.
Significance: This work demonstrates the versatility of this model organism for metabolic engineering purposes.
Keywords: Archaea, Enzymes, Genetics, Metabolic Engineering, Microbiology, Hydrogen, Hyperthermophiles
Abstract
Biohydrogen gas has enormous potential as a source of reductant for the microbial production of biofuels, but its low solubility and poor gas mass transfer rates are limiting factors. These limitations could be circumvented by engineering biofuel production in microorganisms that are also capable of generating H2 from highly soluble chemicals such as formate, which can function as an electron donor. Herein, the model hyperthermophile, Pyrococcus furiosus, which grows optimally near 100 °C by fermenting sugars to produce H2, has been engineered to also efficiently convert formate to H2. Using a bacterial artificial chromosome vector, the 16.9-kb 18-gene cluster encoding the membrane-bound, respiratory formate hydrogen lyase complex of Thermococcus onnurineus was inserted into the P. furiosus chromosome and expressed as a functional unit. This enabled P. furiosus to utilize formate as well as sugars as an H2 source and to do so at both 80° and 95 °C, near the optimum growth temperature of the donor (T. onnurineus) and engineered host (P. furiosus), respectively. This accomplishment also demonstrates the versatility of P. furiosus for metabolic engineering applications.
Introduction
Hydrogen gas (H2) can be generated from a variety of feedstocks and has great potential as a renewable energy carrier (1, 2). There is currently great interest in engineering microorganisms for efficient H2 production (3, 4), particularly from abundant one-carbon compound feedstocks such as formate (5). In addition, H2 is proposed as the energy carrier for converting carbon dioxide into industrial chemicals and liquid biofuels or so-called “electrofuels.” Although gas mass transfer and the rapid supply of sparingly soluble H2 to the site of carbon fixation within a microbe is a major challenge (6), this could be circumvented by the production of biohydrogen from highly soluble H2 donors such as formate, which simultaneously generates CO2. The hyperthermophile Thermococcus onnurineus is capable of formate oxidation coupled to H2 evolution (7). The related organism, Pyrococcus furiosus, cannot utilize formate as a source of H2 (8), but it has many desirable features as a metabolic engineering host for the H2-dependent reduction of CO2 to chemicals and electrofuels (6, 9).
P. furiosus and T. onnurineus are members of the archaeal order Thermococcales, with optimal growth temperatures ranging from 75 to 100 °C. They grow heterotrophically by the fermentation of sugars or peptides with formation of either H2 or H2S, and most require S0 as an electron acceptor. A hallmark of this group is the presence of a respiratory membrane-bound [NiFe]hydrogenase (Mbh)2 complex that is responsible for evolving H2 as a means for disposing of reductant from fermentation. A very close homolog of Mbh, known as Mbx, is involved in reducing S0 to H2S (8). The Mbh and Mbx complexes each occur in conjunction with a Na+/H+ antiporter (Mrp) module, such that H2 and H2S evolution are coupled to the formation of a Na+ ion gradient that allows energy conservation via Na+-dependent ATP synthase (10, 11).
In some Thermococcales species, there are additional modules that function in conjunction with the Mrp-Mbh complexes, such as the formate dehydrogenase (Fdh) of T. onnurineus. This Fdh, together with an Mrp-Mbh, forms a multiprotein membrane-bound formate hydrogen lyase (FHL) system that allows oxidation of formate to generate H2 and CO2, where the CO2 is predominantly bicarbonate (7). The standard reduction potentials of the formate/bicarbonate and H2/H+ couples are very similar, but the thermodynamics of the FHL reaction become favorable (ΔG = −8 to −20 kJ mol−1) at high temperature, high formate concentrations and low partial pressures of H2 (7). Thus, in T. onnurineus, cell growth can be supported by formate-driven ATP synthesis. T. onnurineus contains three different Fdh-containing gene clusters, two of which also encode Mbh and Mrp modules, and it has been shown that only one of these (Mrp-Mbh-Fdh2) is required for formate-dependent growth and H2 evolution (7). This FHL complex is encoded by an 18-gene cluster (TON_1563-1580), which also includes a formate transporter of the FocA formate channel type (12), although the mechanism of formate uptake is unclear. A schematic representation of the membrane-associated T. onnurineus FHL complex is presented in Fig. 1A.
FIGURE 1.
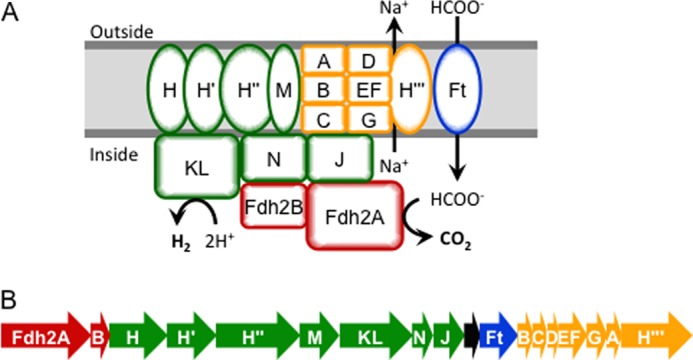
The T. onnurineus FHL complex and gene cluster. A, a schematic representation of the T. onnurineus FHL complex (encoded by TON_1563–1580) based on operon structure and homology with P. furiosus Mrp-Mbh, showing subunits of formate dehydrogenase (Fdh2) in red, membrane-bound hydrogenase (Mbh) in green, formate transporter (Ft) in blue, and Na+/H+ transporter (Mrp) in yellow. The hypothetical protein encoded by TON_1572 is not shown. B, the TON_1563–1580 gene cluster (Fdh2, TON_1563–64, red; Mbh, TON_1565–71, green; hypothetical gene, TON_1572, black; formate transporter, TON_1573, blue; Mrp, TON_1574–80, yellow).
