Background: How microbial agonists induce macrophages to store triglycerides (TAG) for prolonged periods was not known.
Results: Toll-like receptor (TLR)1/2, TLR3, and TLR4 agonists increased fatty acid uptake, increased TAG synthesis, and decreased lipolysis, augmenting TAG storage for ≥72 h.
Conclusion: TAG retention is an active, multifaceted, and long lasting response to sensing microbes.
Significance: Prolonged TAG storage contributes to foam cell formation.
Keywords: Adipose Triglyceride Lipase, Lipid Droplet, Lipolysis, Lipopolysaccharide (LPS), Macrophages, Toll-like Receptors (TLR), Triglyceride, ACSL, DGAT2, Acyl-CoA Synthetase
Abstract
Macrophages in infected tissues may sense microbial molecules that significantly alter their metabolism. In a seeming paradox, these critical host defense cells often respond by increasing glucose catabolism while simultaneously storing fatty acids (FA) as triglycerides (TAG) in lipid droplets. We used a load-chase strategy to study the mechanisms that promote long term retention of TAG in murine and human macrophages. Toll-like receptor (TLR)1/2, TLR3, and TLR4 agonists all induced the cells to retain TAG for ≥3 days. Prolonged TAG retention was accompanied by the following: (a) enhanced FA uptake and FA incorporation into TAG, with long lasting increases in acyl-CoA synthetase long 1 (ACSL1) and diacylglycerol acyltransferase-2 (DGAT2), and (b) decreases in lipolysis and FA β-oxidation that paralleled a prolonged drop in adipose triglyceride lipase (ATGL). TLR agonist-induced TAG storage is a multifaceted process that persists long after most early pro-inflammatory responses have subsided and may contribute to the formation of “lipid-laden” macrophages in infected tissues.
Introduction
Lipid droplets (LD,4 also called lipid bodies) are cytosolic collections of neutral lipids (triglycerides and cholesteryl esters) that may be both storage depots and foci for the production of eicosanoids and other important mediator molecules (1). They may also give macrophages the “foamy” appearance that is often noted within granulomas, xanthogranulomatous lesions, and atherosclerotic vessels. Although triglycerides (TAG) may comprise a substantial fraction of the lipids in LD (2), the mechanisms that promote long term TAG storage within macrophages have received little study.
Glucose is the major food of resting macrophages (3, 4). It provides precursors for the synthesis of glycerolipids, amino acids, and nucleic acids via pathways that also supply the cell with essential NADH, NADPH, and ATP. When macrophages sense bacterial LPS, they greatly increase their uptake and catabolism of glucose. Sensing LPS also increases fatty acid (FA) uptake (5, 6). In a seeming paradox, a large fraction of the newly acquired FA is incorporated into TAG and stored in LD; FA β-oxidation and the production of ATP via oxidative phosphorylation decrease. Intrigued by observing that LD persisted within LPS-exposed macrophages even as the ambient glucose supply was depleted, we used a load-chase model to study the mechanisms that promote long term TAG retention in stimulated primary macrophages.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 and B6.B10ScN-Tlr4lps-del/JthJv (TLR4-deficient) mice were purchased from The Jackson Laboratory and used for experiments when they were 5–12 weeks of age. MyD88−/− mice were kindly provided by Alan Sher, NIAID, National Institutes of Health. All protocols were approved by the IACUC of the Division of Intramural Research, NIAID, National Institutes of Health.
Reagents
Fatty acids were obtained from NuChek, PAM3CysK4 (PAM) from InvivoGen, and poly(I:C) from Sigma or InvivoGen. LPS was prepared in our laboratory from Escherichia coli O14 or E. coli LCD25 (7). The TLR agonists were tested in preliminary studies to establish each compound's ability to elicit IL-6 (MyD88-dependent) and/or RANTES (TRIF-dependent) production by macrophages in vitro and to choose effective stimulatory concentrations of each. Poly(I:C) elicited RANTES but not IL-6; PAM only elicited IL-6, and LPS stimulated the production of both cytokines. Radioisotopes were obtained from PerkinElmer Life Sciences ([3H]palmitate; [U-14C]glucose) or Moravek ([1-14C]palmitate; [9,10-3H]oleate).
Macrophage Cultures
Thioglycollate-elicited peritoneal macrophages (TEPM), harvested 4 or 5 days after injecting 1.0 ml of 3% Brewer's thioglycollate (BD Biosciences/BBL) intraperitoneally, were allowed to adhere to plastic wells for 4–6 h in complete RPMI 1640 medium (8). The cells (typically 5.5 × 105/0.5 ml of medium in 24-well Costar plates) were then washed twice with PBS and cultured further in DMEM (Sigma D5030) that contained 0.5% low endotoxin fetal bovine serum (Hyclone), 1 g/liter (5.5 mm) glucose, 44 mm NaHCO3, 1 mm sodium pyruvate, 2 mm l-glutamine, 100 units/ml penicillin G, and 100 μg/ml streptomycin (“cDMEM”). Cultures were carried in a humidified incubator at 37 °C in an atmosphere of 5% CO2.
Elutriated human monocytes were obtained from normal volunteers by the Clinical Center Blood Bank Elutriation Service, National Institutes of Health, with Institutional Review Board approval and subject consent. Prior to initiating experiments, the cells were cultured at 1 million cells/well in 24-well plastic plates (Costar) for 5 days in DMEM containing 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 5.5 mm d-glucose, 44 mm NaHCO3, and 2.5% human AB serum (generously provided by Dr. Lawrence Wahl, NIDCR, National Institutes of Health).
Fatty acids were suspended in ethanol (50 mm), heated for >30 min at 50 °C, diluted 1:50 into PBS that contained 2.5 mm essentially fatty acid-free human serum albumin (Sigma), and then diluted into warm cDMEM. [3H]Palmitate or [9,10-3H]oleate were dried under argon and added (1–1.5 μCi/ml) to cDMEM that contained nonradioactive palmitate (50 μm) and oleate (100 μm). After overnight incubation, cells were washed twice with 0.8 ml of PBS before adding fresh cDMEM that contained ½ the initial FA concentrations plus agonists as indicated. Cells and media were harvested up to 96 h later for analysis. Lipids were isolated from twice-washed cells into isopropyl alcohol (400 μl, combined with 100 μl wash), dried under argon, resuspended in chloroform that contained carrier triolein, oleate, and diacylglycerol, and separated on Whatman Silica Gel G plates using hexane/ethyl acetate/acetic acid (80:15:1) or hexane/diethyl ether/acetic acid (80:20:1) as solvent. Silica corresponding to TAG, DAG, and the origin was scraped from the plate, suspended in 0.5 ml of 2% SDS, 5 mm EDTA in 3 ml of Bio-Safe II scintillation fluid (Research Products International Corp.), and radioactivity was quantitated using a Beckman LS 6500 scintillation counter with quench and spillover correction. In some experiments, [1-14C]palmitate was used, and TLC band intensity was quantitated using a Typhoon 9410 phosphorimager (Amersham Biosciences). Radiolabeled TAG retention was calculated by multiplying the total 3H or 14C dpm in the lipid extract by the percentage of the 3H or 14C dpm that was recovered in TAG (dpm in TAG); more than 70% of the radioactivity in the lipid extract was recoverable from the scraped silica gel. Methanolic alkaline hydrolysis of labeled TAG and TLC analysis of the products confirmed that the isotopes remained in fatty acids.
To measure glucose uptake and utilization, FA-loaded cells were stimulated with LPS (2.5 ng/ml) overnight, washed, and re-incubated in glucose-free DMEM for 10 min before adding [U-14C]glucose (in 200 μm nonradioactive d-glucose) and continuing incubation for 3 h. Neutral lipids were isolated using TLC, and the 14C dpm in TAG and DAG were summed.
