Abstract
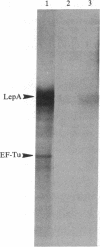
The amino acid sequence of LepA protein, which has been shown to be cotranscribed with signal peptidase I in Escherichia coli, was compared with greater than 2000 known protein sequences. It was revealed that, of the 598 amino acid residues contained in LepA, an amino-terminal domain of 112 residues is homologous to a domain of similar size found in initiation factor 2, elongation factor Tu, and elongation factor G (IF2, EF-Tu, and EF-G), factors required for translation in E. coli. In this domain, 46 and 34 residues align perfectly with the corresponding regions of EF-G and EF-Tu, respectively. If functionally conserved residues within this domain (19 for EF-G and 17 for EF-Tu) are included, the overall resemblance is 58% and 46%, respectively, for EF-G and EF-Tu. A similar domain exists internally in IF2, where there is 42% overall resemblance with the domain of LepA. Immediately adjacent to this region is a small sequence of limited similarity that exists not only in EF-G, EF-Tu, and IF2 but also in the protooncogene c-Ha-ras-1 (from human bladder) and other GTP-binding proteins. Given these homologies, GTP-photoaffinity labeling and subcellular fractionation experiments were undertaken, and it was found that LepA is indeed a membrane-bound GTP-binding protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Date T. Demonstration by a novel genetic technique that leader peptidase is an essential enzyme of Escherichia coli. J Bacteriol. 1983 Apr;154(1):76–83. doi: 10.1128/jb.154.1.76-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Wickner W. Isolation of the Escherichia coli leader peptidase gene and effects of leader peptidase overproduction in vivo. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6106–6110. doi: 10.1073/pnas.78.10.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Duisterwinkel F. J., Kraal B., De Graaf J. M., Talens A., Bosch L., Swart G. W., Parmeggiani A., La Cour T. F., Nyborg J., Clark B. F. Specific alterations of the EF-Tu polypeptide chain considered in the light of its three-dimensional structure. EMBO J. 1984 Jan;3(1):113–120. doi: 10.1002/j.1460-2075.1984.tb01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoyye S., Takeishi K., Lee N., DeMartini M., Hirashima A., Inouye M. Lipoprotein from the outer membrane of Escherichia coli: purification, paracrystallization, and some properties of its free form. J Bacteriol. 1976 Jul;127(1):555–563. doi: 10.1128/jb.127.1.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Czakó-Graham M., Grebenau R., Mok W., Rodriguez-Boulan E., Sabatini D. D. Characterization of the ribosomal binding site in rat liver rough microsomes: ribophorins I and II, two integral membrane proteins related to ribosome binding. J Supramol Struct. 1978;8(3):279–302. doi: 10.1002/jss.400080307. [DOI] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Stringer E. A., Chaudhuri A. Initiation factors in protein biosynthesis. Annu Rev Biochem. 1982;51:869–900. doi: 10.1146/annurev.bi.51.070182.004253. [DOI] [PubMed] [Google Scholar]
- March P. E., Inouye M. Characterization of the lep operon of Escherichia coli. Identification of the promoter and the gene upstream of the signal peptidase I gene. J Biol Chem. 1985 Jun 25;260(12):7206–7213. [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Hachmann J., Weissbach H. The reactions of the sulfhydryl groups on the elongation factors Tu and Ts. Arch Biochem Biophys. 1971 May;144(1):115–121. doi: 10.1016/0003-9861(71)90460-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Masui Y., Inouye M. Use of a lac promoter-operator fragment as a transcriptional control switch for expression of the constitutive lpp gene in Escherichia coli. J Mol Appl Genet. 1982;1(4):289–299. [PubMed] [Google Scholar]
- Papageorge A., Lowy D., Scolnick E. M. Comparative biochemical properties of p21 ras molecules coded for by viral and cellular ras genes. J Virol. 1982 Nov;44(2):509–519. doi: 10.1128/jvi.44.2.509-519.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdot C., Dessen P., Hershey J. W., Plumbridge J. A., Grunberg-Manago M. Sequence of the initiation factor IF2 gene: unusual protein features and homologies with elongation factors. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7787–7791. doi: 10.1073/pnas.81.24.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P., Wickner W. Genetic mapping of the Escherichia coli leader (signal) peptidase gene (lep): a new approach for determining the map position of a cloned gene. J Bacteriol. 1983 May;154(2):569–572. doi: 10.1128/jb.154.2.569-572.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., Robinson P. S., Scolnick E. M. Photoaffinity labeling with GTP of viral p21 ras protein expressed in Escherichia coli. J Virol. 1984 May;50(2):343–351. doi: 10.1128/jvi.50.2.343-351.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M., Laursen R. A., Miller D. L. Amino acid sequence of elongation factor Tu. Sequence of a region containing the thiol group essential for GTP binding. FEBS Lett. 1975 Apr 15;53(1):37–39. doi: 10.1016/0014-5793(75)80676-4. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Archer R. H., Lindahl L. The nucleotide sequence of the Escherichia coli fus gene, coding for elongation factor G. Nucleic Acids Res. 1984 Feb 24;12(4):2181–2192. doi: 10.1093/nar/12.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]