The primary goal of this research was to convert P. furiosus into a formate-oxidizing, H2-evolving microorganism by inserting the 16.9-kb 18-gene FHL operon into its chromosome (Fig. 1B). An efficient genetic system has been developed for P. furiosus, the first for a microorganism that can grow at, or even close to, 100 °C (13). The genetically tractable P. furiosus strain, COM1, has been used for making gene deletions (14–16), overproducing its proteins (17, 18), and for metabolic engineering (9, 19). The COM1 strain is remarkably efficient at both DNA uptake and recombination (13, 20, 21), although the factors responsible are not clear (22). It is also not known how large of a DNA fragment can be functionally inserted into the P. furiosus chromosome. Herein, we demonstrate chromosomal insertion of a DNA fragment of almost 19 kb for heterologous expression of the T. onnurineus FHL operon in P. furiosus. As a member of the Thermococcales, P. furiosus contains an Mbh-Mrp gene cluster that is used for fermentative H2 production during growth on sugars (10, 15). Successful engineering of the FHL operon into P. furiosus enabled this organism the capacity to utilize both sugars and formate as sources of H2 at 80° to 95 °C, at or near the optimum growth temperatures of T. onnurineus (80 °C) and P. furiosus (100 °C).
EXPERIMENTAL PROCEDURES
Vector Construction
PCR products of the mbh1 promoter region (180 b starting immediately upstream of the PF1423 gene start), the T. onnurineus FHL operon (TON1563–1580, 16.9 kb), homologous flanking regions targeting a locus termed genome region 5 (between convergent genes PF1232-PF1233, ∼0.5 kb each), the pyrF pop-out marker cassette (9), and bacterial artificial chromosome (BAC) vector backbone (amplified from pBELOBac-11, obtained from New England Biolabs) were assembled into a single vector, pGL054 (Fig. 2A), using Gibson Assembly (New England Biolabs) (23). pGL054 was sequence-verified.
FIGURE 2.
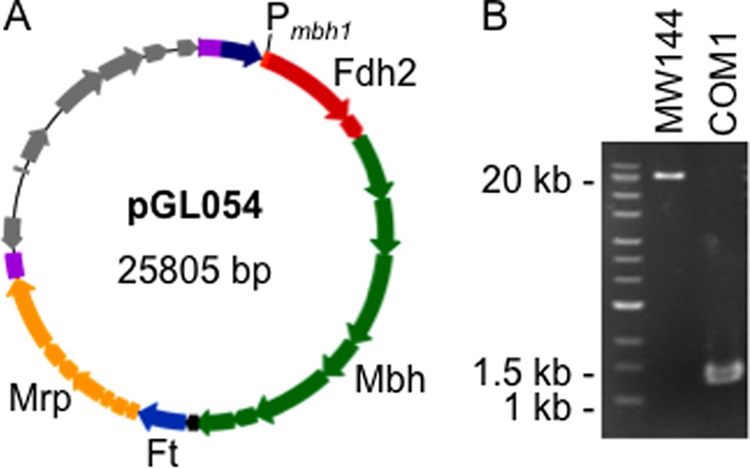
Insertion of the T. onnurineus FHL gene cluster into the P. furiosus chromosome. A, the BAC-based vector pGL054 containing the FHL operon (with genes color-coded according to Fig. 1) under the control of the mbh1 promoter (Pmbh1, orange), with the pyrF marker cassette (indigo) and 5′ and 3′ 0.5-kb homologous recombination regions (purple) used for targeted insertion into the P. furiosus chromosome. The BAC vector backbone features are shown in gray (from left to right: cat marker, oriS, repE, sopA, sopB, sopC, and cos). B, PCR confirmation with genomic DNA of MW144 versus COM1 parent with primers amplifying from ∼100 b outside each homologous flanking region of the target insertion locus.
Strain Construction
P. furiosus COM1 was transformed as described previously (13) with pGL054 linearized using the unique PvuI restriction site (positioned between sopA and sopB on the BAC vector backbone). Transformant colonies were cultured anaerobically in defined cellobiose medium (13), genomic DNA was isolated using the ZymoBeadTM Genomic DNA kit (Zymo Research), and PCR screens were performed with primers ∼100 b outside the homologous flanking regions used to insert the expression construct into the chromosome. PCR-verified isolates were further purified twice on solid defined cellobiose medium prior to saving glycerol stocks. One of the purified pGL054 transformants was designated MW144, and this strain was used for phenotypic analyses. Strains used and constructed in this study are listed in Table 1.
TABLE 1.