To measure lipolysis, cells that had been loaded overnight with FA and [3H]oleate were washed three times and then treated for 24 h with LPS (or PBS vehicle). After washing three times again, the 3H dpm in TAG was measured in each group to determine base-line TAG retention. The remaining cells were chased for an additional 22–24 h in FA-free cDMEM that either contained or did not contain triacsin C (3.3 or 10 μm) to inhibit long chain acyl-CoA synthetases (9–11). The percentage of the [3H]TAG load that was lost during Tc treatment was then compared for cells that were, or were not, exposed to LPS before and during the chase.
Imaging
Cells attached to coverslips were fixed in paraformaldehyde (2.5–4.0% in PBS), washed, and stained with Nile Red (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI, Sigma). Cells were visualized with an OLYMPUS BX61 motorized confocal microscope using a UPlan-FLN ×40 objective equipped with a RETIGA-SRV Deep-Cooled, High Sensitivity Digital CCD Camera (QImaging Company). Images of lipid droplets stained with Nile Red and DAPI were acquired using a sequential excitation of 457 and 528 nm, respectively. Each confocal Z-stack image consisted of 15 optical slices taken at 0.4-nm intervals with a dimension of 224.5 × 167.7 × 6.0 μm (x,y,z) and a total magnification of ×400. Exposure settings remained exactly the same between treatments in the same experiment (10 ms of DAPI and 1,000–1,200 ms of FITC were used for all samples). All images were acquired with Slide-book 5.0 software (3I Co.) and extracted at a resolution of 1,392 × 1040.
Lipid Droplet Quantification
Assessment of the average number of LDs per cell was carried out on confocal Z-stack images using Imaris (Bitplane) 7.4.2 software. Background subtraction was performed prior to detection using the “spot-object” command, manipulating the detection threshold when necessary to quantify only spots associated with lipid droplets that had ≥500 nm diameter within cells. The average number of LDs per cell was determined by dividing the total number of LDs in the field by the number of nuclei detected by the volume-rendering command in the software. A minimum of four fields was analyzed for each condition with an overall average of 200–300 cells analyzed per treatment.
Inhibitors
In some experiments, etomoxir (Sigma) (0.2 mm) was added to inhibit carnitine palmitoyltransferase 1 (CPT1) (12). 3H2O in the medium was measured as described (13) using Pierce spin cups with cellulose acetate filter (Pierce 69702) and Dowex 1-X8 adsorbent (Sigma). Triacsin C was from ENZO Life Sciences.
Assays
After isopropyl alcohol extraction, cell proteins were solubilized in 1 n NaOH and quantitated using the bicinchoninic acid assay (Pierce, Thermo Scientific). Triglycerides and glucose were measured using kits from Abcam and Sigma, respectively.
Microarray Analysis
Control and FA-loaded macrophages (2 × 106) were treated with LPS (10 ng/ml) or vehicle for 24 h prior to extraction of the RNA. Quality control was performed using the Agilent BioAnalyzer. Amplification and labeling of the RNA samples were performed using the Illumina TotalPrep RNA Amplification (Applied Biosystems) with an input of 500 ng of total RNA per sample. Biotinylated cRNA was hybridized to Illumina MouseWT-6 Version 2.0 Expression BeadChip (NCBI GEO accession GPL6887) having 45,281 unique probes and then scanned using the Illumina HiScan.
Statistical Analysis
Raw data were exported from Illumina Genome Studio software without background subtraction or normalization. Signal levels for each array were normalized using quantile normalization after transforming to log base 2. Using a simple mixed effect analysis of variance model (treatment as fixed effect and independent experiment as random effect), log ratios were calculated comparing each treatment to the unstimulated control. The false discovery rate method was used to adjusted p values for multiple testing (multiple microarray probes to test the same biological contrast). Normalization and mixed effects analysis of variance were computed using JMP/Genomics software version 5.1 (SAS Institute). Raw tabular data and normalized expression profiles have been deposited in NCBI GEO database (accession GSE42190). Differences between groups were estimated using Student's t test unless indicated otherwise.
Quantitative PCR
Q-PCR was performed using Fast SYBR Green PCR Master Mix in an ABI PRISM Fast 7500 sequence detection system (Applied Biosystems). Briefly, total RNA was isolated using RNeasyPlus mini kit with genomic DNA elimination (Qiagen) and reverse-transcribed into cDNA using iScript cDNA synthesis kit (Bio-Rad). PCRs were amplified for 40 cycles (30 s at 95 °C and then 60 s at 60 °C). Cyclophilin was used as the internal control, and data were analyzed by the relative quantification (ΔΔCt) method. Primer sequences are available from the authors on request. In some experiments, TaqMan primers and probes and TaqMan master mix were from ABI. β-Actin was used as the internal control.
Western Blotting
Cells were lysed directly with RIPA buffer supplemented with 1 mm PMSF, complete-mini protease inhibitor mixture (Roche Applied Science), and phosphatase inhibitor mixture 2 (Sigma). Antibodies used were as follows: anti-β-actin (sc-1615) and anti-ACSL1 (sc-98925) from Santa Cruz Biotechnology; anti-ATGL (2138S) from Cell Signaling Technology; and anti-DGAT2 from Pierce (PAS-21722). For two-color quantitative Western analysis, the membranes were incubated with infrared-conjugated secondary antibodies and scanned in an Odyssey SA scanner (LICOR Biosciences). Band intensities were quantified using Odyssey software. Super Signal Western blot enhancer (Pierce) was used.
RESULTS
Microbial Agonists Promote Prolonged TAG Retention in Macrophages
To identify mechanisms that promote long term TAG retention when macrophages that contain LD are exposed to microbes, we used a load-chase approach (Fig. 1A); after elicited macrophages had adhered to plastic culture wells for 4–6 h, they were washed and incubated overnight with cDMEM plus 50 μm palmitate, 100 μm oleate, and 1–2 μCi/ml radiolabeled palmitate or oleate (LOAD). Approximately 50% of the label was taken up by the cells overnight; 50–70% of the cell-associated label was then recovered in TAG, whereas less than 5% was found in cholesteryl esters. The cells were washed and re-incubated in fresh medium (CHASE) that contained ½ the original concentration of nonradioactive FA and either a microbial agonist or media control. The cells were harvested up to 96 h later, and the amount of label that remained in TAG was measured. In many experiments, LD abundance and intensity were also quantitated by Nile Red staining and image analysis, or TAG mass was measured using an enzymatic assay.
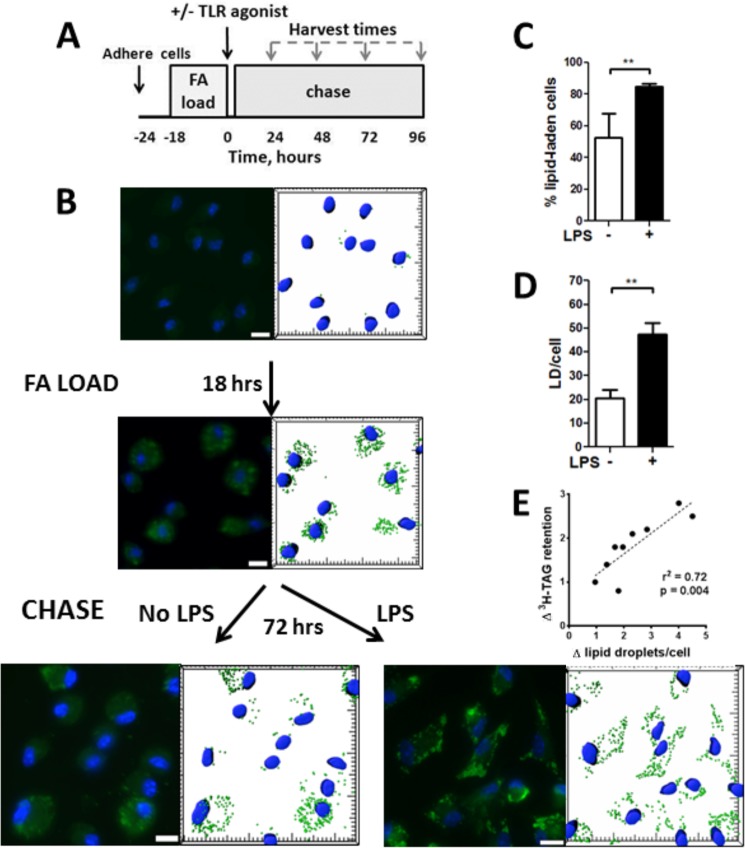
FIGURE 1.
