Abstract
Background
Pulmonary arterial hypertension (PAH) is characterized by dysregulated proliferation of pulmonary artery smooth muscle cells leading to (mal)adaptive vascular remodeling. In the systemic circulation, vascular injury is associated with downregulation of sarcoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) and alterations in Ca2+ homeostasis in vascular smooth muscle cells that stimulate proliferation. We, therefore, hypothesized that downregulation of SERCA2a is permissive for pulmonary vascular remodeling and the development of PAH.
Methods and Results
SERCA2a expression was decreased significantly in remodeled pulmonary arteries from patients with PAH and the rat monocrotaline model of PAH in comparison with controls. In human pulmonary artery smooth muscle cells in vitro, SERCA2a overexpression by gene transfer decreased proliferation and migration significantly by inhibiting NFAT/STAT3. Overexpresion of SERCA2a in human pulmonary artery endothelial cells in vitro increased endothelial nitric oxide synthase expression and activation. In monocrotaline rats with established PAH, gene transfer of SERCA2a via intratracheal delivery of aerosolized adeno-associated virus serotype 1 (AAV1) carrying the human SERCA2a gene (AAV1.SERCA2a) decreased pulmonary artery pressure, vascular remodeling, right ventricular hypertrophy, and fibrosis in comparison with monocrotaline-PAH rats treated with a control AAV1 carrying β-galactosidase or saline. In a prevention protocol, aerosolized AAV1.SERCA2a delivered at the time of monocrotaline administration limited adverse hemodynamic profiles and indices of pulmonary and cardiac remodeling in comparison with rats administered AAV1 carrying β-galactosidase or saline.
Conclusions
Downregulation of SERCA2a plays a critical role in modulating the vascular and right ventricular pathophenotype associated with PAH. Selective pulmonary SERCA2a gene transfer may offer benefit as a therapeutic intervention in PAH.
Keywords: calcium; gene therapy; heart failure; muscle, smooth; pulmonary hypertension; ventricular remodeling
Pulmonary arterial hypertension (PAH) is characterized by dysregulated pulmonary vascular remodeling that leads to an increase in pulmonary vascular resistance, right ventricular hypertrophy, dysfunction and uncompensated right heart failure, and ultimately death.1 Vascular remodeling occurs as a result of pulmonary vascular endothelial dysfunction, pulmonary artery smooth muscle cell (PASMC) proliferation and migration, medial hypertrophy, inflammation, and thrombosis in situ leading to the formation of plexiform lesions, which are a hallmark of the disease.2,3 These remodeled vessels, together with an imbalance in vasodilators and vasoconstrictors, contribute to increased pulmonary vascular resistance, which, in turn, strains and remodels the right heart. Despite our understanding of these pathobiological processes, pharmacotherapies to treat the disease are limited, and survival outcomes have improved little over the past few decades.4
Clinical Perspective on p 523
In vascular smooth muscle cells, sarcoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) modulates calcium homeostasis. Under basal conditions, SERCA2a sequesters calcium in the endoplasmic reticulum; when SERCA2a is downregulated, intracellular calcium [Ca2+]i levels are increased. In rat vascular injury models, SERCA2a expression is downregulated in the vascular media leading to increased [Ca2+]i and activation of calcineurin (protein phosphatase 2B)/cytoplasmic nuclear factor of activated T cells (NFAT) signaling to stimulate vascular smooth muscle cell proliferation and promote neointima formation.5 Similar findings have also been observed in human coronary artery smooth muscle cells in vitro and balloon-injured human internal mammary arteries ex vivo.6,7
Akin to what is observed in the systemic vasculature, there is evidence to indicate that SERCA2a is downregulated in PAH. In PASMCs isolated from PAH patients and animal models of PAH, [Ca2+]i levels are elevated, and this stimulates PASMC proliferation and migration. Furthermore, PASMCs isolated from patients with idiopathic PAH demonstrate increased activation of the signal transducer and activator of transcription-3 (STAT3) and nuclear localization of NFATc2 suggesting that [Ca2+]i-dependent NFAT activation is associated with vascular remodeling in PAH.8–10 Thus, strategies to maintain SERCA2a expression and regulate [Ca2+]i should offer a therapeutic benefit in PAH by limiting pulmonary vascular cell proliferation and, thereby, vessel remodeling.