Strains used and constructed in this study
Growth and Cell Protein Quantitation
P. furiosus strains were cultured under strict anaerobic conditions in artificial seawater medium containing per liter: 1× base salts (24), 1× trace minerals (24), 0.26 μm sodium tungstate, 0.25 μg of resazurin, 0.5 g of cysteine, 1 g of sodium bicarbonate, and 1 mm potassium phosphate buffer, with pH adjusted to 6.8 prior to bottling. Medium was adjusted to contain equivalent amounts of tungstate and molybdate because it is unknown whether the cofactor utilized by Fdh contains molybdenum or tungsten Media was aliquoted into serum bottles, and the headspace was replaced with argon after three cycles of vacuum and argon. For growth curves, this medium was supplemented with 0 or 5 g maltose with 0.5 g of casein hydrolysate (USB) and 1× vitamin mix per liter (13). Sodium formate was added at a concentration of 50 mm from an anaerobic stock solution. Medium was inoculated to ∼3 × 106 cells ml−1, and cultures were incubated at 98 °C or 80 °C with shaking. For extract preparation, growth medium was supplemented with 5 g of maltose and 5 g of yeast extract per liter, and cultures were incubated at 80 °C with stirring. When necessary, cell growth was monitored by cell counts using a Petroff-Hausser counting chamber. Cell protein was quantified from 1-ml culture samples using the Bradford protein assay kit (Bio-Rad). Briefly, cells were harvested by centrifugation from 1-ml culture samples and lysed by osmotic shock in an equal volume of water, with vortexing and one freeze-thaw cycle. Lysate was centrifuged at 10,000 × g for 1 min to pellet insoluble cell debris prior to quantitation of soluble cell protein.
Cell-free Extract Preparation and Enzyme Assays
Cell-free extracts of P. furiosus cell pellets were prepared as follows using strict anaerobic conditions in an anaerobic chamber (Coy Laboratory Products). Cells were suspended in 50 mm MOPS, pH 7.5 (3 ml of buffer per gram of cells) and lysed by osmotic shock and sonication (Qsonica model Q55, 1 min at amplitude 30). To prepare membrane extracts, the cell-free extract was centrifuged at 100,000 × g for 1 h. The resulting pellet was suspended in 9 ml of buffer and centrifuged again, and the pellet was suspended in 0.5 ml of buffer and stored at 4 °C in stoppered glass vials until assayed. Formate hydrogen lyase activity was measured by the production of H2 from formate. A buffer (50 mm MOPS, pH 7.5, 20 mm NaCl, 2 mm MgCl2) containing 25 mm sodium formate was preheated to 80 °C, and the reaction was initiated by the addition of extracts (∼0.5 mg ml−1), and gas samples were analyzed by gas chromatography (Shimadzu GC8A with TCD detector, oven 70 °C, injector/detector 120 °C, Alltech Molecular Sieve column 5A 80/100). Membrane bound hydrogenase (Mrp-Mbh) activity was measured by the production of H2 from pyruvate via reduced ferredoxin (regenerated by pyruvate ferredoxin oxidoreductase, POR), and total hydrogenase activity was measured using sodium dithionite with methyl viologen (10). Care was taken to maintain strict anaerobic conditions during extract preparation and enzyme assays.
H2 Quantitation
H2 was measured by sampling the headspace of closed bottle cultures with a pressure-lock syringe. Samples from identical bottles of medium (without cells) containing known amounts of H2 were used as standards. H2 content from headspace samples was analyzed by gas chromatography (see above). We assumed H2 solubility in the liquid phase as negligible; therefore, H2 production is expressed as moles produced per liquid phase, using the ideal gas law (assuming 1 mole of gas at 25 °C and 1 atmosphere equals 24.5 liters).
Formate Quantitation
Formate was quantified via HPLC either by derivatization with 2,4-dibromoacetophenone (25) or direct analysis on an Aminex 87H column (Bio-Rad). For derivatization, spent media samples of 20 μl were diluted 5- to 10-fold with water, and pH was adjusted to ∼9. Subsequently 100 μl acetonitrile, 200 μl of 50 mm 2,4-dibromoacetophenone (in acetonitrile), and 50 μl of 1 m 15-crown-5 ether (in acetonitrile) were added, the mixture was incubated for 45 min at 80 °C, and 10-μl aliquots were analyzed by HPLC (Agilent 1260 Infinity; Poroshell 120 EC-C18 column; solvents, 0.05% (v/v) trifluoroacetic acid and acetonitrile; gradient, 90/10 to 40/60 in 8 min at 2 ml min−1). The Aminex 87H column was run with an isocratic mobile phase of 5 mm H2SO4 for 30 min, and organic acids were detected by UV absorbance at 210 nm.
RNA Extraction and Quantitative RT-PCR
RNA was extracted from 10 ml samples of culture using a phenol:chloroform extraction method as previously described (26). Genomic DNA was digested using TURBO DNase (Ambion). Synthesis of cDNA was performed with 1 μg of purified RNA using the Affinity Script QPCR cDNA synthesis kit (Agilent). The Brilliant II SYBR® Green QPCR Master Mix (Agilent) was used for quantitative RT-PCR experiments with primers designed to amplify a ∼150 b product within the target gene: PF0971 (PORγ gene), PF1423 (mbh1), TON1563, TON1567, TON1573, TON1580.