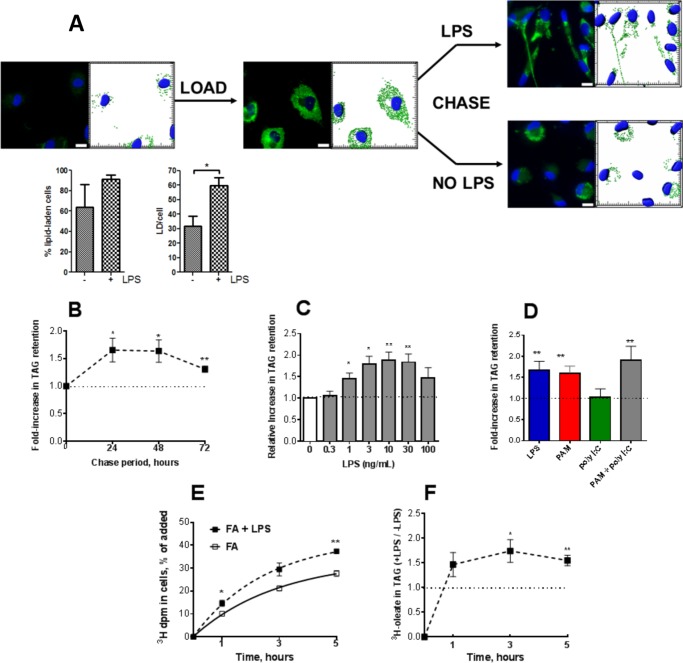
LPS stimulates lipid body retention in macrophages. A, flow diagram for the experiments. B, cells showing Nile Red fluorescence (green) and stained nuclei (DAPI, blue) are shown to the left of an image of the same field that shows lipid droplets as analyzed using Imaris software. C, percentage of the cells that were lipid-laden (>15 lipid droplets/cell) after chase for 72 h. D, average number of lipid droplets per cell. Data are from four independent experiments. E, fold-change (Δ) in lipid droplets/cell (+LPS/no LPS) positively correlated with the fold-change (Δ) in [3H]TAG retention. **, p < 0.01.
The retention of radiolabeled TAG is influenced by the factors that govern the synthesis and catabolism of TAG. As more molecules of unlabeled TAG are synthesized and incorporated into LD during the chase period, the radiolabeled TAG undergoes dilution and its rate of lipolysis decreases pari passu. When FA are released from TAG, one potential fate is thioesterification and re-incorporation into TAG. Although measuring [3H]TAG does not indicate the relative contributions of FA uptake, thioesterification, TAG synthesis, lipolysis, and recycling, it was useful for determining whether or not each of these steps plays a part in promoting TAG storage.
We first noted that FA-loaded macrophages retained LD much longer if they were exposed to LPS. LPS exposure increased both the percentage of the cells that retained LD and the number of droplets retained per cell (Fig. 1, B–D). There was significant positive correlation between the average number of LD/cell and the retention of radiolabeled oleate in TAG after 48–72 h of chase (n = 9, r2 = 0.72, p = 0.004) (Fig. 1E). In three other experiments, we found a strong correlation between the amount of [3H]TAG and TAG mass (n = 48, r2 = 0.90, p = 0.001).
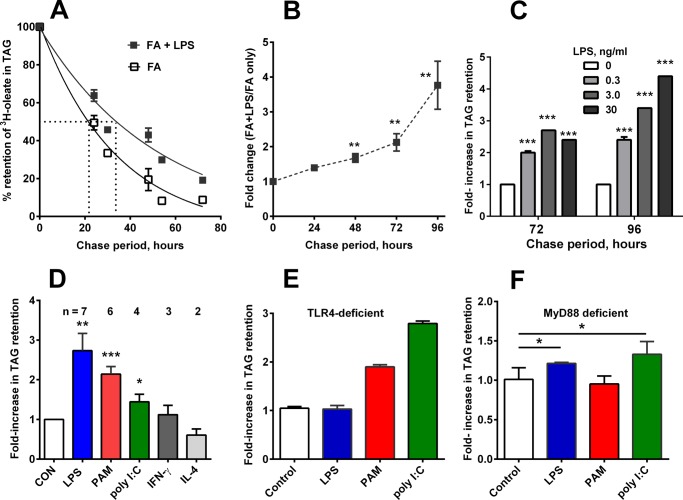
Following an overnight FA load, the fraction of the labeled FA that remained in TAG decreased with a half-life of ∼20 h (Fig. 2A). LPS exposure after the load increased TAG retention for at least 4 days, with an overall increase in the [3H]TAG half-life of ∼50%. By 96 h of chase, LPS-exposed cells had >3-fold more [3H]TAG than did cells that were incubated without LPS (Fig. 2B). The response to LPS was dose-dependent (Fig. 2C), with significant increases in TAG retention observed after exposure to as little as 0.3 ng of LPS/ml. PAM3CysK4 (TLR1/2 agonist) and poly(I:C) (TLR3 agonist) also increased TAG retention, whereas interferon-γ did not, and IL-4 allowed TAG loss (Fig. 2D). LPS did not stimulate TAG retention in TLR4-deficient macrophages (Fig. 2E), whereas PAM was stimulatory toward both wild type and TLR4-deficient cells (Fig. 2, D and E) but not in MyD88−/− cells (Fig. 2F), endorsing the importance of the MyD88-dependent pathway in inducing TAG retention. LPS stimulated TAG retention in MyD88−/− macrophages, as did poly(I:C), confirming a role for the MyD88-independent (TRIF) pathway as well (Fig. 2F) (14).
FIGURE 2.
LPS, PAM, and poly(I:C) stimulate TAG retention. A, time course of TAG retention following exposure to LPS (5–10 ng/ml). Data were combined from six independent experiments, each with n = 4/group/time point. B, relative TAG retention increased in LPS-stimulated cells over time. Data are from two to six experiments per time point. C, LPS dose-response. Results are expressed relative to cells that were only loaded with FA (no LPS). n = 4/dose. D, TLR4 (LPS, 5 ng/ml), TLR1/2 (PAM, 1 μg/ml), and TLR3 (poly(I:C), 15 μg/ml) stimulated TAG retention; interferon-γ (200 units/ml) did not, and IL-4 (10 ng/ml; induces M2 phenotype) allowed TAG loss. Chase period = 72 h. The number of independent experiments is indicated above each bar. Mean + 1 S.D. E, PAM (1 μg/ml) and poly(I:C) (15 μg/ml) stimulated TAG retention in TLR4-deficient macrophages, whereas LPS (5 ng/ml) did not. 72-h chase. Two experiments, each with n = 4. Bar, mean + upper value. F, LPS and poly(I:C) stimulated TAG retention in MyD88−/− cells, whereas PAM did not. n = 3; similar results were observed in a second experiment. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Increased FA Uptake and Incorporation into TAG
TAG retention increased when nonradioactive oleate was added to the medium during the chase (Fig. 3A), suggesting that FA were taken up from the medium and incorporated into TAG, increasing the TAG pool and slowing the loss of radiolabeled TAG. To confirm agonist-induced FA uptake, we loaded macrophages with nonradioactive oleate and palmitate overnight, added 2.5 ng/ml LPS (or PBS) for an additional 20 h, and then measured the uptake of [3H]palmitate over the next 5 h. LPS exposure significantly increased both [3H]palmitate uptake by the cells (1.21 ± 0.07 (S.D.)-fold) and its incorporation into triglyceride (1.32 ± 0.06 (S.D.)-fold) (Fig. 3, B and C); similar results were found for [3H]oleate (data not shown). Because glucose is the major glycerol precursor but does not contribute substantially to de novo fatty acid synthesis (15), we also measured the incorporation of 14C from [14C]glucose into glycerolipids in the presence and absence of LPS. When we incubated washed cells for 10 min in glucose-free medium, then added [14C]glucose for 3 h, recovery of 14C in TAG and DAG was 1.8-fold higher in FA-loaded cells that had been exposed overnight to 2.5 ng/ml LPS than in cells only loaded with FA (Fig. 3D). Twenty hours after it was added to cells, LPS thus stimulated FA uptake, FA incorporation into TAG, and the utilization of glucose for glycerolipid synthesis. LPS stimulation of FA uptake and incorporation into TAG continued for at least 72 h (Fig. 3E); although at this time point [14C]palmitate was principally utilized for phospholipid synthesis, a substantial fraction of both palmitate and oleate was incorporated into TAG, and the LPS present throughout the chase was still stimulatory.