Gene transfer of SERCA2a has demonstrated in vivo therapeutic efficacy in small- and large-animal models of vascular injury. Forced expression of SERCA2a in vascular smooth muscle cells restores sarco/endoplasmic reticulum Ca2+ handling, decreases [Ca2+]i, and inhibits NFAT transcriptional activity to limit proliferation, migration, and neointima formation. SERCA2a gene transfer has also been shown to abrogate endothelial dysfunction by increasing endothelial nitric oxide synthase (eNOS) expression and activity.5,7 SERCA2a gene transfer using adeno-associated virus serotype 1 (AAV1. SERCA2a) has been validated extensively in the ventricular myocardium and shown to improve left ventricular systolic function and ventricular remodeling in rat and swine preclinical models of heart failure and in a phase 2 clinical trial of patients with New York Heart Association stage III/IV congestive heart failure.11 The therapeutic potential of SERCA2a gene transfer to target pulmonary vascular dysfunction in PAH has not been tested to date. We therefore hypothesized that SERCA2a is downregulated in pulmonary arterioles in PAH and that gene transfer of SERCA2a via intratracheal administration of aerosolized AAV1.SERCA2a would provide selective gene transfer to the pulmonary circulation to prevent or ameliorate pulmonary vascular remodeling and right ventricular myocardial dysfunction in PAH.
Methods
Please refer to the expanded Methods section in the online-only Data Supplement.
Human Lung Tissue Samples
Lung tissue specimens were obtained at the time of lung transplantation from 8 patients with idiopathic PAH, and at the time of thoracic surgery (lobectomy or pneumonectomy for localized lung cancer) from 5 patients without PAH who served as controls. Preoperative echocardiography was performed in the control patients to rule out pulmonary hypertension. Lung tissue from the control patients was collected at a site remote from tumor foci. The study was approved by the local ethics committee (Comité de Protection des Personnes, CPP Ile de France VII, Le Kremlin Bicêtre, France), and patients gave informed consent.
Cell Culture
Human PASMCs and pulmonary artery endothelial cells (PAECs) were purchased from Lonza, Inc. (Allendale, NJ). PASMCs were cultured in SmBM medium supplemented with 5% fetal bovine serum (FBS) and SmGM-2 SingleQuots (Lonza). PAECs were grown in EBM-2 medium supplemented with 5% FBS supplemented with EGM-2 SingleQuots (Lonza). Cells were grown in 5% CO2 at 37°C and passaged at confluence.
Adenovirus and AAV1 Vectors
Ad-SERCA2a encoding human SERCA2a and green fluorescence protein under control of the cytomegalovirus promoter12; Ad-βGal, encoding β-galactosidase and green fluorescence protein under control of the cytomegalovirus promoter12; and Ad-VIVIT, encoding the NFAT competing peptide VIVIT and green fluorescence protein under control of the cytomegalovirus promoter were produced as described previously.13,14 Cells were infected with adenovirus at 100 pfu/cell. The efficiency of infection was assessed by green fluorescence protein.
Human AAV1.SERCA2a and AAV1.βGal were produced as described previously.15 The rAAV1.SERCA2a vector used in this study contains an AAV1 viral capsid and a single-stranded ≈4.5-kb DNA containing the human SERCA2a cDNA driven by a cytomegalovirus immediate-early promoter/enhancer, a hybrid intron, and a bovine growth hormone polyadenylation signal, all flanked by 145-nucleotide AAV2 inverted terminal repeat sequences necessary for replication and packaging of the vector DNA in the capsid. The vector was manufactured by using standard calcium phosphate transfection methods in adherent 293 cells. Three plasmids were used, 1 containing helper functions from adenovirus, 1 containing the AAV rep2 and cap1 genes, and 1 containing the vector genome. AAV1.βGal was constructed in a similar fashion by using the β-galactosidase gene.
Rat Monocrotaline-PAH Model
All animal experiments were approved by the Icahn School of Medicine at Mount Sinai institutional animal use and care committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (Charles River, MA) weighing 350 to 400 g were fed standard chow and administered an intraperitoneal injection of monocrotaline (MCT) 60 mg/kg (Sigma-Aldrich) (MCT group) or 0.9% saline as a control group (sham, n=6). In the treatment protocol, 15 days after the MCT or vehicle control injection, rats from the MCT group (n=30) were randomly assigned to 1 of 3 treatment groups: saline (n =10); AAV1.βGal (3.5×1011 vg/mL, n=10) or AAV1.SERCA2a (3.5×1011 vg/mL, n=10). Treatments were aerosolized in 300 µL for single-dose intratracheal delivery by using an IA-1C Microsprayer (PennCentury, Wyndmoor, PA). In the prevention protocol, immediately after MCT or vehicle control (n=6) injection, rats were randomly assigned into the 3 treatment groups: saline (n=15), AAV1.SERCA2a (n=15), or AAV1.βGal (n=6), delivered as described for the treatment protocol. Hemodynamic studies were conducted 45 days after the administration of MCT or vehicle control after which the rats were euthanized for tissue collection.
