Abstract
Progenitor cells for the endothelial lineage have been widely investigated for more than a decade, but continue to be controversial since no unique identifying marker has yet been identified. This review will begin with a discussion of the basic tenets originally proposed for proof that a cell displays properties of an endothelial progenitor cell. We then provide an overview of the methods for putative endothelial progenitor cell derivation, expansion, and enumeration. This discussion includes consideration of cells that are present in the circulation as well as cells resident in the vascular endothelial intima. Finally, we provide some suggested changes in nomenclature that would greatly clarify and demystify the cellular elements involved in vascular repair.
Introduction
If one had to point to a single paper that established the field of endothelial progenitor cell (EPC) biology, the 1997 paper by Asahara et al. (6) would have to be the sentinel choice. In this work, the authors reasoned that since angioblasts and hematopoietic cells emerged near simultaneously in extra-embryonic blood islands during mouse development and shared expression of many cell surface antigens, these lineages may have been derived from a common precursor. They also inferred that the ability of circulating hematopoietic stem cells (HSC) to reconstitute the hematopoietic system of recipient mice as evidence that some circulating stem cells were present in the systemic bloodstream. Thus, the authors sought to determine whether circulating adult human peripheral blood cells could differentiate into endothelial cells. Magnetic beads were used to isolate cells expressing CD34 and/or Flk-1 (vascular endothelial growth factor receptor-2) and purity of the isolated fractions were noted to be 15.7% and 20%, respectively. Overall, a limited number of CD34+ cells attached, became spindle shaped, and proliferated over 4 weeks of in vitro culture. When the CD34+ cells were labeled with a fluorescent dye (DiI) and then co-cultured with CD34− peripheral blood cells, a 10-fold increase in cell proliferation was observed compared to the plated CD34+ cells alone. Furthermore, the CD34+ cells in co-culture with CD34− cells for only 12 hours formed numerous cell clusters. After 5 days of co-culture the CD34+ cells began to ingest acetylated low density lipoprotein (ac-LDL) and emerged as spindle shaped cells from the base of the cell clusters (whereas the round cells atop the clusters failed to ingest ac-LDL). Since the spindle shaped CD34+ derived cells also displayed less CD45, but greater amounts of CD31, CD34, Flk-1, Tie-2, and E-selectin than the freshly isolated CD34+ cells and were stimulated to express nitric oxide in response to acetylcholine and vascular endothelial growth factor (VEGF) administration, the authors postulated that the peripheral blood cells had become endothelial cell-like during the culture. When 500,000 freshly isolated human peripheral blood CD34+ cells (co-labeled with DiI) were injected intravenously into athymic mice that had undergone unilateral femoral artery excision, numerous DiI-labeled cells were detected 1–6 weeks in the injured limb but not the contralateral healthy limb. DiI-labeled cells were detected in 13.7% of the host capillaries in the injured limb and were determined to co-express CD31, Tie-2, and the receptor for Ulex europaeus agglutinin-1 lectin (UEA-1). As a secondary proof of concept, Flk-1+ cells were isolated from the whole blood of 10 donor transgenic mice expressing β-galactosidase and injected into host syngeneic mice following unilateral femoral artery excision. Some β-galactosidase expressing cells were identified in the ischemic limbs post-injection in close apposition with host capillaries and small arteries that co-stained with CD31 and Bandeiraea simplicifolia-1 lectin (BS-1). These results were interpreted as evidence that the circulating human CD34+ cells and murine Flk-1+ cells were capable of contributing to vascular structures in ischemic limbs of host mice in vivo. In sum, this seminal paper proposed that some circulating cells are capable of serving as progenitors of the endothelial lineage both in vitro and in vivo. The fact that the circulating cells could contribute to vascular regeneration in vivo suggested these cells were undergoing postnatal vasculogenic responses.
Key elements selected from this seminal paper (6) that subsequently became foundational and/or controversial concepts of features that define a circulating EPC included: 1) human and murine blood contains circulating EPC, 2) human and murine putative EPC displayed different markers; human EPC were CD34+, Tie-2+, CD31+, UEA-1+, ac-LDL+, and some expressed CD45 while murine EPC were Flk-1+, BS-1+, and CD31+, 3) circulating putative EPC integrated into regenerating host vessels in an area of injury (but not in uninjured sites), 4) putative human EPC displayed low proliferative potential that was augmented by other non-EPC peripheral blood cells, 5) putative human EPC attached to non-EPC to form cord-like structures in vitro, 6) putative EPC formed clusters when co-cultured in vitro with non-EPC, and 7) circulating putative EPC contributed via postnatal vasculogenesis to vascular repair and tissue regeneration. Given this introduction to some of these foundational concepts, we will examine the methods that are currently used to identify and define putative EPC.