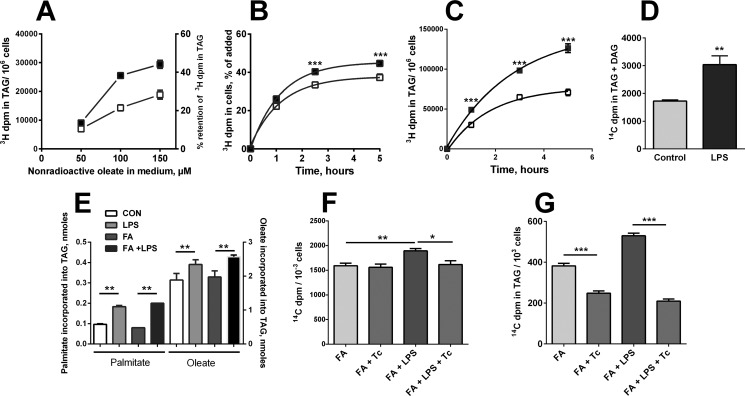
FIGURE 3.
LPS stimulates FA uptake and incorporation into DAG and TAG. A, adding FA during the chase increased the retention of [3H]TAG. Cells were loaded with FA and [3H]oleate overnight, washed, and re-incubated for 96 h in cDMEM containing 25 μm palmitate and 50, 100, or 150 μm oleate. Open box, no LPS; closed box, LPS (5 ng/ml). n = 4, p < 0.0001, two-way analysis of variance. B, LPS stimulates FA uptake and incorporation into TAG. FA-loaded macrophages were incubated with or without LPS (5 ng/ml) for 18 h prior to adding nonradioactive palmitate (20 μm) and 1 μCi/ml [3H]palmitate. 3H dpm were measured in the lipid extract at each time point. n = 4. C, in the same experiments, LPS significantly increased the incorporation of [3H]palmitate into TAG. Symbols (as in A) show mean + 1 S.D. Similar results were obtained when [3H]oleate was used instead of [3H]palmitate (not shown). D, LPS stimulated incorporation of 14C from [14C]glucose into DAG and TAG. Bars show mean and the upper value from two experiments; n = 3–4 in each experiment, p < 0.001 for each comparison of control and LPS-exposed cells. E, after LPS exposure for 72 h, [14C]palmitate and [3H]oleate were still taken up and incorporated into TAG. Radiolabeled FA were added to washed cells in cDMEM containing 20 μm nonradioactive oleate and palmitate (4 nmol each/well). Cells were harvested for analysis 5 h later. F and G, after FA-loaded cells had been exposed to LPS or vehicle for 18 h, Tc (7.5 μm), [14C]palmitate (1 μCi/ml), and 20 μm unlabeled palmitate were added for 4 h. Tc prevented LPS-induced increases in fatty acid uptake (F) and palmitate incorporation into TAG (G). n = 4/group; similar results were obtained for palmitate incorporation into DAG and in two additional experiments. Mean + 1 S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FA utilization requires thioesterification of the FA to CoA, a reaction performed largely by acyl-CoA synthetases (ACSLs). We loaded cells with FA overnight and incubated them with or without LPS for 20 h. We then added [14C]palmitate (in 20 μm nonradioactive palmitate), along with 7.5 μm triacsin C (Tc), an inhibitor of three of the four ACSL isoforms expressed in murine macrophages (10, 16), for 5 h. Triacsin C inhibited [14C]palmitate uptake by LPS-stimulated cells (Fig. 3F) and its incorporation into TAG (Fig. 3G) and DAG (data not shown) by both unstimulated and LPS-exposed cells. LPS-induced ACSL expression (see below) or activation thus plays a role in promoting both FA uptake (possibly by vectorial acylation (17)) and FA esterification to glycerol.
Decreased Lipolysis and FA Oxidation
Lipolytic activity, assayed by measuring the release of [3H]oleate from [3H]triolein by cell lysates, decreased by ∼50% in cells that were exposed to LPS, PAM, or poly(I:C) for 24–48 h (Fig. 4A). FA released from TAG by lipolysis may undergo thioesterification and be (a) transported into mitochondria by carnitine palmitoyltransferase 1 (CPT1) and undergo β-oxidation, releasing CO2 and H2O, (b) re-incorporated into triglyceride (the triglyceride-fatty acid cycle) (4, 18–20), or (c) incorporated into other lipids (11). Nonesterified FA may also leave the cell. To measure the impact of LPS stimulation on lipolysis, we loaded cells overnight with [3H]oleate and then treated them with LPS (10 ng/ml) or not (control) for 22–24 h. The [3H]TAG present in LPS-treated and control cells was then measured to determine base-line TAG retention. We then washed and re-incubated the remaining cells in FA-free medium with or without triacsin C to block thioesterification. [3H]TAG was measured 22–24 h later in cells that were and were not exposed to Tc. Cells that were exposed to LPS prior to and during Tc treatment lost 10 ± 2% (S.D.) less [3H]TAG than did control (no LPS) cells (five determinations in four independent experiments, each with n = 3 or 4/group; includes determinations using 3.3 or 10 μm Tc, which gave similar results). Because blocking thioesterification allows lipolysis while preventing FA incorporation into TAG (11), this result is additional evidence that LPS stimulation decreases lipolysis.
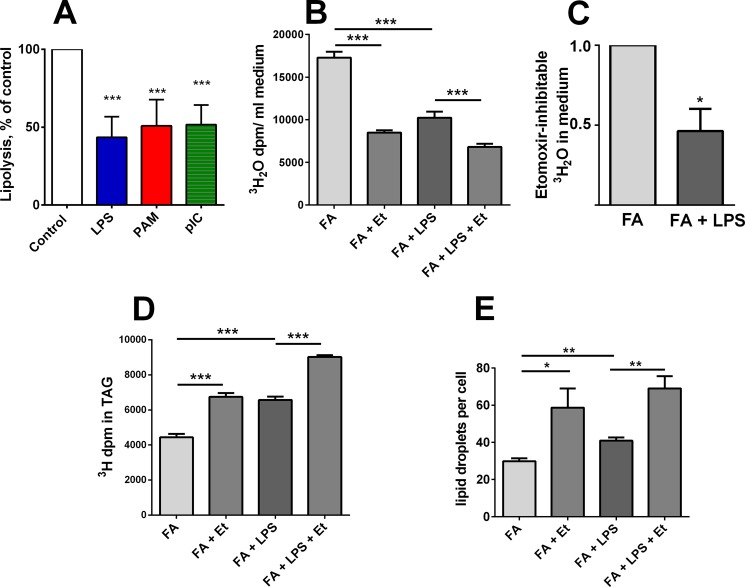
FIGURE 4.