Right and Left Heart Hemodynamic Studies
Rats were anesthetized with 1% isoflurane, intubated via a tracheotomy, and mechanically ventilated (tidal volume=10 mL/kg; respiratory rate=30 breaths per minute). The thoracic cavity was opened by using a midsternal approach, a Scisense catheter was inserted directly into the right or left ventricle, and an ultrasonic flow probe (flow probe 2.5S176; Transonic Systems Inc., Ithaca, NY) was placed on the ascending root of the aorta. The heart rate, mean pulmonary artery pressure, aortic systolic arterial pressure, right and left ventricular end-systolic and diastolic pressures were measured directly. Hemodynamic data were recorded by using a Scisense P–V Control Unit (Scisense, Ontario, Canada).
Statistical Analysis
Results are presented as mean±standard error of the mean. Normality of the data was assumed based on previous study of the outcome variables subjected to Shapiro-Wilk testing.16 Data were analyzed with the use of an unpaired t test for comparisons between means or a 1-way analysis of variance with the Bonferroni correction for comparisons between >2 means. Data comparison between the AAV1. SERCA2a and AAV1.βGal groups was analyzed by using a post hoc Tukey test. P<0.05 was considered significant. Statistical analysis was performed by the use of GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA).
Results
SERCA2a Expression Is Decreased in Human PAH
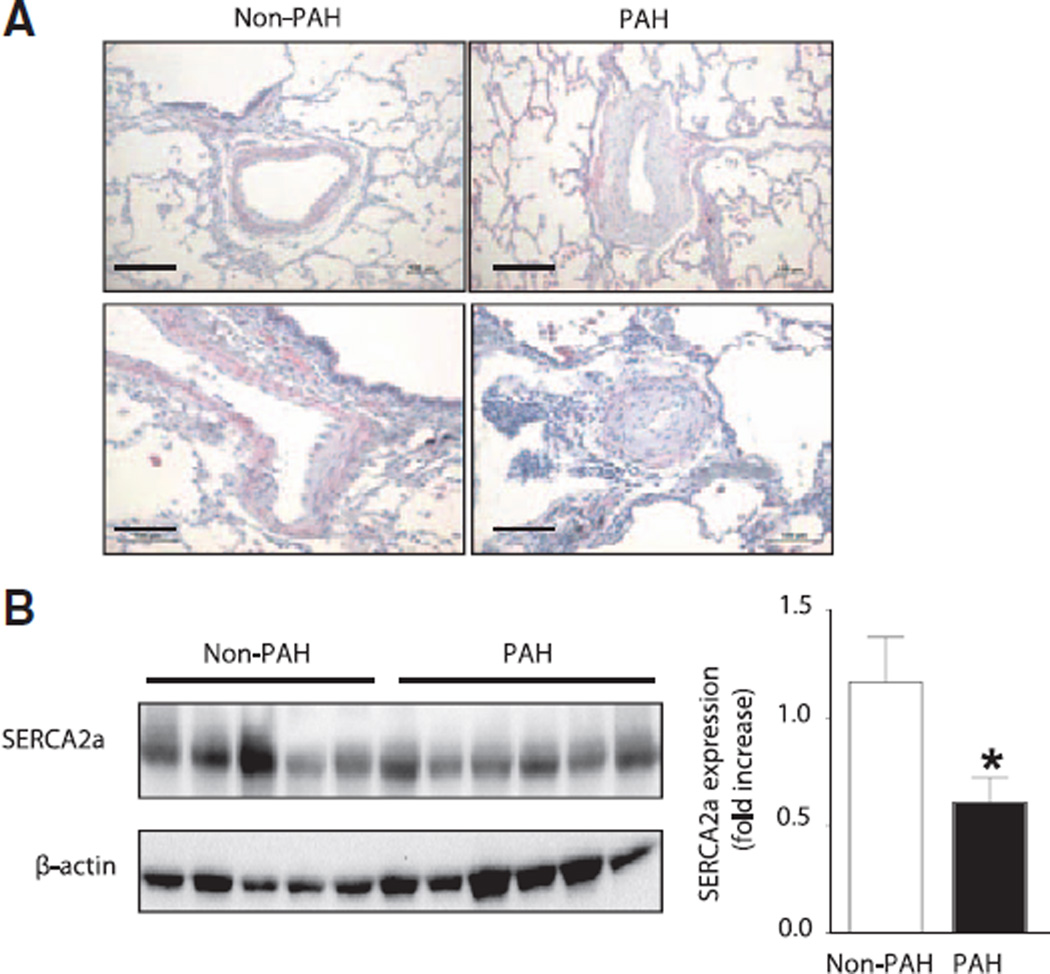
To determine whether SERCA2a expression is decreased in PAH, we examined pulmonary vessels and lung tissue from patients with documented PAH (n=6) and compared them with tissues from patients without PAH (n=5) harvested at the time of thoracic surgery (Tables I and II in the online-only Data Supplement). Tissue sections from PAH patients were typical for the disease with hypertrophy of small pulmonary arteries, which was not observed in vessels of non-PAH patients. Hypertrophied PAH vessels demonstrated decreased expression of SERCA2a in the vessel intima and media layers consistent with downregulation of SERCA2a in PAECs and PASMCs (Figure 1A). Decreased expression of SERCA2a was also confirmed in whole lung tissue lysates in PAH in comparison with non-PAH patients (Figure 1B). Taken together, these findings indicate that SERCA2a expression is decreased in small hypertrophied pulmonary arteries in PAH and suggest that downregulation of SERCA2a may play a role in the pathological vascular remodeling that is characteristic of the disease.