Methods to define human EPCs
Putative human EPCs have been identified using several approaches. Human peripheral blood cell mononuclear cells can be plated into culture plates coated with fibronectin and are commonly grown in a commercial medium (Endothelial growth medium 2 [EGM2], Lonza) with addition of varying growth factors that include vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF2), insulin-like growth factor 1 (IGF1), and epidermal growth factor (EGF) (34, 42). The adherent cells that persist for 5–7 days and display the capacity to take up ac-LDL and bind UEA-1 have been defined as EPCs, based in large part on the original observations of Asahara et al. (6). However, circulating platelets commonly contaminate the plated mononuclear cells and may release membrane particles that bind to the adherent putative EPC (67). Since the platelet membranes and vascular endothelial cells share expression of many cell surface proteins, it is not uncommon for putative EPC that are not actively transcribing these proteins to display the platelet-endothelial protein markers on their cell surface. Thus, this assay for adherent putative EPCs cannot be considered valid (too many false positive cells) unless contaminating platelets can be completely eradicated from the starting cell material (67). Furthermore, peripheral blood monocytes are known to be isolated using attachment to fibronectin coated dishes and may therefore also contaminate the putative EPC subset (32). Since monocytes cultured with VEGF express numerous endothelial-like cell surface proteins (VEGF receptors, CD144, CD31, and von Willebrand factor(vWF), upregulate endothelial nitric oxide synthase (eNOS), and take up ac-LDL, these cells display all of the in vitro features of the putative EPC (74, 75). Indeed, EPCs isolated by the methods described above have been reported to more closely resemble VEGF treated monocytes than cultured endothelial cells when examined at a whole transcriptome level (1, 22, 59). Nonetheless, the cultured adherent cells isolated by this method demonstrate a variety of angiogenic promoting properties in vitro and in vivo and thus, may be more accurately defined as circulating angiogenic cells (CACs) (69, 72). Despite these clear published limitations and deficiencies, this now “unreliable” assay continues to be used to “identify” putative EPC (Fig. 1).
Figure 1. Comparison of the phenotypic and functional characteristics of putative EPC determined by different assays.
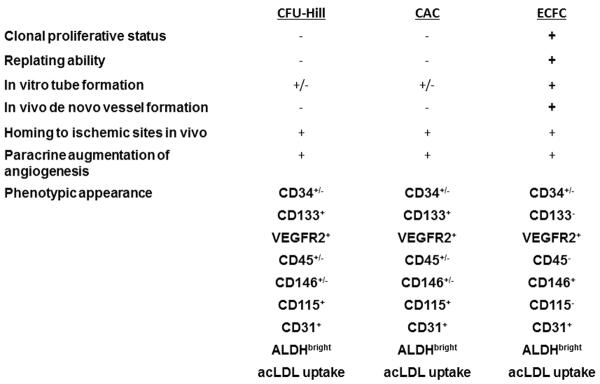
The cells isolated by the CFU-Hill and CAC assays identify essentially similar proangiogenic hematopoietic subsets. In contrast the cells isolated in the ECFC assay differ from the proangiogenic cells by displaying clonal proliferative potential, replating ability, and in vivo vessel forming ability. In addition the phenotype of the ECFC can be distinguished from the proangiogenic cells by the lack of CD133, CD45, and CD115 expression by the ECFC. Abbreviations: ALDH: aldehyde dehydrogenase, acLDL: acetylated low density lipoprotein, VEGFR2: vascular endothelial growth factor receptor 2.
A second method used to define putative EPCs relies upon fluorochrome-labeled antibodies and fluorescence activated cell (FACS) analysis to discriminate peripheral blood subsets. Human peripheral blood cells expressing CD34, AC133, and KDR have been proposed as a fundamental EPC phenotype (6, 66), but, none of these proteins is specifically restricted in expression to the EPCs and all are expressed by hematopoietic stem and progenitor cells (2, 13). Other antigens (CD105, CD144, CD106, CD117, and CD45) and some enzymatic activities (aldehyde dehydrogenase) have also been proposed as EPC markers (Fig. 1), but none discriminate the EPCs from circulating blood cells (27, 35). Thus, in the absence of unique antigens to identify the EPC, one must consider that cells previously identified as EPC actually represent circulating hematopoietic cells. Since a variety of hematopoietic stem and progenitor cell subsets have recently been reported to display pro-angiogenic activities (89) it is quite possible these cells play the role of the circulating cell subsets with vascular reparative activities previously attributed to EPC (27, 51, 64, 84).
Finally, two colony forming assays have been used to identify putative EPCs. Peripheral blood cells are plated on fibronectin-coated dishes and 1–2 days later, the cells that fail to attach are removed and then replated on dishes coated with fibronectin. Using features originally described in the Asahara et al. paper (1), clusters of cells emerging in 4–9 days that display spindle-shaped cells emerging from the base are referred to as colony forming unit-Hill (CFU-Hill) EPCs (Fig. 1) (34). The CFU-Hill frequency in human peripheral blood highly predicts adverse cardiovascular risk in human subjects with cardiovascular disease (34). Both myeloid progenitor and lymphoid cells participate in the formation of these CFU-Hill clusters (70, 71). Rohde et al. (70) have suggested that lymphoid cell derived chemokines stimulate the myeloid progenitor cells comprising the clustered cells that mature into the spindle-shaped macrophages that emerge from the clusters. The pattern of gene expression in the CFU-Hill clusters is indistinguishable from CAC (above) or cultured monocytes, but distinctly different from cultured endothelial cells (1, 22, 59). CFU-Hill derived cells, like CAC cells, do not display replating potential and display low proliferative potential. Thus, this colony assay does not identify a cell that serves as a progenitor of an endothelial cell, but rather measures a mixture of peripheral blood hematopoietic subsets that can be informative as a biomarker of cardiovascular risk status.