Agonist exposure decreases lipolysis and etomoxir-inhibitable β-oxidation. A, TLR agonists decrease lipolytic activity in cell lysates. Macrophages were incubated for 48 h with or without LPS (5 ng/ml), PAM (1 μg/ml), or poly(I:C) (15 μg/ml), lysed, and assayed to measure their ability to release [3H]oleate from [3H]triolein. Bars show mean + 1 S.E. from three independent experiments, each with n = 3. Each agonist decreased lysate lipolytic activity relative to unstimulated control cells (p < 0.001 for all comparisons with control). B, LPS exposure decreases FA β-oxidation. Cells were loaded overnight with FA containing trace amounts of [3H]oleate and [3H]palmitate. They were then exposed to LPS (5 ng/ml) or media control for 18 h before they were washed; etomoxir (0.2 mm) was added to block mitochondrial FA uptake, and incubation was continued for 18–24 h. FA-loaded, LPS-exposed cells released less 3H2O from retained [3H]oleate and [3H]palmitate than did FA-loaded cells (no LPS). Etomoxir blocked 3H2O accumulation in the presence and absence of LPS. n = 4. C, Etomoxir-inhibitable 3H2O accumulation in the media was significantly less in cells exposed to LPS. Mean + 1 S.D. of three independent experiments. D, etomoxir treatment increased the amount of 3H-fatty acid that was retained in TAG, with and without LPS exposure. Data are representative of three experiments, each with n = 4. E, etomoxir-induced lipid retention was confirmed by counting the lipid droplets in cells stained with Nile Red and DAPI. >200 cells were analyzed per condition in each of four experiments. Et, etomoxir. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To measure FA catabolism, we loaded cells with nonradioactive FA and trace amounts of [3H]oleate and/or [3H]palmitate. After incubation for 18 h in medium with or without LPS, the cells were washed and re-incubated in medium that contained etomoxir to inhibit CPT1. We found that LPS exposure significantly reduced the total amount of 3H2O released into the media (Fig. 4B), and that the “etomoxir-inhibitable 3H2O release” was significantly reduced by LPS exposure (Fig. 4, B and C). This result is consistent with both the observed decrement in lipolytic rate and an LPS-induced decrease in CPT1a mRNA abundance (see below). Etomoxir-treated cells also retained significantly more TAG (Fig. 4D) and lipid droplets/cell (Fig. 4E) than did untreated cells, whether or not they had been exposed to LPS. FA released from TAG may undergo thioesterification and become re-incorporated into TAG; this process may increase when the entry of acyl-CoA into mitochondria is blocked by etomoxir.
The results presented in Figs. 3 and 4 suggest that LPS promotes prolonged TAG retention during the chase period by stimulating FA uptake, thioesterification, and incorporation into TAG, by increasing glucose utilization to produce glycerides, by reducing TAG lipolysis, and possibly by maintaining or enhancing TAG-FA recycling.
Changes in mRNA Abundance
To help identify the molecules that promote TAG retention, we performed microarray analysis (Table 1). Relatively few differences were found between unstimulated (Unstim) and FA-loaded (“FA only”) cells, and the LPS-stimulated changes in mRNA abundance were very similar in cells that had been loaded with FA (“FA + LPS”) and in cells stimulated without FA loading (“LPS”) (Table 1). The differences of greatest relevance to agonist-induced TAG retention were those between (FA) and (FA + LPS) cells; we used Q-PCR to confirm many of these changes at 6, 24, 48, and/or 72 h after exposure to LPS, PAM, or poly(I:C) (Table 1 and Figs. 5, A, B, and C, and 6).
TABLE 1.
Microarray and Q-PCR analysis of mRNA abundance after 24-h chase
Q-PCR analysis used the ΔΔCt method relative to the average of “FA only” replicates (=1.0). The Q-PCR was not performed if the last three columns are empty.
| Microarray analysisa |
Q-PCR analysis (FA+LPS)/FA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unstimulated | FA only | LPS only | FA+LPS | Ratio FA/unstimulated | Ratio (FA+LPS)/LPS | Ratio (FA+LPS)/FA | Mean | S.D. | nb | |
| Fatty acid uptake | ||||||||||
| Acsl1 | 112 | 101 | 621 | 854 | 0.90 | 1.4 | 8.5 | 19.5 | 6.3 | |
| Acsl4 | 156 | 136 | 113 | 141 | 0.87 | 1.2 | 1.0 | 1.3 | 1 | |
| Acsl5 | 66 | 80 | 83 | 69 | 1.20 | 0.8 | 0.9 | 2.2 | 1 | |
| CD36 | 135 | 192 | 63 | 89 | 1.42 | 1.4 | 0.5 | 0.2 | 2 | |
| Slc27a1 (FATP1) | 244 | 259 | 254 | 280 | 1.07 | 1.1 | 1.1 | 0.5 | 0.2 | 4 |
| Slc27a4 (FATP4) | 87 | 91 | 95 | 105 | 1.05 | 1.1 | 1.2 | |||
| Slc27a6 (FATP6) | 91 | 83 | 54 | 54 | 0.91 | 1.0 | 0.7 | |||
| Fabp3 | 243 | 214 | 897 | 773 | 0.88 | 0.9 | 3.6 | 9.5 | 4.2 | 4 |
| Fabp4 (aP2) | 62 | 60 | 50 | 52 | 0.87 | 1.0 | 0.9 | |||
| Fabp5 (mal1) | 505 | 519 | 114 | 151 | 0.87 | 1.3 | 0.3 | |||
| TAG synthesis | ||||||||||
| Dgat1 | 73 | 86 | 72 | 83 | 1.18 | 1.2 | 1.0 | 0.6 | 1 | |
| Dgat2 | 50 | 48 | 60 | 66 | 0.98 | 1.1 | 1.4 | 8.6 | 4.2 | 5 |
| Lpgat1 | 59 | 55 | 53 | 52 | 0.93 | 1.0 | 0.9 | |||
| Glucose uptake | ||||||||||
| Slc2a1 (GLUT1) | 832 | 816 | 1833 | 1847 | 0.87 | 1.0 | 2.3 | |||
| Acylglycerolphosphate synthesis | ||||||||||
| Gyk | 129 | 124 | 98 | 99 | 0.96 | 1.0 | 0.8 | 0.9 | 1 | |
| PEPCK | 61 | 58 | 58 | 57 | 0.94 | 1.0 | 1.0 | |||
| Agpat2 | 283 | 247 | 281 | 256 | 0.87 | 0.9 | 1.0 | |||
| Agpat6 | 990 | 950 | 1272 | 1232 | 0.96 | 1.0 | 1.3 | |||
| Agpat9 (GPAT3) | 60 | 59 | 62 | 67 | 0.99 | 1.1 | 1.1 | |||
| Lipid droplet proteins | ||||||||||
| Plin2 | 851 | 1194 | 267 | 854 | 1.40 | 3.2 | 0.7 | 0.7 | 0.3 | 7 |
| Triglyceride lipolysis | ||||||||||
| Pnpla2 (ATGL) | 1852 | 2257 | 1155 | 1242 | 1.22 | 1.1 | 0.6 | 0.5 | 0.1 | 3 |
| Lipe | 60 | 69 | 67 | 64 | 1.15 | 1.0 | 0.9 | 1.2 | 2 | |
| Mgll | 400 | 432 | 83 | 88 | 1.08 | 1.1 | 0.2 | 0.1 | 0.04 | 3 |
| G0S2 | 63 | 60 | 61 | 59 | 0.95 | 1.0 | 1.0 | |||
| Abhd5 | 333 | 323 | 180 | 232 | 0.97 | 1.3 | 0.7 | 1.0 | 1 | |
| Mitochondrial fatty acid uptake, function | ||||||||||
| Cpt1a | 56 | 58 | 54 | 54 | 1.04 | 1.0 | 0.9 | 0.8 | 0.3 | 3 |
| Ppargc1b | 99 | 113 | 82 | 111 | 1.14 | 1.4 | 1.0 | 1.2 | 1 | |
| Regulatory proteins | ||||||||||
| Hif1a | 105 | 102 | 114 | 140 | 0.97 | 1.2 | 1.4 | 1.85 | 0.21 | 5 |
| Sirt1 | 149 | 148 | 119 | 118 | 1.00 | 1.0 | 0.8 | 0.6 | 2 | |
| Pparg | 812 | 731 | 87 | 106 | 0.90 | 1.2 | 0.1 | 0.1 | 0.1 | 3 |
| Ampk (Prkaa1) | 60 | 60 | 64 | 60 | 1.00 | 0.9 | 1 | |||
| Nfe2l2 | 404 | 339 | 181 | 169 | 0.84 | 0.9 | 0.5 | 0.5 | 1 | |
a Means are of four independent experiments. LPS = 10 ng/ml. Cells were studied 48 h after starting FA load and 24 h after adding LPS.
b n = number of independent experiments, each with 3 replicates/condition.