Figure 1.
SERCA2a expression in decreased in pulmonary vessels from patients with PAH. Lung tissue was harvested from patients with confirmed PAH (n=6) and control non-PAH patients. (n=5). A, SERCA2a expression in pulmonary arterioles and bronchial smooth muscle was assessed by immunohistochemistry. SERCA2a was expressed in the endothelial and smooth muscle cell layers of control vessels (top left) but decreased in remodeled pulmonary vessels (right top and bottom). SERCA2a expression was also detected in bronchial smooth muscle cells (bottom left). Representative images are shown. Scale bar, 100 µm. B, Downregulation of SERCA2a in PAH was confirmed by Western blot of lung tissue homogenates. *P=0.034 vs non-PAH. PAH indicates pulmonary arterial hypertension; and SERCA2a, sarcoplasmic reticulum Ca2+-ATPase 2a.
SERCA2a Modulates NFAT and STAT3 to Regulate Human PASMC Proliferation and Migration
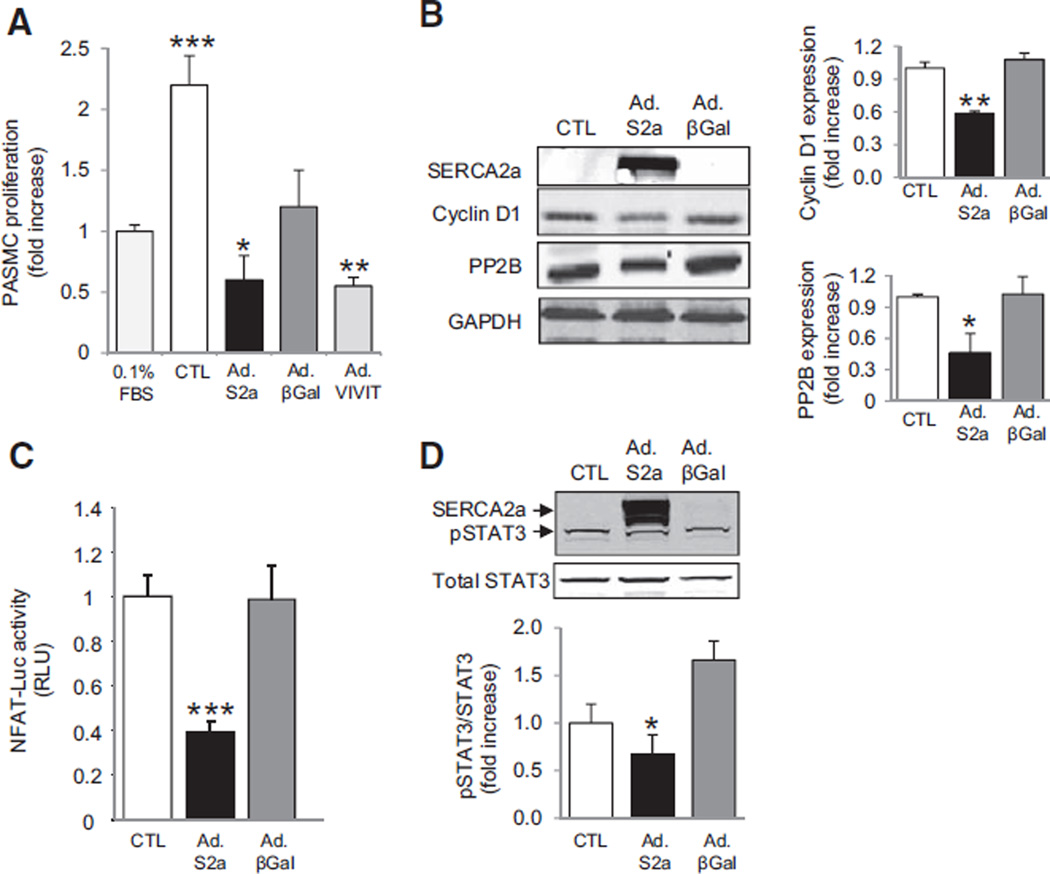
To understand the role of SERCA2a in pulmonary vascular remodeling, we first examined the effect of modulating SERCA2a expression on PASMC proliferation and migration. PASMCs were infected for 48 hours with a control adenovirus encoding β-galactosidase (Ad.βGal) or an adenovirus encoding human SERCA2a (Ad.SERCA2a) to increase expression by 95% and stimulated with 5% FBS. Serum stimulation increased proliferation of PASMCs by 2.25±0.39-fold (P<0.001 versus 0.1% FBS), whereas forced expression of SERCA2a by gene transfer diminished this response significantly (0.61±0.30-fold, P<0.03 versus 5% FBS) (Figure 2A). Serum stimulation also increased cell migration (1.61±0.11-fold, P<0.001 versus 0.1% FBS), an effect that was abrogated by SERCA2a overexpression (0.92±0.13, P=0.027 versus control, 5% FBS; Figure I in the online-only Data Supplement). These findings correlated with the SERCA2a expression profile in serum-stimulated PASMCs: SERCA2a was undetectable in noninfected or Ad.βGal-infected cells but highly expressed in Ad.SERCA2a-infected PASMCs (Figure 2B). Increased SERCA2a expression was associated with a significant decrease in calcineurin/protein phosphatase 2B and Cyclin D1 expression in comparison with control or Ad.