The second colony forming assay identifies circulating endothelial cells that possess proliferative potential. Adult peripheral blood cells are placed in culture wells coated with type 1 collagen and culture medium is added (41). After 2–3 weeks in culture, adherent endothelial cell colonies become visible. The emergent colonies can be removed and plated to expand the cells or analyzed for endothelial characteristics. The progeny of the isolated colonies display typical endothelial antigens similar to vascular endothelial cells, form capillary-like structures when plated on Matrigel, but also form human blood vessels when suspended in collagen or Matrigel scaffolds and implanted in immunodeficient mice (16, 48, 60, 61, 95). Analysis of the individual cells comprising the colonies indicates that some of the cells display heterogeneous clonal proliferative potential (41, 95). Some of the single cultured cells do not divide over a two week culture period while other clones form small clusters of progeny (2–50 cells) and some of the clones display high proliferative potential (>2000 progeny per colony). Endothelial colony forming cells (ECFCs) with high proliferative potential (Fig. 1) display robust replating potential and high telomerase activity and appear to generate more human vessels when implanted into immunodeficient mice than ECFC with lower proliferative potential (95). Infusion of ECFC into pre-clinical animal models of hindlimb ischemia (96), myocardial infarction (23), retinal ischemia (59), and islet transplantation (45) promote enhanced vascular recovery with evidence of ECFC formed vessels in most models. Thus, ECFCs appear to display many features of circulating cells that are consistent with the original criteria for an EPC (Fig. 1); circulating cells that give rise in vitro to colonies of endothelial cells with inherent heterogeneous proliferative potential, high replating activity, high telomerase activity, endothelial cell surface phenotype, in vivo vessel forming ability, and demonstrated ability to play a role in vascular repair or regeneration in pre-clinical animal models of human vascular disorders.
Methods to identify rodent EPCs
The approaches to identify putative murine EPC are similar in concept but deviate considerably in detail from the methods employed in human subjects. The circulating murine EPC is thought to be derived from the bone marrow compartment. The cell surface markers used to identify putative EPC are highly variable and have included different combinations of stem cell antigen-1 (Sca-1), c-Kit (CD117), Flk-1, vascular endothelial growth factor receptor-1 (Flt-1), CD31, Tie-2, CXCR4, CD133, or CD144 expression (7, 19, 30, 31, 33, 43, 46, 49, 56, 62, 65, 81, 88, 90, 91). While some of these cell surface markers are similar to those utilized originally used to identify human EPC, many are unique to the murine system. An alternative approach currently employed is to plate the mouse peripheral blood or bone marrow cells onto fibronectin-coated culture wells and to identify the adherent cells that persist in the culture at 4–7 days that display uptake of ac-LDL and the plant lectin BS-1 (14, 15) similar to the approach used in the human CAC assay (above). Like the human CAC assay, it is now well recognized that myeloid cells are the predominant cells isolated in the fibronectin-coated dishes and Tie-2 monocytes are the predominant pro-angiogenic cells comprising these cultures (18, 21, 36). While the proliferative ECFC from human adult peripheral blood are rare, murine circulating blood cells do not appear to possess any ECFC at an individual animal level. Specifically, whole blood cells pooled from 4–6 mice >8 weeks of age resulted in successful outgrowth of a single ECFC in 28% of attempts; a frequency far below a single event per animal (79). Thus, mice like human subjects display circulating hematopoietic cells with pro-angiogenic activity that participate in vascular repair, but differ from human subjects in failing to exhibit circulation of proliferative endothelial cells under homeostatic conditions. Selection of blood samples from younger mice or mice infused with VEGF does significantly augment the capacity to identify circulating ECFC (79). Understanding the origin of the circulating endothelial cells with proliferative potential would facilitate better understanding of the regulation of this process in both mouse and man.
Identification of tissue resident ECFCs
The identification of ECFCs can be placed within the context of established data regarding known endothelial turnover rates based on studies using whole animal tritiated thymidine labeling (77, 78). Mapping of proliferating cells in rat aorta reveals that they are not localized diffusely and heterogeneously, but rather are found in discrete foci in the endothelial intima (77). Such observations are consistent with the presence of a hierarchy of ECFC which may be distributed throughout the tissue and activated upon demand to meet the reparative needs of the endothelium (93). While normal steady-state endothelial turnover is slow, endothelial proliferation rates are increased in response to injury and reparative potential may be viewed as a function of cells within the tissue wall (12, 29, 80). For example, in disease prone states, where reduced endothelial repair may represent a component of the pathophysiology (as may occur for example in atherosclerosis), one would predict a reduction in the percentage of tissue resident ECFC and diminished capacity for endothelial proliferation (12, 29, 80, 93). Likewise, such situations may be associated with diminished recovery of circulating endothelial cells from the bloodstream (11).