FIGURE 5.
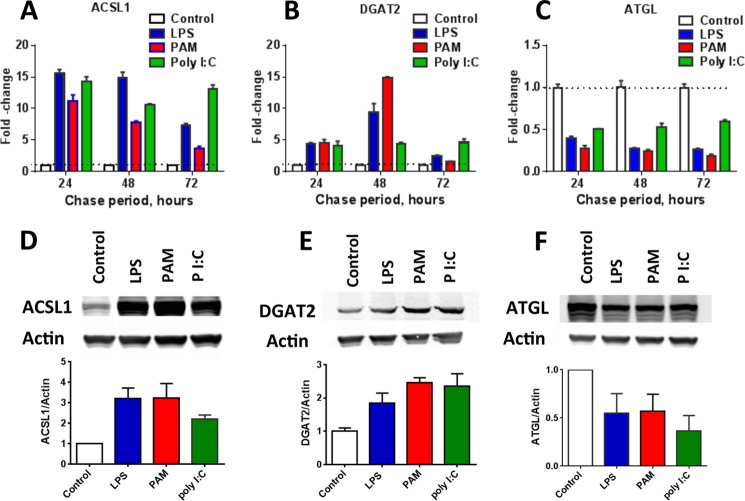
TLR agonists alter mRNA transcript and protein abundance. A–C, time course of mRNA transcript abundance, determined using Q-PCR, in FA-loaded cells that were stimulated with LPS (5 ng/ml), PAM (1 μg/ml), or poly(I:C) (15 μg/ml). Bars show mean + 1 S.E. for determinations from three independent experiments, each with n = 2 or 3. Agonist-induced changes persisted for 72 h. D–F, Western blots performed after 72 h chase showing LPS-induced increases in ACSL1 (D) and DGAT2 (E) and decreases in ATGL (F). Protein quantitation is shown below each Western blot. Bars show mean +1 S.D. from three or more analyses. In each panel, the changes shown for each agonist were different from control (FA only, no agonist) at each time point (A–C) and in each Western quantitation (D–F) (p ≤ 0.05).
FIGURE 6.
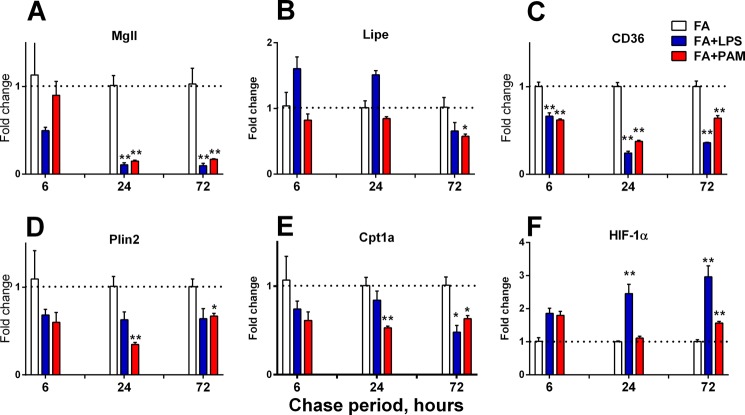
Time course of changes in mRNA abundance for selected murine genes. mRNA abundance was measured using Q-PCR. The results obtained for cells stimulated with LPS (FA+LPS) (blue) or PAM (FA+PAM) (red) are expressed with respect to the results obtained in each assay from cells that were loaded with FA and not exposed to LPS (FA) (white). Each bar represents the mean + 1 S.E. of at least three determinations per time point/condition. Shown are genes that contribute to lipolysis (Mgll, Lipe) (A and B), fatty acid uptake (CD36, C), lipid droplet formation (Plin2, D), mitochondrial acyl-CoA uptake (Cpt1a, E), and metabolic regulation (Hif-1α, F). Bars indicate mean + 1 S.E. *, p < 0.05; **, p < 0.01.
FA Uptake, FA Thioesterification, and TAG Synthesis
Cells that had been exposed to LPS for 24 h after FA loading had 10–15-fold higher levels of long chain acyl-CoA synthetase 1 (ACSL1) mRNA than did cells that were only loaded with FA (Fig. 5A and Table 1). PAM and poly(I:C) also increased ACSL1 mRNA abundance for 24–72 h by >3-fold (Fig. 5A). When quantitated using Western blotting, ACSL1 protein abundance increased ∼3-fold in LPS- or PAM-stimulated cells and 2-fold in cells treated with poly(I:C) (Fig. 5D). Elevated levels of diacylglycerol acyltransferase 2 (DGAT2) were also maintained for 72 h in cells exposed to each of the TLR agonists (Fig. 5, B and E).
Lipolysis
Cells exposed to each of the agonists had significantly lower levels of mRNAs for ATGL (Pnpla2) (Fig. 5C; Table 1). Decreased ATGL abundance after a 72-h chase was confirmed using Western blotting (Fig. 5F). There was also a significant drop in Mgll (monoacylglycerol lipase) mRNA abundance but not in Lipe (hormone-sensitive lipase) (Fig. 6, A and B).
We also looked for time-dependent, agonist-induced changes in mRNA abundance for other genes related to FA and TAG metabolism. LPS stimulated a significant decrease in mRNA abundance for CD36 (Fig. 6C), a gene previously associated with LPS-induced FA uptake by a macrophage cell line (6). There were 4–10-fold higher levels of fatty acid-binding protein 3 (FABP3) mRNA (Table 1). Perilipin-2 (adipophilin or Plin2, lipid droplet protein) mRNA decreased (Fig. 6D). Carnitine palmitoyltransferase-1a (Cpt1a) mRNA was lower at 24–72 h (Fig. 6E), whereas mRNA for Hif-1α, an important metabolic regulator, was increased as long as 72 h after LPS or PAM exposure (Fig. 6F). Changes in mRNA abundance for other genes are shown in Table 1.
The analysis of mRNA and protein abundance was consistent with the biochemical findings. Increases in ACSL1 may increase thioesterification, FA uptake, FA incorporation into TAG, and TAG-FA recycling. DGAT2 carries out the final step in TAG synthesis. Decreases in ATGL and Mgll are consistent with the observed reduction in lipolytic rate. Both decreased lipolysis and reduced CPT1 abundance may contribute to the lower rate of β-oxidation. All of these changes persisted for ≥72 h.
Prolonged TAG Retention in Human Monocyte-Macrophages
We also studied elutriated human monocytes after they had differentiated into macrophages in vitro. In general, the findings were similar to those described above. Although LPS-stimulated human macrophages did not show a significant increase in the percentage of cells that had LD, a 2-fold increase in LD per cell was observed after 48 h of LPS exposure (Fig. 7A). LPS exposure increased TAG retention (Fig. 7, B and C) in human macrophages, as did PAM (Fig. 7D). Poly(I:C) did not stimulate TAG retention by human monocyte-macrophages, yet it reproducibly augmented the ability of PAM to do so (Fig. 7D), suggesting cooperation between the MyD88 and TRIF pathways in these cells. TLR4 activation increased FA uptake and FA incorporation into TAG (Fig. 7, E and F). LPS also stimulated significant increases in ACSL1 and DGAT2 mRNA abundance (data not shown). The degree to which LPS and PAM stimulate TAG retention was lower in human monocyte-macrophages than in murine macrophages, however, there was no change in ATGL mRNA abundance (data not shown), and TAG retention was increased for a shorter time.
FIGURE 7.