βGal-infected cells (Figure 2B). Similarly, inhibition of protein phosphatase 2B signaling by adenovirus-mediated expression of VIVIT, a peptide that competes with NFAT for binding to protein phosphatase 2B, decreased PASMC proliferation significantly, suggesting that SERCA2a inhibits proliferation by regulating NFAT activity (Figure 2A). Therefore, we assessed the effect of SERCA2a on NFAT transcriptional activity. In comparison with Ad.βGal-infected or noninfected cells, SERCA2a overexpression decreased NFAT transcriptional activity by 2.5-fold (P=0.0008) (Figure 2C). We next examined the effect of SERCA2a on STAT3, which has been shown to regulate NFAT and is activated in PAH.10 Overexpression of SERCA2a decreased phosphorylation and activation of STAT3 (Figure 2D). Thus, these findings indicate that SERCA2a inhibits PASMC proliferation through a mechanism that involves decreased activation of STAT3 and NFAT.
Figure 2.
SERCA2a gene transfer inhibits the proliferation of PASMCs. PASMCs were infected for 48 hours with Ad.SERCA2a, Ad.βGal, or Ad.VIVIT and then cultured for 48 hours in virus-free medium. A, Proliferation was determined by labeling cells with BrdU and supplementing the medium with 5% FBS to stimulate proliferation. Noninfected PASMCs and PASMCs cultured with 0.1% FBS to maintain quiescence cultured under the same conditions served as controls (n=6) *P=0.03 vs CTL. **P<0.005 vs CTL. ***P<0.001 vs CTL or 0.1% FBS. B, Western immunoblotting was performed to evaluate SERCA2a, protein phosphatase 2B (PP2B), and Cyclin D1 expression (n=3). Protein expression was normalized to GAPDH. *P<0.03 vs CTL. **P<0.008 vs CTL. C, NFAT transcriptional activity was assessed by using a luciferase promoter-reporter assay. Data are expressed in relative luciferase units (RLU; n=5) ***P<0.001 vs CTL. D, Phosphorylated and total STAT3 expression were determined by immunoblotting. Phosphorylated STAT3 was normalized to total STAT3. GAPDH was used as the loading control (n=3). Representative blots are shown. *P=0.04 vs CTL. BrdU indicates bromodeoxyuridine; CTL, control; FBS, fetal bovine serum; NFAT, nuclear factor of activated T cells; and PASMC, pulmonary artery smooth muscle cell.