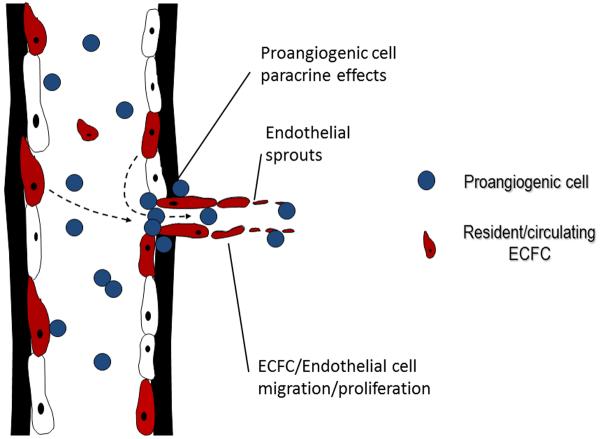
As described above, while pro-angiogenic hematopoietic cells (PACs) do not represent actual endothelial progenitors, they are recruited to sites of active angiogenesis following injury. Adoptive transfer of these PACs facilitates vascular repair without stable integration into the remodeled endothelium (5). A model of cooperative interaction between PAC and ECFC during injury and/or physiological vascular remodeling has been proposed (Fig. 2) (40, 94). In the proposed model, hematopoietic PAC rapidly home to sites vascular injury to create a proangiogenic environment (39). The secretion of paracrine factors by PACs recruits ECFCs from either the circulation or the local vascular wall. Migration and proliferation of ECFCs are guided by PAC activity leading to vascular repair and/or angiogenesis (40). In support of this hypothesis, co-adoptive transfer of both cell types results in superior in vivo neo-angiogenesis and restoration of blood flow when compared to single cell transfers alone (96). When viewed from this perspective, the degree of vascular remodeling stimulated by PAC is dependent, in part, on the resident proliferative potential of ECFC within the tissue or the amount of circulating ECFC that can be recruited into the tissue (Fig. 2). If this is true, one would predict a correlation between the tissue ECFC content and the angiogenic or vascular proliferative capacity of a specific tissue bed. Different vascular beds have different angiogenic and repair capacities, reflecting their unique physiology and/or susceptibility to disease (93). A greater understanding of the cellular basis for such heterogeneity has been proposed to result from regulation of the local growth and angiogenic cues contained within the perivascular environment (68). However, the induction of local paracrine factors alone may not be sufficient to initiate or sustain angiogenesis since the complement of ECFC with proliferative potential may be limiting.
Figure 2. Collaborative interaction of proangiogenic hematopoietic cells and ECFC in the formation of new blood vessels.
Bone marrow-derived proangiogenic cells are recruited to sites of tissue ischemia or damaged endothelium and secrete paracrine molecules to recruit circulating and tissue resident ECFC to participate in new blood vessel formation.
The lung microvasculature is well-known to actively engage in physiological and pathological angiogenic remodeling, for example, following acute lung injury or secondary to pulmonary hypertension or asthma (85). In addition to the proliferation of new cells, pulmonary microvascular cells may become apoptosis resistant in pulmonary hypertension, leading to excessive endothelial proliferation and angioproliferative occlusion (85). Rodent models of pulmonary ischemia (87) or inflammation (58) can recapitulate the robust angiogenesis of the lung microvasculature, suggesting that an understanding of rodent lung angiogenesis has relevance to human disorders. Pulmonary microvascular endothelial cells are a frequently used model system for understanding endothelial cell growth in vitro because they are relatively easy to establish in culture (57). Recently, Alvarez et al. (3), compared the growth potential of EC derived from the rat pulmonary microvasculature (RPMVEC) with that of endothelial cells derived from the rat pulmonary artery (RPAEC). Endothelial cells from both sources maintained cobblestone morphology, incorporated ac-LDL, and expressed CD31, and VEGFR-2, but not CD45 (4). In culture, these cells maintained a high degree of electrical resistance consistent with establishment of barrier function and were capable of forming blood-perfused vessels when suspended in collagen gels and implanted in vivo. When subjected to single-cell colony forming assays, the majority (~60%) of RPAEC remained as non-dividing cells and only ~ 15% of the RPAECs were capable of forming large colonies indicative of emergence from single cells with high proliferative potential (>2000 cells/colony). In contrast, only a small percentage of RPMVEC were non-dividing cells, while greater than 50% of RPMVEC formed large colonies (>2000 cells/colony). Thus, one explanation for the overall differences in growth potential of the RPMVEC versus the RPAEC resides in the increased content of HPP-ECFC in the RPMVEC (4).
Other investigators have also identified robust colony forming capacity in endothelial cells isolated from mouse lung. For example, Schneidermann et al (76), isolated CD31+ mouse lung endothelial cells and discovered a small subpopulation capable of forming large endothelial colonies in vitro. The mouse lung endothelial cells were also capable of forming blood-perfused vessels when suspended in Matrigel and implanted in vivo (76). The molecular pathways that permitted these rare lung ECFC to retain proliferative potential, while the vast majority of the isolated lung endothelial cells displayed minimal proliferative potential, have not been identified. However, alterations in specific pro-growth or progenitor regulatory proteins in some lung ECFC have been described. For example, the regulatory protein nucleosome associated protein-1 (NAP-1) was shown to be preferentially more expressed in rat lung HPP-ECFC and RPMVEC compared to the level of expression in RPAEC (17). In addition, Fang et al., suggested that lung derived ECFC activity co-segregated with a CD117/c-Kit+ subpopulation of pulmonary vasculature (28).
Despite the fact that the pulmonary microvasculature appears enriched with a complement of HPP-ECFC activity, further exploration of the hypothesis that angiogenic/remodeling potential is a function of local tissue resident ECFC content requires analysis of ECFCs from a variety of tissue vascular sources. One would predict that tissues capable of undergoing physiological angiogenesis such as brain, corpus luteum, placenta, or skeletal muscle would be enriched in HPP-ECFCs. While previous investigators in many cases propagated EC cultures from these various sources demonstrating robust growth properties (37, 44, 63, 86), a systematic evaluation to identify resident ECFC populations should be conducted in these tissues.