TLR-induced TAG retention in human monocyte-derived macrophages. A, LPS induces human macrophages to retain LD. After incubation overnight in medium containing radiolabeled FA, the cells were washed and chased for 48 h in medium that contained LPS (10 ng/ml) or no LPS. LD were analyzed as described under “Experimental Procedures” and the legend to Fig. 1. Data averaged from three donors. Bars indicate mean + 1 S.D. B, relative increase in TAG retention in LPS-stimulated cells over time (unstimulated cells = 1). 10 ng/ml LPS was added after overnight FA loading. Mean ± 1 S.E. for three different donors, each studied in quadruplicate. C, FA-loaded macrophages were exposed to the indicated concentrations of LPS for 48 h. D, [3H]FA remaining in TAG 48 h after adding LPS (10 ng/ml), PAM (1 μg/ml), poly(I:C) (5 μg/ml). Fold increase above FA-loaded cells is shown (no stimulus). Mean + 1 S.D. of six experiments using macrophages from different donors, each with n = 4. E and F, uptake (E) and incorporation into TAG (F) of [14C]oleate by cells that were loaded with FA 48 h earlier and stimulated with LPS (or not) for 24 h prior to study. Data are from four donors, each with n = 4 determinations/time point. Bars indicate mean + 1 S.D. *, p < 0.05; **, p < 0.01.
DISCUSSION
We used TEPM for these experiments because they are recently recruited (21) cells that can live for several days in culture. In addition, many of the foundational studies on macrophage metabolism were performed using TEPM (3, 15, 22, 23), which undergo minimal cell division (24) and are thus suitable for studying the behavior of differentiated macrophages over several days ex vivo.
To our knowledge, these are the first experiments to show that TLR agonist-induced TAG storage can persist for several days. In summary, we found that TAG retention in TEPM was: 1) enhanced by sensing TLR agonists via either the MyD88-dependent or the MyD88-independent (TRIF) signaling pathway; 2) greatly enhanced when FA were present in the medium; 3) promoted by agonist-induced changes in FA uptake, glucose-derived glyceride synthesis, TAG synthesis, lipolysis, and possibly FA recycling; 4) accompanied by changes in the abundance of proteins that carry out TAG synthesis or lipolysis; and 5) long lasting (at least 72–96 h).
Much of the previous evidence that TLR agonists increase FA accumulation by macrophages was found using immortalized cell lines (RAW 264.7 and J774) (5, 6, 25, 26), and the results we obtained using TEPM differed from these findings in several ways. In agreement with others (6, 24), for example, we did not detect a significant increase in mRNA abundance in TEPM for CD36, the FA transporter that increases dramatically in LPS-stimulated murine macrophage cell lines. The impressive increase in GPAT3 (agpat-9) noted by Feingold et al. (6) in RAW 264.7 cells was not observed here. These workers also found LPS-induced inhibition of TAG lipolysis in RAW 264.7 cells without noticing a decrement in ATGL mRNA expression (6), whereas we found agonist-induced decreases in ATGL mRNA and protein expression that lasted at least 72 h. TLR agonists induced aP2 (FABP4) expression in RAW 264.7 cells (26) but not in the primary macrophages studied here. Another study found TLR agonist-induced increases in mRNA and protein levels of adipophilin (Plin2) and Mal1 (fatty acid-binding protein, FABP5) in both RAW 264.7 and J774 murine macrophages (25). We did not find these changes in TEPM when we studied them 20 h or more after initiating LPS exposure (Table 1). Finally, we found that poly(I:C) induced significant increases in DGAT2 mRNA and protein in TEPM; this was not seen in RAW 264.7 cells (6). The extent to which these discrepancies reflect the differences between dividing (transformed) and nondividing cells is uncertain; we focused here on longer term changes whereas the studies of cell lines addressed acute (≤24 h) events; and the LPS concentrations used to stimulate the cell lines were also often much higher (≥100 ng/ml) than those we used (2.5–10 ng/ml). Substantial differences between RAW 264.7 cells and TEPM in other aspects of LPS-stimulated lipid metabolism were recently reported (24).
Several different mechanisms combined to prolong TAG retention. First, agonist-exposed macrophages maintained increased FA uptake and FA incorporation into TAG for at least 72 h. A role for ACSL1 in these phenomena was suggested by prior evidence that ACSL1 is both the major ACSL1 isoform in macrophages and the ACSL isoform most closely linked to TAG synthesis (27). In addition, basal levels of 16:0-CoA and 18:1-CoA were reduced in Acsl1−/− macrophages (14), and ACSL1-deficient macrophages incorporated significantly less 14C-18:1 into TAG when compared with wild type controls (28). Here, we found the following: 1) LPS, PAM, and poly(I:C) all stimulated significant elevations in ACSL1 mRNA and protein (Fig. 5, A and D), and 2) triacsin C inhibited LPS-induced increases in FA uptake and FA incorporation into DAG and TAG (Fig. 3, F and G) (29). These findings suggest a prominent role for ACSL1 in TAG retention. High levels of ACSL1 protein have been found in foamy macrophages within caseous granulomas, the hallmark lesion elicited by Mycobacterium tuberculosis (2); the most potent mycobacterial molecule for promoting lipid accumulation is trehalose dimycolate, which stimulates macrophages via TLR2 (2, 30). Poly(I:C) induced large increases in ACSL1 mRNA, confirming an important role for the TRIF pathway in ACSL1 regulation (14), yet its ability to prolong TAG retention was weaker than that of LPS or PAM (Fig. 2D).
In addition, cells exposed to LPS, PAM, or poly(I:C) had significant and sustained increases in DGAT2 mRNA and protein abundance (Fig. 5, B and E) that accompanied significant increases in FA incorporation into TAG, and blocking mitochondrial uptake of acyl-CoA increased LD number/cell and TAG retention, suggesting increased FA recycling. Finally, all of the agonists induced significant reductions in lipolytic activity (Fig. 4A) (6), and in ATGL, the lipase that cleaves the initial FA from TAG (Fig. 5, C and F) and LPS decreased lipolysis in cells during the chase period.
In contrast to the acute inflammatory response program seen in LPS-stimulated macrophages (14, 31, 32), TAG retention is thus a prolonged response to TLR sensing. TAG retention was relative, however; TLR-activated cells stored FA in TAG significantly longer than did unstimulated cells, but FA were gradually lost from TAG whether or not the cells were exposed to TLR agonists (Fig. 2A). Adding FA to the medium greatly enhanced the impact of TLR4 activation on TAG retention (Fig. 3A); when FA are continuously present in the cells' environment, as occurs in vivo, the impact of TLR agonists on TAG storage may be apparent much longer than the 72–96-h time period studied here.
TLR1/2 and TLR4 agonists stimulate lipid body formation in the presence and absence of lipoprotein supplementation (33). The ability of TLR agonists to prolong TAG retention, in addition to augmenting the uptake of oxidized LDL (and promoting desmosterol synthesis (34)), suggests that both phenomena may contribute to the formation of macrophage foam cells in vivo.
Acknowledgments
We thank Diane Adler-Wailes, NICHD, National Institutes of Health, for much helpful advice, and Francisco Otaizo-Carrasquero, Genomic Technologies Section, NIAID, National Institutes of Health, for microarray hybridizations.
This work was supported, in whole or in part, by National Institutes of Health, Division of Intramural Research, NIAID.
- LD
- lipid droplet
- TLR
- toll-like receptor
- TAG
- triglyceride
- DAG
- diacylglyceride
- FA
- fatty acid
- PAM
- PAM3CysK4
- ACSL
- acyl-CoA synthetase-long
- DGAT2
- diacylglycerol acyltransferase 2
- ATGL
- adipose triglyceride lipase
- Tc
- triacsin C
- QPCR
- quantitative PCR
- TEPM
- thioglycollate-elicited peritoneal macrophages
- TRIF
- Toll-like receptor adaptor molecule
- RANTES
- regulated on activation, normally T cell expressed and secreted, Ccl5.