SERCA2a Limits Proliferation and Migration of PAECs and Restores Activation of eNOS
Because remodeled vessels demonstrate intimal hypertrophy, we also examined the effect of modulating SERCA2a expression on proliferation and migration of PAECs. Similar to what was observed in PASMCs, SERCA2a overexpression decreased Cyclin D1 expression in comparison with Ad.βGal-infected cells, consistent with the inhibition of proliferation. SERCA2a overexpression also prevented the serum-stimulated increase in PAEC migration (0.93±0.04, P<0.02 versus control, 5% FBS). We also observed that increased SERCA2a expression was associated with a concomitant 1.5-fold (P<0.03 versus control) increase in eNOS activation as demonstrated by increased phosphorylation of eNOS at Ser1177 (Figure II in the online-only Data Supplement). These findings suggest that SERCA2a regulates PAEC proliferation and migration, which may occur as a result of enhanced eNOS activation.
SERCA2a Gene Transfer Via Intratracheal Delivery of AAV1.SERCA2a Reverses Established Pulmonary Hypertension in the MCT-PAH Rat Model
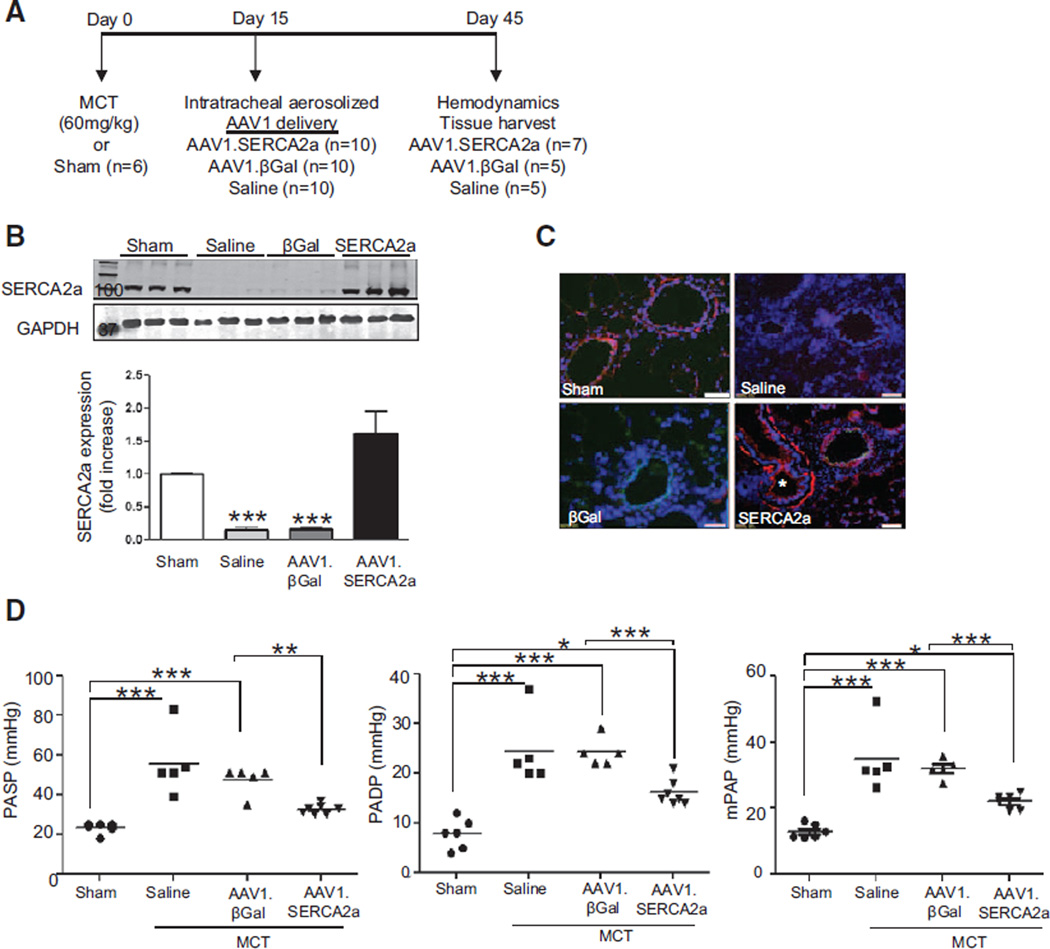
Having found that SERCA2a is downregulated in PAH, we next evaluated the effect of targeted vascular gene transfer of SERCA2a on pulmonary hemodynamics and vascular remodeling in the rat MCT model of PAH. This model develops elevated pulmonary pressures with evidence of pulmonary vascular remodeling 15 days after a single injection of MCT.16–19 To determine the therapeutic efficacy of forced pulmonary vascular SERCA2a expression in a model of established PAH, rats received a single subcutaneous injection of MCT and 15 days later were randomly assigned to receive intratracheal aerosolized saline, AAV1 carrying LacZ encoding for β-galactosidase (AAV1.βGal) or AAV1.SERCA2a. Sham rats that were injected with vehicle only served as the controls (Figure 3A).