On the opposite end of the proposed spectrum, one would predict that a tissue with poor vascular reparative function would be depleted of HPP-ECFC. Such is the case with the renal vasculature. Renal capillary rarefaction promotes renal hypoxia and fuels progression of renal fibrosis in acute kidney injury (AKI) (8). Rarefaction is commonly observed in all models of chronic kidney disease as well as human patients with CKD (8). Capillary rarefaction is also observed in patients following AKI (50). In rats following experimental AKI, parenchymal cells undergo repair, but capillaries remain rarefied and show no evidence of vascular repair (8). The reason for impaired capillary regeneration is unclear. Several studies have been carried out to determine if hematopoietic pro-angiogenic cells may participate and facilitate vascular repair following AKI. Bone marrow (BM)-derived cells expressing endothelial cell markers (likely corresponding to PAC) infiltrate into the renal vasculature following acute injury due to ischemia reperfusion, Adriamycin, or administration of the anti Thy1.1 antibody (24, 25, 38, 54, 55). In these studies, evidence of revascularization by BM-derived cells was assessed by colocalization of the donor cells with a vascular marker such as CD31 or vWF. However, it is well known that the expression of surface endothelial markers in bone marrow cells is not sufficient to define an endothelial cell (92). Moreover, in a study by Li et al (53), BM-derived cells expressing endothelial cell markers peak within 14 days and decline by 28 days in kidney following adriamycin injury. Such a result is consistent with the transient homing of PACs to support local vascular remodeling, rather than stable incorporation and differentiation into more long-lived vascular endothelial cells.
Adoptive transfer studies of cells likely corresponding to PAC have been shown to preserve renal function in response to various acute injuries (52, 65, 83). These responses appear largely protective of the initiating insult rather than sustaining a growth regenerating response since there is no evidence that adoptively transferred cells stably integrate into vessels (83). Moreover, there are no studies in rodent models in which PACs have been shown to stimulate vascular regeneration following an established injury. In summary, despite significant interest in the trafficking of endogenous or exogenous PACs to injured kidney, such activity seems largely ineffective since vascular rarefaction remains the predominant feature of these models.
We have suggested that the lack of vascular repair in kidney may be reflective of low resident ECFC proliferative capacity. In contrast to the lung microvascular cells or HUVECs, which are widely utilized because of they are relatively easy to establish and maintain in culture, kidney endothelial cells (KEC) are notorious for being difficult to establish and maintain in long term culture conditions (82). Until recently, the only reports of long-term cultures of rodent kidney endothelial cells described cell lines derived secondary to transformation (73). After optimization, our laboratory was recently able to generate and maintain primary rat KEC in long term cultures (10). Multiple KEC were obtained but all were characterized by exceptionally slow growth rates relative to RPAEC, RPMVEC and HUVECs. Colony forming assay demonstrated that ~90% of cells remained as single/non-dividing colonies, while only a small percentage of cells were capable of forming moderately sized colonies, and there was no evidence of any population that would be defined as an HPP-ECFC (ie., colony > 2000 cells). The nature of the impaired growth of KEC is not clear, but it does not appear to be due to senescence or a reduction in the VEGFR, since VEGF-R expression is significantly greater in KEC vs PMVEC (10). The lack of ECFC proliferative capability is consistent with the relatively low proliferation rates observed in the kidney in vivo. In recent studies, using repetitive BrdU administration to rats, the identification of proliferating KEC was shown to be exceedingly rare and was not affected by kidney injury or that exogenous administration of VEGF (9). Although other studies have also identified some proliferating KEC using PCNA immunohistochemistry, these structures are also typically few in number (47). Therefore, in the setting of acute renal injury, persistent vascular loss occurs not because of a lack of bone marrow derived PACs, but rather that the resident ECFC population is either inherently low in number or actively under some form of proliferative repression that is dominant over PAC paracrine effects.
Summary
Our understanding of the circulating cells in the bloodstream that contribute to vascular repair has increased a great deal over the past 15 years. In many ways, the reported identification of an EPC has spurred this interest in the field. However, we have now accumulated sufficient evidence to demonstrate that the only circulating cell that displays all the features of the originally defined EPC are circulating ECFC. While numerous hematopoietic cells play key roles as pro-angiogenic paracrine activators of vascular repair and regeneration, they do not become specified to an endothelial state or fate in vivo and thus, are not EPC. Going forward the field should more fully analyze the specific molecules secreted by the proangiogenic hematopoietic cell subsets to design potential molecules that may be used to augment vascular repair. In addition, the field must learn how to interrogate the small pool of ECFC residing in the vascular endothelium to understand how these progenitors protect the vasculature from injury, senescence, and disease.
Reviewing the foundational and/or controversial concepts of features that were originally penned to define a circulating EPC we can now state: 1) human blood contains circulating ECFC and human and murine blood contains circulating PACs, 2) human and murine PACs display different markers; human PAC are CD34+CD133+CD45+CD31+CD14-CD235a- but also express other markers (20, 26, 27) while murine PACs are not strictly defined but generally display a Sca-1+Flk-1+CD31+ phenotype (above), 3) circulating PACs may lodge, emigrate, and accumulate in a peri-endothelial location at sites of vascular injury but do not become integrated as long-lived endothelial cells (ECFC have not yet been shown to integrate into vessels following prospective isolation without culture), 4) human PACs display low proliferative potential (ECFC display a hierarchy of proliferative potentials), 5) human PACs attach to HUVEC- or ECFC-derived capillary-like structures in vitro (cultured ECFC form vessels when injected in vivo), 6) human PACs form clusters in vitro in the presence of lymphoid cells or certain cytokines, and 7) circulating human and murine PACs promote vascular repair and regeneration via paracrine secretion of molecules that affect resident endothelial cells with residual ECFC activity. Given the status of the field, we are better served to use the above terminology or define a specific cellular subset by phenotype and function, rather than use the less precise term EPC.