RESULTS
Insertion of the T. onnurineus FHL Operon into the P. furiosus Chromosome
The FHL cluster was assumed to be a single operon as all but one of its intergenic spaces are less than 12 bp in size (the exception is 42 bp). The native promoter of FHL was replaced with that of the P. furiosus Mbh operon (Pmbh1), the transcription of which is activated by the SurR regulator during growth in the absence of S0, thereby allowing Mbh to catalyze H2 evolution during sugar fermentation (27, 28). A BAC vector was used to facilitate cloning of the construct for chromosomal insertion of the FHL operon (Fig. 2A). Insertion of the expression construct was targeted to a region of the P. furiosus chromosome (between convergent genes PF1232-PF1233) having little to no transcriptional activity as determined by analysis of tiling array data (29). Using 0.5-kb homologous recombination regions, the resulting ∼19-kb fragment of DNA containing the FHL operon along with the pyrF selectable marker was inserted into the chromosome in one transformation event, generating the strain MW144 (Fig. 2B).
In Vitro Evaluation of FHL Complex Activity
To examine the functionality of the T. onnurineus FHL system in P. furiosus, strain MW144 was grown on the disaccharide maltose in the absence of S0 to activate expression of genes controlled by the mbh1 promoter, which included both the FHL and Mbh operons. Although P. furiosus grows optimally near 100 °C, the MW144 strain was grown at 80 °C, the optimal growth temperature of T. onnurineus, as our previous work has demonstrated that heterologously-produced enzymes in P. furiosus have the highest specific activities when produced near the optimum growth temperature of their organism of origin (9). Quantitative RT-PCR indicated that expression of the FHL operon in MW144 was at or above the level of mbh1 gene expression, and similar to that of the major sugar metabolism enzyme pyruvate ferredoxin oxidoreductase (POR), which generates the reduced ferredoxin that is oxidized by P. furiosus Mbh to evolve H2 (Fig. 3A). Furthermore, formate-dependent H2 evolution activity (0.3 ± 0.02 units mg−1) was present in membrane preparations of MW144 cells but not in the P. furiosus parental control strain MW004 (<0.01 unit mg−1). However, both MW144 and control strains exhibited comparable pyruvate-dependent H2 evolving activity, which is a measure of the activity of POR and the Mbh complex, but not of the FHL complex (Fig. 3B). In contrast, total hydrogenase activity, as measured using the artificial electron donor dithionite-reduced methyl viologen, was almost 2-fold higher in MW144 membrane preparations compared with MW004 (Fig. 3B). Taken together, these data indicate that the T. onnurineus FHL complex neither replaces nor reduces the activity of the native Mbh, and that in contrast to Mbh, the FHL complex does not efficiently use reduced ferredoxin as an electron donor. Hence, the MW144 strain has twice the Mbh activity (P. furiosus Mbh plus T. onnurineus FHL) but similar pyruvate-dependent ferredoxin-linked Mbh activity (Mbh only) as the parent strain.
FIGURE 3.
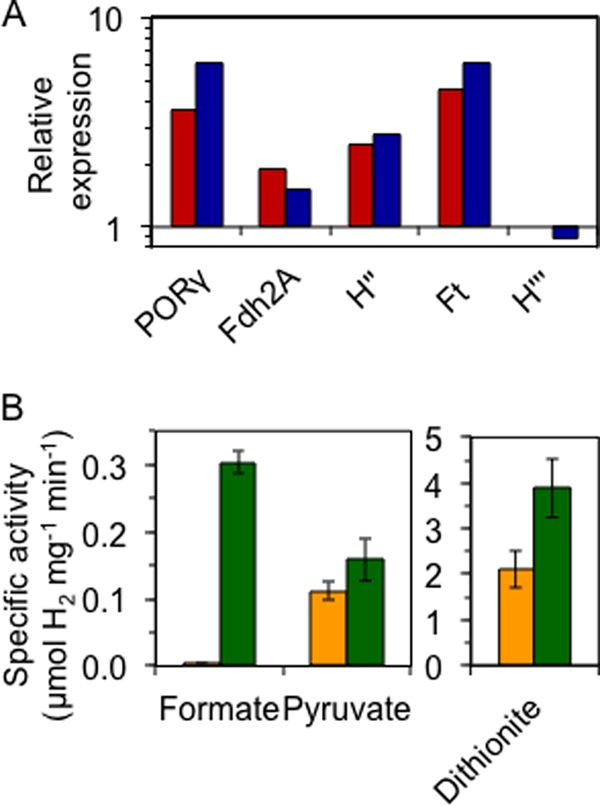
The T. onnurineus FHL complex is actively expressed in P. furiosus. A, quantitative RT-PCR expression of select FHL genes in MW144, relative to mbh1 expression, at both 98 °C (red) and 80 °C (blue). Relative expression of the P. furiosus PORγ gene is shown for comparison. B, specific activities from membrane extracts of MW004 (yellow) and MW144 (green) using the indicated electron donor. Error bars represent S.D. (n = 2).