Figure 3.
Intratracheal delivery of aerosolized AAV1.SERCA2a improves pulmonary hemodynamics in the rat MCT model of PAH. A, Schematic of the treatment protocol to assess the therapeutic efficacy of AAV1.SERCA2a gene therapy when given after PAH is established. B, SERCA2a protein expression in lung homogenates from the sham (n=6) and MCT-PAH rats treated with aerosolized saline (n=5), AAV1.βGal (n=5), or AAV1.SERCA2a (n=7) was examined by Western blot. GAPDH was used as a loading control. A representative blot (n=3) is shown. ***P<0.001 vs saline, AAV1.SERCA2a. C, SERCA2a expression (red) in pulmonary arterioles was detected by immunofluorescence. Nuclei were counterstained with DAPI (blue). * (lower right) refers to airway with visible SERCA2a expression in the smooth muscle layer. Scale bar, 100 µm. D, The effect of AAV1.SERCA2a gene therapy on pulmonary artery systolic pressure (PASP), diastolic pressure (PADP), and mean pulmonary pressure (mPAP) was measured invasively at day 45. *P<0.04 vs sham or AAV1.βGal, **P<0.004 vs AAV1.βGal, ***P<0.001 vs sham or AAV1.βGal. DAPI indicates 4',6-diamidino-2-phenylindole; MCT, monocrotaline; and PAH, pulmonary arterial hypertension.
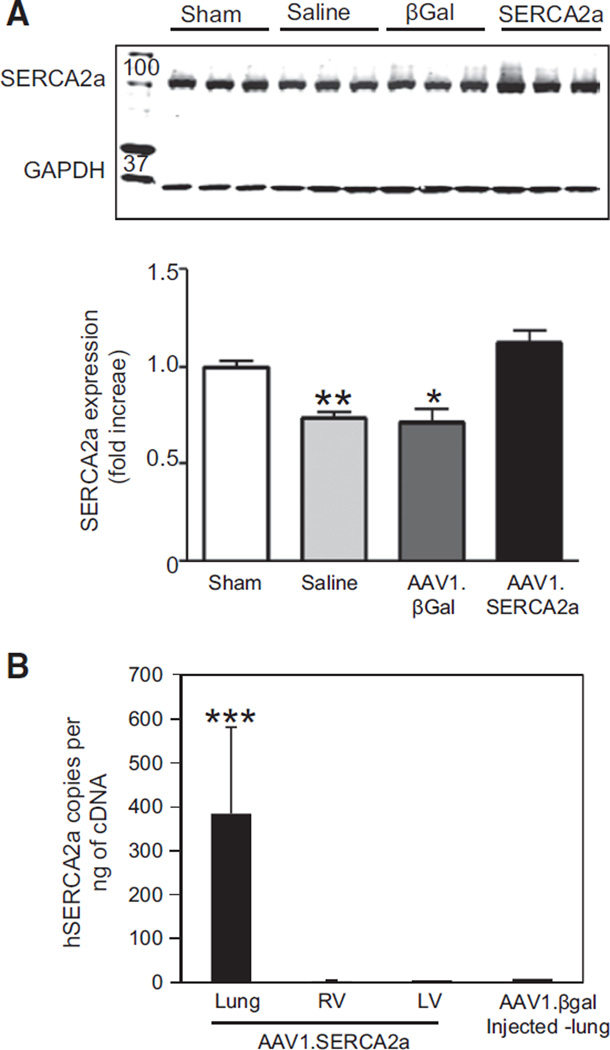
We first assessed the efficiency of AAV1-mediated gene transfer by performing X-Gal staining to corroborate transduction in lung tissue harvested 30 days after the intratracheal delivery of AAV1.βGal in MCT rats. β-Galactosidase was expressed abundantly throughout the intima and media of small pulmonary arteries, indicating successful gene transfer to the pulmonary vessels by using AAV1 and aerosolized intratracheal delivery. β-Galactosidase was also detected in bronchial smooth muscle cells (Figure IIIA in the online-only Data Supplement). We also examined SERCA2a mRNA levels and protein expression in the lungs at the end of the 30-day treatment period; human SERCA2a mRNA levels were detected only in MCT-PAH rats randomly assigned to aerosolized AAV1.SERCA2a (Figure IIIB in the online-only Data Supplement). Next, SERCA2a protein levels were assessed in lung homogenates by using an antibody that recognized both the human and the rat SERCA2a isoform. SERCA2a expression was downregulated in MCT-PAH rats treated with aerosolized saline or AAV1.βGal but was highly expressed in AAV1.SERCA2a-treated MCT-PAH rats (Figure 3B). These findings were confirmed by immunofluorescent staining of remodeled pulmonary arteries. SERCA2a expression was downregulated in MCT-PAH rats treated with saline or AAV1.βGal but detectable in rats treated with AAV1.SERCA2a (Figure 3C). These findings confirm that SERCA2a is downregulated in pulmonary vessels from rats with MCT-induced PAH, and intratracheal aerosol delivery of AAV1.SERCA2a is sufficient to transduce pulmonary vessels and increase pulmonary arteriole SERCA2a expression.