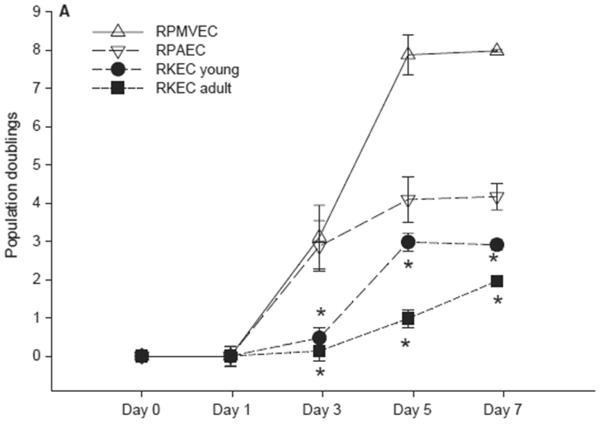
Figure 3. Tissue specific and age-related differences in proliferative potential of endothelial cells.
Rat pulmonary microvascular endothelial cells (PMVEC) and pulmonary arterial endothelial cells (PAEC) proliferate more rapidly than endothelial cells isolated from 9–11 day old rat kidney (RKEC young) and 8–10 week old adult rat kidney (RKEC adult). Modified from, Basile DP, et al. Microcirculation 19:598–609, 2012.
REFERENCES
- 1.Ahrens I, Domeij H, Topcic D, Haviv I, Merivirta RM, Agrotis A, Leitner E, Jowett JB, Bode C, Lappas M, Peter K. Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS One. 2011;6:e23210. doi: 10.1371/journal.pone.0023210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010;156:112–129. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2008;294:L419–430. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez DF, Huang L, King JA, Elzarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 9.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F721–733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basile DP, Zeng P, Friedrich JL, Leonard EC, Yoder MC. Low proliferative potential and impaired angiogenesis of cultured rat kidney endothelial cells. Microcirculation. 2012;19:598–609. doi: 10.1111/j.1549-8719.2012.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P, Sampol J, Solovey A, Dignat-George F. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 12.Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973;17:401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 13.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Xiao X, Chen S, Zhang C, Yi D, Shenoy V, Raizada MK, Zhao B, Chen Y. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 2013;61:681–689. doi: 10.1161/HYPERTENSIONAHA.111.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JY, Feng L, Zhang HL, Li JC, Yang XW, Cao XL, Liu L, Qin HY, Liang YM, Han H. Differential regulation of bone marrow-derived endothelial progenitor cells and endothelial outgrowth cells by the Notch signaling pathway. PLoS One. 2012;7:e43643. doi: 10.1371/journal.pone.0043643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng G, Liao S, Kit Wong H, Lacorre DA, di Tomaso E, Au P, Fukumura D, Jain RK, Munn LL. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood. 2011;118:4740–4749. doi: 10.1182/blood-2011-02-338426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark J, Alvarez DF, Alexeyev M, King JA, Huang L, Yoder MC, Stevens T. Regulatory role for nucleosome assembly protein-1 in the proliferative and vasculogenic phenotype of pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L431–439. doi: 10.1152/ajplung.00316.2007. [DOI] [PubMed] [Google Scholar]
- 18.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y, Lewis CE. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 19.Davidoff AM, Leary MA, Ng CY, Spurbeck WW, Frare P, Vanhove M, Nienhuis AW, Vanin EF. Autocrine expression of both endostatin and green fluorescent protein provides a synergistic antitumor effect in a murine neuroblastoma model. Cancer Gene Ther. 2001;8:537–545. doi: 10.1038/sj.cgt.7700346. [DOI] [PubMed] [Google Scholar]
- 20.de Boer HC, Hovens MM, van Oeveren-Rietdijk AM, Snoep JD, de Koning EJ, Tamsma JT, Huisman MV, Rabelink AJ, van Zonneveld AJ. Human CD34+/KDR+ cells are generated from circulating CD34+ cells after immobilization on activated platelets. Arterioscler Thromb Vasc Biol. 2011;31:408–415. doi: 10.1161/ATVBAHA.110.216879. [DOI] [PubMed] [Google Scholar]
- 21.De Palma M, Naldini L. Tie2-expressing monocytes (TEMs): Novel targets and vehicles of anticancer therapy? Biochim Biophys Acta. 2009 doi: 10.1016/j.bbcan.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Desai A, Glaser A, Liu D, Raghavachari N, Blum A, Zalos G, Lippincott M, McCoy JP, Munson PJ, Solomon MA, Danner RL, Cannon RO., 3rd Microarray-based characterization of a colony assay used to investigate endothelial progenitor cells and relevance to endothelial function in humans. Arterioscler Thromb Vasc Biol. 2009;29:121–127. doi: 10.1161/ATVBAHA.108.174573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P, Vandenwijngaert S, Depelteau H, Streb W, Chaothawee L, Maes F, Gheysens O, Debyser Z, Gillijns H, Pellens M, Vandendriessche T, Chuah M, Collen D, Verbeken E, Belmans A, Van de Werf F, Bogaert J, Janssens S. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol. 2010;55:2232–2243. doi: 10.1016/j.jacc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 24.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estes ML, Mund JA, Mead LE, Prater DN, Cai S, Wang H, Pollok KE, Murphy MP, An CS, Srour EF, Ingram DA, Jr., Case J. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A. 2010;77:831–839. doi: 10.1002/cyto.a.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10:e1001407. doi: 10.1371/journal.pbio.1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Florentin RA, Nam SC, Lee KT, Thomas WA. Increased 3H-thymidine incorporation into endothelial cells of swine fed cholesterol for 3 days. Exp Mol Pathol. 1969;10:250–255. doi: 10.1016/0014-4800(69)90055-0. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 32.Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 33.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher A, DImmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 34.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 35.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hristov M, Schmitz S, Schuhmann C, Leyendecker T, von Hundelshausen P, Krotz F, Sohn HY, Nauwelaers FA, Weber C. An optimized flow cytometry protocol for analysis of angiogenic monocytes and endothelial progenitor cells in peripheral blood. Cytometry A. 2009;75:848–853. doi: 10.1002/cyto.a.20772. [DOI] [PubMed] [Google Scholar]