Analysis of in Vivo H2 Production from Formate
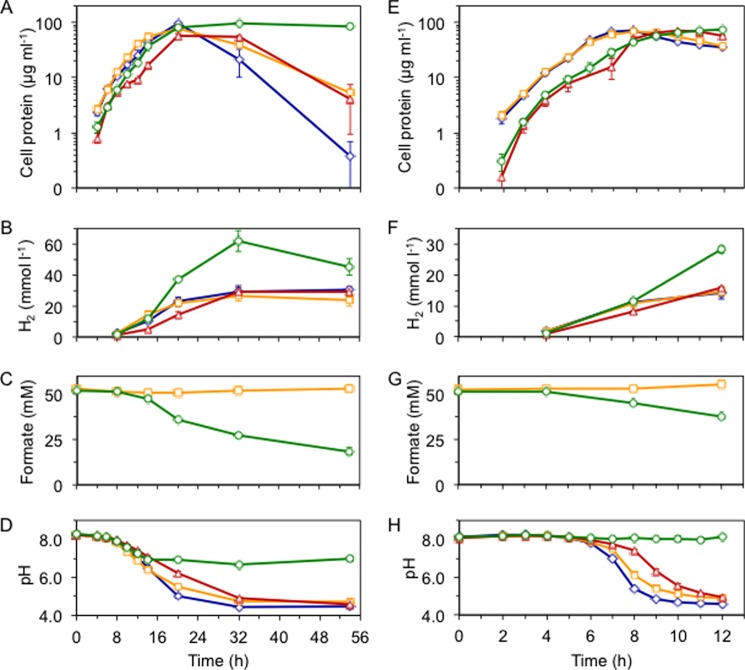
To determine the physiological effects of expression of the T. onnurineus FHL complex in P. furiosus, strain MW144 and the control strain MW004 were grown on maltose at 80 °C in the presence and absence of 50 mm formate. According to previously reported work, the doubling time of wild type P. furiosus increases by ∼2-fold at 80 °C compared with 100 °C (∼80 min versus ∼40 min, respectively) (30). Both strains grew similarly with and without formate, reaching stationary phase after ∼20 h of growth (as measured by soluble cell protein; Fig. 4A). As can be seen in Fig. 4B, compared with the control strain and no formate conditions, MW144 grown in the presence of formate produced almost twice as much H2 after 20 h (37 versus 22 mm), reaching a maximum of 62 mm H2 by 32 h (although by 54 h, high gas pressures caused difficulty in accurate headspace sampling of MW144 with formate). Additionally, MW144 consumed formate as shown by the decrease in the measured concentration of formate in the culture medium from 50 mm to <20 mm after 54 h (Fig. 4C). These data clearly show that the FHL complex is functionally expressed in P. furiosus and is able to efficiently oxidize formate with concomitant H2 evolution. Interestingly, even though the optimal growth temperature of T. onnurineus is 80 °C, the FHL complex was still produced and active in P. furiosus at 95 °C. After 12 h of growth at 95 °C (Fig. 4E), MW144 produced >2-fold more H2 in the presence of formate rather than in its absence (28 mm versus 16 mm; Fig. 4F) and consumed a corresponding amount of formate (∼14 mm; Fig. 4G). The maximum rate of H2 production for MW144 grown with formate was the same at 95 and 80 °C (4.2 mmol H2 liter−1 h−1) and was at least 2-fold higher than growth without formate.
FIGURE 4.
The T. onnurineus FHL complex is active in vivo in P. furiosus. Growth (as measured by soluble cell protein, A and E), H2 produced per liter of culture (B and F), formate in spent medium (C and G), and culture pH (D and H) of MW004 cultured in the absence (blue diamonds) or presence (yellow squares) of 50 mm formate compared with MW144 in the absence (red triangles) or presence (green circles) of 50 mm formate, at 80 °C (A–D) or 95 °C (E–H) in medium supplemented with 5 g of maltose, 0.5 g of tryptone, and 1× vitamin mixture per liter. Error bars represent S.D. (n = 3).
P. furiosus produces acetate during growth, causing a decrease in growth medium pH (to near pH 4); however, the pH medium remained near neutral for MW144 grown with formate (Fig. 4, D and H). The drop in growth medium pH for the control strain and MW144 in the absence of formate caused a decrease in soluble cell protein (presumably due to protein aggregation resulting from low intracellular pH) and a subsequent loss in cell viability, as measured by subculturing exponential and stationary phase cells and monitoring growth (data not shown). The pH stabilizing effect of the T. onnurineus FHL complex in P. furiosus is akin to that of the analogous FHL system found in Escherichia coli and related microorganisms (31). The E. coli FHL is important for cell survival under anaerobic acid stress conditions whereby FHL increases intracellular pH by converting endogenously produced formate to H2 and CO2 (HCOO− + H+ → CO2 + H2).
In T. onnurineus, formate oxidation is coupled to ATP generation via ion pumping (see Fig. 1A), and there is evidence that it is also assimilated into cellular carbon (32). In the absence of S0, T. onnurineus displays no growth on peptides (supplied as 0.5–1 g of yeast extract per liter); however, cells do grow when formate is supplied (7). P. furiosus also shows very poor growth on peptides (without S0), although unlike T. onnurineus, it can utilize the sugars found in yeast extract (24). To determine whether the FHL complex could supply energy to P. furiosus via formate oxidation, the MW144 strain was grown at 80 °C solely on peptides (supplied as 0.5 g of tryptone per liter). Cell growth was similarly poor for both the MW144 and control P. furiosus strains grown with and without formate, indicating MW144 could not use formate as an energy source (cell densities of ∼5 × 107 cells ml−1 or cell protein of 10 μg ml−1 were obtained, Fig. 5A). Nevertheless, although MW144 did not gain energy from formate oxidation, the FHL complex was highly active, with MW144 producing ∼6-fold more H2 (24 versus 4 mm, Fig. 5B) and consuming a corresponding amount of formate (12 mm, Fig. 5C). Although the formate-dependent H2 production activity of the FHL complex is similar in both the native organism and P. furiosus, the energy-conserving feature observed in T. onnurineus must result from other factors distinct from the FHL operon but functioning in combination with the FHL complex.