REFERENCES
- 1. Bozza P. T., Magalhães K. G., Weller P. F. (2009) Leukocyte lipid bodies–Biogenesis and functions in inflammation. Biochim. Biophys. Acta 1791, 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim M. J., Wainwright H. C., Locketz M., Bekker L. G., Walther G. B., Dittrich C., Visser A., Wang W., Hsu F. F., Wiehart U., Tsenova L., Kaplan G., Russell D. G. (2010) Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol. Med. 2, 258–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newsholme P., Curi R., Gordon S., Newsholme E. A. (1986) Metabolism of glucose, glutamine, long chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 239, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newsholme E. A., Crabtree B. (1976) Substrate cycles in metabolic regulation and in heat generation. Biochem. Soc. Symp. 41, 61–109 [PubMed] [Google Scholar]
- 5. Funk J. L., Feingold K. R., Moser A. H., Grunfeld C. (1993) Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis 98, 67–82 [DOI] [PubMed] [Google Scholar]
- 6. Feingold K. R., Shigenaga J. K., Kazemi M. R., McDonald C. M., Patzek S. M., Cross A. S., Moser A., Grunfeld C. (2012) Mechanisms of triglyceride accumulation in activated macrophages. J. Leukocyte Biol. 92, 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munford R. S., DeVeaux L. C., Cronan J. E., Jr., Rick P. D. (1992) Biosynthetic radiolabeling of bacterial lipopolysaccharide to high specific activity. J. Immunol. Methods 148, 115–120 [DOI] [PubMed] [Google Scholar]
- 8. Lu M., Zhang M., Kitchens R. L., Fosmire S., Takashima A., Munford R. S. (2003) Stimulus-dependent deacylation of bacterial lipopolysaccharide by dendritic cells. J. Exp. Med. 197, 1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tansey J. T., Huml A. M., Vogt R., Davis K. E., Jones J. M., Fraser K. A., Brasaemle D. L., Kimmel A. R., Londos C. (2003) Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J. Biol. Chem. 278, 8401–8406 [DOI] [PubMed] [Google Scholar]
- 10. Saraswathi V., Hasty A. H. (2009) Inhibition of long chain acyl coenzyme a synthetases during fatty acid loading induces lipotoxicity in macrophages. Arterioscler. Thromb. Vasc. Biol. 29, 1937–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Igal R. A., Wang P., Coleman R. A. (1997) Triacsin C blocks de novo synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholipid: evidence for functionally separate pools of acyl-CoA. Biochem. J. 324, 529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deberardinis R. J., Lum J. J., Thompson C. B. (2006) Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J. Biol. Chem. 281, 37372–37380 [DOI] [PubMed] [Google Scholar]
- 13. Krawczyk C. M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R. J., Cross J. R., Jung E., Thompson C. B., Jones R. G., Pearce E. J. (2010) Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubinow K. B., Wall V. Z., Nelson J., Mar D., Bomsztyk K., Askari B., Lai M. A., Smith K. D., Han M. S., Vivekanandan-Giri A., Pennathur S., Albert C. J., Ford D. A., Davis R. J., Bornfeldt K. E. (2013) Acyl-CoA synthetase 1 is induced by Gram-negative bacteria and lipopolysaccharide and is required for phospholipid turnover in stimulated macrophages. J. Biol. Chem. 288, 9957–9970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newsholme P., Newsholme E. A. (1989) Rates of utilization of glucose, glutamine and oleate and formation of end-products by mouse peritoneal macrophages in culture. Biochem. J. 261, 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soupene E., Kuypers F. A. (2008) Mammalian long chain acyl-CoA synthetases. Exp. Biol. Med. 233, 507–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arias-Barrau E., Dirusso C. C., Black P. N. (2009) Methods to monitor fatty acid transport proceeding through vectorial acylation. Methods Mol. Biol. 580, 233–249 [DOI] [PubMed] [Google Scholar]
- 18. Nye C., Kim J., Kalhan S. C., Hanson R. W. (2008) Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol. Metab. 19, 356–361 [DOI] [PubMed] [Google Scholar]
- 19. Prentki M., Madiraju S. R. (2008) Glycerolipid metabolism and signaling in health and disease. Endocr. Rev. 29, 647–676 [DOI] [PubMed] [Google Scholar]
- 20. Adler-Wailes D. C., Guiney E. L., Wolins N. E., Yanovski J. A. (2010) Long term ritonavir exposure increases fatty acid and glycerol recycling in 3T3-L1 adipocytes as compensatory mechanisms for increased triacylglycerol hydrolysis. Endocrinology 151, 2097–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghosn E. E., Cassado A. A., Govoni G. R., Fukuhara T., Yang Y., Monack D. M., Bortoluci K. R., Almeida S. R., Herzenberg L. A., Herzenberg L. A. (2010) Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl. Acad. Sci. U.S.A. 107, 2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Newsholme P., Gordon S., Newsholme E. A. (1987) Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem. J. 242, 631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loike J. D., Kaback E., Silverstein S. C., Steinberg T. H. (1993) Lactate transport in macrophages. J. Immunol. 150, 1951–1958 [PubMed] [Google Scholar]
- 24. Maurya M. R., Gupta S., Li X., Fahy E., Dinasarapu A. R., Sud M., Brown H. A., Glass C. K., Murphy R. C., Russell D. W., Dennis E. A., Subramaniam S. (2013) Analysis of inflammatory and lipid metabolic networks across RAW264.7 and thioglycolate-elicited macrophages. J. Lipid Res. 54, 2525–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feingold K. R., Kazemi M. R., Magra A. L., McDonald C. M., Chui L. G., Shigenaga J. K., Patzek S. M., Chan Z. W., Londos C., Grunfeld C. (2010) ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis 209, 81–88 [DOI] [PubMed] [Google Scholar]
- 26. Kazemi M. R., McDonald C. M., Shigenaga J. K., Grunfeld C., Feingold K. R. (2005) Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler. Thromb. Vasc. Biol. 25, 1220–1224 [DOI] [PubMed] [Google Scholar]
- 27. Li L. O., Klett E. L., Coleman R. A. (2010) Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta 1801, 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanter J. E., Kramer F., Barnhart S., Averill M. M., Vivekanandan-Giri A., Vickery T., Li L. O., Becker L., Yuan W., Chait A., Braun K. R., Potter-Perigo S., Sanda S., Wight T. N., Pennathur S., Serhan C. N., Heinecke J. W., Coleman R. A., Bornfeldt K. E. (2012) Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc. Natl. Acad. Sci. U.S.A. 109, E715–E724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Namatame I., Tomoda H., Arai H., Inoue K., Omura S. (1999) Complete inhibition of mouse macrophage-derived foam cell formation by triacsin C. J. Biochem. 125, 319–327 [DOI] [PubMed] [Google Scholar]
- 30. D'Avila H., Melo R. C., Parreira G. G., Werneck-Barroso E., Castro-Faria-Neto H. C., Bozza P. T. (2006) Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 176, 3087–3097 [DOI] [PubMed] [Google Scholar]
- 31. Gilchrist M., Thorsson V., Li B., Rust A. G., Korb M., Roach J. C., Kennedy K., Hai T., Bolouri H., Aderem A. (2006) Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441, 173–178 [DOI] [PubMed] [Google Scholar]
- 32. Nilsson R., Bajic V. B., Suzuki H., di Bernardo D., Björkegren J., Katayama S., Reid J. F., Sweet M. J., Gariboldi M., Carninci P., Hayashizaki Y., Hume D. A., Tegner J., Ravasi T. (2006) Transcriptional network dynamics in macrophage activation. Genomics 88, 133–142 [DOI] [PubMed] [Google Scholar]
- 33. Nicolaou G., Goodall A. H., Erridge C. (2012) Diverse bacteria promote macrophage foam cell formation via toll-like receptor-dependent lipid body biosynthesis. J. Atheroscler. Thromb. 19, 137–148 [DOI] [PubMed] [Google Scholar]
- 34. Spann N. J., Garmire L. X., McDonald J. G., Myers D. S., Milne S. B., Shibata N., Reichart D., Fox J. N., Shaked I., Heudobler D., Raetz C. R., Wang E. W., Kelly S. L., Sullards M. C., Murphy R. C., Merrill A. H., Jr., Brown H. A., Dennis E. A., Li A. C., Ley K., Tsimikas S., Fahy E., Subramaniam S., Quehenberger O., Russell D. W., Glass C. K. (2012) Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 [DOI] [PMC free article] [PubMed] [Google Scholar]