- 37.Ieronimakis N, Balasundaram G, Reyes M. Direct isolation, culture and transplant of mouse skeletal muscle derived endothelial cells with angiogenic potential. PLoS One. 2008;3:e0001753. doi: 10.1371/journal.pone.0001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikarashi K, Li B, Suwa M, Kawamura K, Morioka T, Yao J, Khan F, Uchiyama M, Oite T. Bone marrow cells contribute to regeneration of damaged glomerular endothelial cells. Kidney Int. 2005;67:1925–1933. doi: 10.1111/j.1523-1755.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 39.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 40.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 41.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 42.Ito H, Rovira II, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA, Finkel T. Endothelial progenitor cells as putative targets for angiostatin. Cancer Res. 1999;59:5875–5877. [PubMed] [Google Scholar]
- 43.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 44.Jin Y, Liu Y, Antonyak M, Peng X. Isolation and characterization of vascular endothelial cells from murine heart and lung. Methods Mol Biol. 2012;843:147–154. doi: 10.1007/978-1-61779-523-7_14. [DOI] [PubMed] [Google Scholar]
- 45.Jung HS, Kim MJ, Hong SH, Lee YJ, Kanga S, Lee H, Chung SS, Park JS, Park KS. The Potential of Endothelial Colony-Forming Cells to Improve Early Graft Loss after Intraportal Islet Transplantation. Cell Transplant. 2013 doi: 10.3727/096368912X661364. [DOI] [PubMed] [Google Scholar]
- 46.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 47.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24:559–572. doi: 10.1681/ASN.2012080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirschstein R. Public Health Report. 2001;116:515–516. doi: 10.1016/S0033-3549(04)50082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280–1289. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon O, Hong SM, Ramesh G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am J Physiol Renal Physiol. 2009;296:F25–33. doi: 10.1152/ajprenal.90531.2008. [DOI] [PubMed] [Google Scholar]
- 51.Kwon SM, Lee YK, Yokoyama A, Jung SY, Masuda H, Kawamoto A, Lee YM, Asahara T. Differential activity of bone marrow hematopoietic stem cell subpopulations for EPC development and ischemic neovascularization. J Mol Cell Cardiol. 2011;51:308–317. doi: 10.1016/j.yjmcc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Deane JA, Campanale NV, Bertram JF, Ricardo SD. Blockade of p38 mitogen-activated protein kinase and TGF-beta1/Smad signaling pathways rescues bone marrow-derived peritubular capillary endothelial cells in adriamycin-induced nephrosis. J Am Soc Nephrol. 2006;17:2799–2811. doi: 10.1681/ASN.2006020130. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Deane JA, Campanale NV, Bertram JF, Ricardo SD. The contribution of bone marrow-derived cells to the development of renal interstitial fibrosis. Stem Cells. 2007;25:697–706. doi: 10.1634/stemcells.2006-0133. [DOI] [PubMed] [Google Scholar]
- 55.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 57.Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2000;244:205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 58.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 59.Medina RJ, O'Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, Stitt AW. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima M, Ogawa M, Shimoda Y, Hiraoka S, Iida M, Koseki H, Shirasawa T, Furukawa K. Presenilin-1 controls the growth and differentiation of endothelial progenitor cells through its beta-catenin-binding region. Cell Biol Int. 2006;30:239–243. doi: 10.1016/j.cellbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Navone SE, Marfia G, Nava S, Invernici G, Cristini S, Balbi S, Sangiorgi S, Ciusani E, Bosutti A, Alessandri G, Slevin M, Parati EA. Human and mouse brain-derived endothelial cells require high levels of growth factors medium for their isolation, in vitro maintenance and survival. Vasc Cell. 2013;5:10. doi: 10.1186/2045-824X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohtani K, Vlachojannis GJ, Koyanagi M, Boeckel JN, Urbich C, Farcas R, Bonig H, Marquez VE, Zeiher AM, Dimmeler S. Epigenetic regulation of endothelial lineage committed genes in pro-angiogenic hematopoietic and endothelial progenitor cells. Circ Res. 2011;109:1219–1229. doi: 10.1161/CIRCRESAHA.111.247304. [DOI] [PubMed] [Google Scholar]