FIGURE 5.
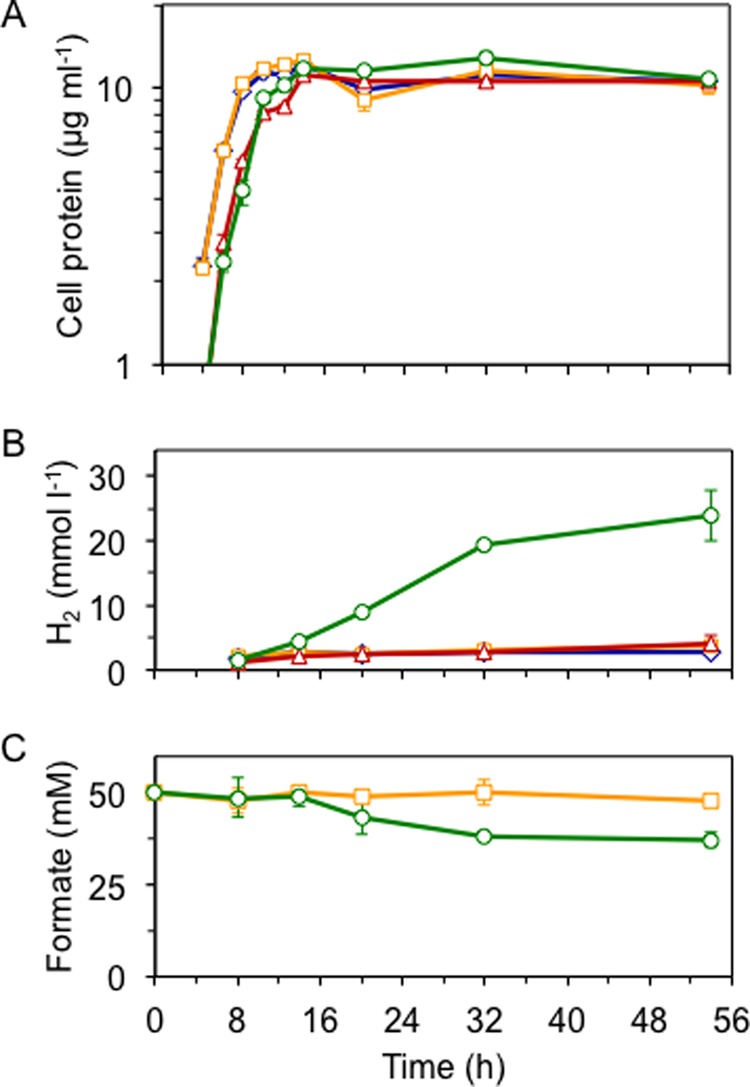
Formate oxidation and H2 production function independently from primary metabolism. Growth (as measured by soluble cell protein, A), H2 produced per liter of culture (B), formate in spent medium (C) of MW004 cultured in the absence (blue diamonds) or presence (yellow squares) of 50 mm formate compared with MW144 in the absence (red triangles) or presence (green circles) of 50 mm formate, at 80 °C in medium supplemented with 0.5 g of tryptone and 1× vitamin mixture per liter. Error bars represent S.D. (n = 3).
DISCUSSION
The T. onnurineus FHL complex in P. furiosus is a self-contained unit that functions independently of cellular metabolism when formate is present, displaying close to stoichiometric conversion of formate to H2. Moreover, the maximum rate (4.2 mmol H2 liter−1 h−1) and amount (∼45 mmol H2 liter−1) of H2 produced by recombinant P. furiosus strain MW144 after 24 h (in the presence of maltose, Fig. 4B) is comparable with that reported for T. onnurineus (3.8 and ∼38, respectively) (5), although the rate and amount of H2 produced are at least 3-fold lower than T. onnurineus when MW144 is grown with formate on peptide-containing medium (without maltose). When maltose was supplied in the P. furiosus growth medium, the mole ratio of H2 evolved to formate utilized was not stoichiometric because sugar fermentation also leads to H2 production. The ratio of excess H2 produced by MW144 when formate is present compared with the amount of formate utilized ranges from 1 to 1.5 during growth (with or without maltose), which is close to the theoretical rate of one mole of H2 produced per mole of formate consumed.