- 65.Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 66.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 67.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, Boulanger CM, Westwood N, Urbich C, Willeit J, Steiner M, Breuss J, Xu Q, Kiechl S, Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 68.Regan ER, Aird WC. Dynamical systems approach to endothelial heterogeneity. Circ Res. 2012;111:110–130. doi: 10.1161/CIRCRESAHA.111.261701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 70.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–1752. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 71.Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 72.Rohde E, Schallmoser K, Reinisch A, Hofmann NA, Pfeifer T, Frohlich E, Rechberger G, Lanzer G, Kratky D, Strunk D. Pro-angiogenic induction of myeloid cells for therapeutic angiogenesis can induce mitogen-activated protein kinase p38-dependent foam cell formation. Cytotherapy. 2011;13:503–512. doi: 10.3109/14653249.2010.536214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rops AL, van der Vlag J, Jacobs CW, Dijkman HB, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, Berden JH. Isolation and characterization of conditionally immortalized mouse glomerular endothelial cell lines. Kidney Int. 2004;66:2193–2201. doi: 10.1111/j.1523-1755.2004.66009.x. [DOI] [PubMed] [Google Scholar]
- 74.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 75.Schmeisser A, Graffy C, Daniel WG, Strasser RH. Phenotypic overlap between monocytes and vascular endothelial cells. Adv Exp Med Biol. 2003;522:59–74. doi: 10.1007/978-1-4615-0169-5_7. [DOI] [PubMed] [Google Scholar]
- 76.Schniedermann J, Rennecke M, Buttler K, Richter G, Stadtler AM, Norgall S, Badar M, Barleon B, May T, Wilting J, Weich HA. Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol. 2010;11:50. doi: 10.1186/1471-2121-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwartz SM, Benditt EP. Clustering of replicating cells in aortic endothelium. Proc Natl Acad Sci U S A. 1976;73:651–653. doi: 10.1073/pnas.73.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwartz SM, Benditt EP. Aortic endothelial cell replication. I. Effects of age and hypertension in the rat. Circ Res. 1977;41:248–255. doi: 10.1161/01.res.41.2.248. [DOI] [PubMed] [Google Scholar]
- 79.Somani A, Nguyen J, Milbauer LC, Solovey A, Sajja S, Hebbel RP. The establishment of murine blood outgrowth endothelial cells and observations relevant to gene therapy. Transl Res. 2007;150:30–39. doi: 10.1016/j.trsl.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Taylor RG, Lewis JC. Endothelial cell proliferation and monocyte adhesion to atherosclerotic lesions of white carneau pigeons. Am J Pathol. 1986;125:152–160. [PMC free article] [PubMed] [Google Scholar]
- 81.Thal MA, Krishnamurthy P, Mackie AR, Hoxha E, Lambers E, Verma S, Ramirez V, Qin G, Losordo DW, Kishore R. Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ Res. 2012;111:180–190. doi: 10.1161/CIRCRESAHA.112.270462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994;45:598–604. doi: 10.1038/ki.1994.78. [DOI] [PubMed] [Google Scholar]
- 83.Uchimura H, Marumo T, Takase O, Kawachi H, Shimizu F, Hayashi M, Saruta T, Hishikawa K, Fujita T. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol. 2005;16:997–1004. doi: 10.1681/ASN.2004050367. [DOI] [PubMed] [Google Scholar]
- 84.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 85.Voelkel NF, Douglas IS, Nicolls M. Angiogenesis in chronic lung disease. Chest. 2007;131:874–879. doi: 10.1378/chest.06-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vorontchikhina MA, Zimmermann RC, Shawber CJ, Tang H, Kitajewski J. Unique patterns of Notch1, Notch4 and Jagged1 expression in ovarian vessels during folliculogenesis and corpus luteum formation. Gene Expr Patterns. 2005;5:701–709. doi: 10.1016/j.modgep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Wagner EM, Jenkins J, Perino MG, Sukkar A, Mitzner W. Lung and vascular function during chronic severe pulmonary ischemia. J Appl Physiol. 2011;110:538–544. doi: 10.1152/japplphysiol.01308.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang CH, Verma S, Hsieh IC, Chen YJ, Kuo LT, Yang NI, Wang SY, Wu MY, Hsu CM, Cheng CW, Cherng WJ. Enalapril increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. J Mol Cell Cardiol. 2006;41:34–43. doi: 10.1016/j.yjmcc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Wara AK, Croce K, Foo S, Sun X, Icli B, Tesmenitsky Y, Esen F, Rosenzweig A, Feinberg MW. Bone marrow-derived CMPs and GMPs represent highly functional proangiogenic cells: implications for ischemic cardiovascular disease. Blood. 2011;118:6461–6464. doi: 10.1182/blood-2011-06-363457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Westerweel PE, Teraa M, Rafii S, Jaspers JE, White IA, Hooper AT, Doevendans PA, Verhaar MC. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS One. 2013;8:e60357. doi: 10.1371/journal.pone.0060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang YH, Zhou H, Binmadi NO, Proia P, Basile JR. Plexin-B1 activates NF-kappaB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS One. 2011;6:e25826. doi: 10.1371/journal.pone.0025826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7(Suppl 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 93.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–1103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 94.Yoder MC, Ingram DA. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim Biophys Acta. 2009;1796:50–54. doi: 10.1016/j.bbcan.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon C-H, Hur J, Park K-W, Kim J-H, Lee C-S, Oh I-Y, Kim T-Y, Cho H-J, Kang H-J, Chae I-H, Yang H-K, Oh B-H, Park Y-B, Kim H-S. Synergistic Neovascularization by Mixed Transplantation of Early Endothelial Progenitor Cells and Late Outgrowth Endothelial Cells: The Role of Angiogenic Cytokines and Matrix Metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]