The functionality of the engineered FHL complex in P. furiosus therefore appears to be very similar to that of T. onnurineus for production of H2 from formate. However, P. furiosus has the advantage over T. onnurineus in that it can also generate H2 by sugar fermentation. Moreover, this H2 production occurs by a completely independent pathway (via P. furiosus Mbh) that does not interfere with formate to H2 conversion (via the FHL Mbh). Thus, the engineered P. furiosus strain MW144 has the unique property of being able to independently convert both sugars and formate to H2 at temperatures ranging from 80 to near 100 °C. Moreover, this was achieved by the stable insertion of a large DNA element into the P. furiosus chromosome via use of a BAC vector. Indeed, the ability to recombine almost 20 kb of DNA into the genome in a single step with relative ease is a remarkable feature of the P. furiosus genetic system and rivals technologies available for chromosomal integration of foreign DNA in model microorganisms such as E. coli (33, 34). This new genetic tool will enable sophisticated genetic engineering of heterologous metabolic pathways in this model hyperthermophile. Furthermore, enabling P. furiosus with the capacity to use formate in place of exogenously supplied H2 gas significantly improves its prospects for serving as an effective metabolic engineering host for chemical and electrofuel production (6, 9).
This work was supported by the United States Department of Energy as part of the Electrofuels Project of ARPA-E (DE-AR0000081) for strain construction and analysis and by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences (DE-FG05–95ER20175) for strain characterization.
- Mbh
- membrane-bound hydrogenase
- Mrp
- Na+/H+ antiporter
- Fdh
- formate dehydrogenase
- FHL
- formate hydrogen lyase
- BAC
- bacterial artificial chromosome
- POR
- pyruvate ferredoxin oxidoreductase.
REFERENCES
- 1. Navarro R. M., Peña M. A., Fierro J. L. (2007) Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass. Chem. Rev. 107, 3952–3991 [DOI] [PubMed] [Google Scholar]
- 2. Armaroli N., Balzani V. (2011) The hydrogen issue. ChemSusChem. 4, 21–36 [DOI] [PubMed] [Google Scholar]
- 3. Maeda T., Sanchez-Torres V., Wood T. K. (2012) Hydrogen production by recombinant Escherichia coli strains. Microb. Biotechnol. 5, 214–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crable B. R., Plugge C. M., McInerney M. J., Stams A. J. (2011) Formate formation and formate conversion in biological fuels production. Enzyme Res. 2011, 532536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bae S. S., Kim T. W., Lee H. S., Kwon K. K., Kim Y. J., Kim M. S., Lee J. H., Kang S. G. (2012) H2 production from CO, formate or starch using the hyperthermophilic archaeon, Thermococcus onnurineus. Biotechnol. Lett. 34, 75–79 [DOI] [PubMed] [Google Scholar]
- 6. Hawkins A. S., McTernan P. M., Lian H., Kelly R. M., Adams M. W. (2013) Biological conversion of carbon dioxide and hydrogen into liquid fuels and industrial chemicals. Curr. Opin. Biotechnol. 24, 376–384 [DOI] [PubMed] [Google Scholar]
- 7. Kim Y. J., Lee H. S., Kim E. S., Bae S. S., Lim J. K., Matsumi R., Lebedinsky A. V., Sokolova T. G., Kozhevnikova D. A., Cha S. S., Kim S. J., Kwon K. K., Imanaka T., Atomi H., Bonch-Osmolovskaya E. A., Lee J. H., Kang S. G. (2010) Formate-driven growth coupled with H2 production. Nature 467, 352–355 [DOI] [PubMed] [Google Scholar]
- 8. Schut G. J., Boyd E. S., Peters J. W., Adams M. W. (2013) The modular respiratory complexes involved in hydrogen and sulfur metabolism by heterotrophic hyperthermophilic archaea and their evolutionary implications. FEMS Microbiol. Rev. 37, 182–203 [DOI] [PubMed] [Google Scholar]
- 9. Keller M. W., Schut G. J., Lipscomb G. L., Menon A. L., Iwuchukwu I. J., Leuko T. T., Thorgersen M. P., Nixon W. J., Hawkins A. S., Kelly R. M., Adams M. W. (2013) Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc. Natl. Acad. Sci. U.S.A. 110, 5840–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sapra R., Bagramyan K., Adams M. W. (2003) A simple energy-conserving system: proton reduction coupled to proton translocation. Proc. Natl. Acad. Sci. U.S.A. 100, 7545–7550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sapra R., Verhagen M. F., Adams M. W. (2000) Purification and characterization of a membrane-bound hydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 182, 3423–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lü W., Du J., Wacker T., Gerbig-Smentek E., Andrade S. L., Einsle O. (2011) pH-dependent gating in a FocA formate channel. Science 332, 352–354 [DOI] [PubMed] [Google Scholar]
- 13. Lipscomb G. L., Stirrett K., Schut G. J., Yang F., Jenney F. E., Jr., Scott R. A., Adams M. W., Westpheling J. (2011) Natural competence in the hyperthermophilic archaeon Pyrococcus furiosus facilitates genetic manipulation: construction of markerless deletions of genes encoding the two cytoplasmic hydrogenases. Appl. Environ. Microbiol. 77, 2232–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bridger S. L., Clarkson S. M., Stirrett K., DeBarry M. B., Lipscomb G. L., Schut G. J., Westpheling J., Scott R. A., Adams M. W. (2011) Deletion strains reveal metabolic roles for key elemental sulfur-responsive proteins in Pyrococcus furiosus. J. Bacteriol. 193, 6498–6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schut G. J., Nixon W. J., Lipscomb G. L., Scott R. A., Adams M. W. (2012) Mutational analyses of the enzymes Involved in the metabolism of hydrogen by the hyperthermophilic archaeon Pyrococcus furiosus. Front. Microbiol. 3, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thorgersen M. P., Stirrett K., Scott R. A., Adams M. W. (2012) Mechanism of oxygen detoxification by the surprisingly oxygen-tolerant hyperthermophilic archaeon, Pyrococcus furiosus. Proc. Natl. Acad. Sci. U.S.A. 109, 18547–18552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hopkins R. C., Sun J., Jenney F. E., Jr., Chandrayan S. K., McTernan P. M., Adams M. W. (2011) Homologous expression of a subcomplex of Pyrococcus furiosus hydrogenase that interacts with pyruvate ferredoxin oxidoreductase. PLoS One 6, e26569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandrayan S. K., McTernan P. M., Hopkins R. C., Sun J., Jenney F. E., Jr., Adams M. W. (2012) Engineering hyperthermophilic archaeon Pyrococcus furiosus to overproduce its cytoplasmic [NiFe]-hydrogenase. J. Biol. Chem. 287, 3257–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basen M., Sun J., Adams M. W. (2012) Engineering a hyperthermophilic archaeon for temperature-dependent product formation. MBio 3, e00053–00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farkas J., Chung D., DeBarry M., Adams M. W., Westpheling J. (2011) Defining components of the chromosomal origin of replication of the hyperthermophilic archaeon Pyrococcus furiosus needed for construction of a stable replicating shuttle vector. Appl. Environ. Microbiol. 77, 6343–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farkas J., Stirrett K., Lipscomb G. L., Nixon W., Scott R. A., Adams M. W., Westpheling J. (2012) Recombinogenic properties of Pyrococcus furiosus strain COM1 enable rapid selection of targeted mutants. Appl. Environ. Microbiol. 78, 4669–4676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bridger S. L., Lancaster W. A., Poole F. L., 2nd, Schut G. J., Adams M. W. (2012) Genome sequencing of a genetically tractable Pyrococcus furiosus strain reveals a highly dynamic genome. J. Bacteriol. 194, 4097–4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merryman C., Gibson D. G. (2012) Methods and applications for assembling large DNA constructs. Metab. Eng. 14, 196–204 [DOI] [PubMed] [Google Scholar]
- 24. Adams M. W., Holden J. F., Menon A. L., Schut G. J., Grunden A. M., Hou C., Hutchins A. M., Jenney F. E., Jr., Kim C., Ma K., Pan G., Roy R., Sapra R., Story S. V., Verhagen M. F. (2001) Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183, 716–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Durst H. D., Milano M., Kikta E. J., Jr., Connelly S. A., Grushka E. (1975) Phenacyl esters of fatty acids via crown ether catalysts for enhanced ultraviolet detection in liquid chromatography. Anal. Chem. 47, 1797–1801 [DOI] [PubMed] [Google Scholar]
- 26. Schut G. J., Zhou J., Adams M. W. (2001) DNA microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus: evidence for an new type of sulfur-reducing enzyme complex. J. Bacteriol. 183, 7027–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipscomb G. L., Keese A. M., Cowart D. M., Schut G. J., Thomm M., Adams M. W., Scott R. A. (2009) SurR: a transcriptional activator and repressor controlling hydrogen and elemental sulphur metabolism in Pyrococcus furiosus. Mol. Microbiol. 71, 332–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang H., Lipscomb G. L., Keese A. M., Schut G. J., Thomm M., Adams M. W., Wang B. C., Scott R. A. (2010) SurR regulates hydrogen production in Pyrococcus furiosus by a sulfur-dependent redox switch. Mol. Microbiol. 77, 1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoon S. H., Reiss D. J., Bare J. C., Tenenbaum D., Pan M., Slagel J., Moritz R. L., Lim S., Hackett M., Menon A. L., Adams M. W., Barnebey A., Yannone S. M., Leigh J. A., Baliga N. S. (2011) Parallel evolution of transcriptome architecture during genome reorganization. Genome Res. 21, 1892–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiala G., Stetter K. O. (1986) Pyrococcus furiosus sp. nov., represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 145, 56–61 [Google Scholar]
- 31. Kanjee U., Houry W. A. (2013) Mechanisms of acid resistance in Escherichia coli. Annu. Rev. Microbiol. 67, 65–81 [DOI] [PubMed] [Google Scholar]
- 32. Moon Y. J., Kwon J., Yun S. H., Lim H. L., Kim M. S., Kang S. G., Lee J. H., Choi J. S., Kim S. I., Chung Y. H. (2012) Proteome analyses of hydrogen-producing hyperthermophilic archaeon Thermococcus onnurineus NA1 in different one-carbon substrate culture conditions. Mol. Cell. Proteomics 10.1074/mcp.M111.015420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyazaki R., van der Meer J. R. (2013) A new large-DNA-fragment delivery system based on integrase activity from an integrative and conjugative element. Appl. Environ. Microbiol. 79, 4440–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sabri S., Steen J. A., Bongers M., Nielsen L. K., Vickers C. E. (2013) Knock-in/Knock-out (KIKO) vectors for rapid integration of large DNA sequences, including whole metabolic pathways, onto the Escherichia coli chromosome at well-characterised loci. Microb. Cell Fact. 12, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]