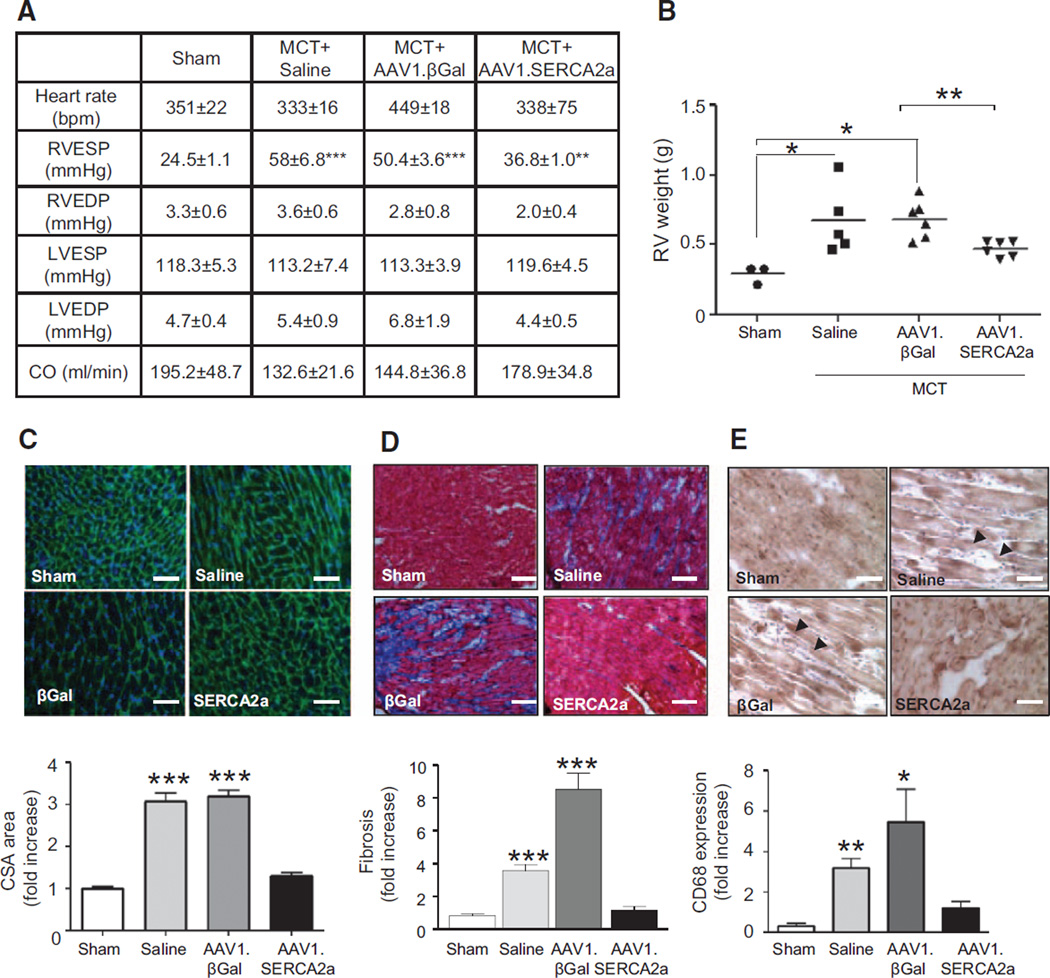
To determine the effect of increased pulmonary vascular SERCA2a expression on pulmonary hemodynamics, we measured pulmonary artery pressures on day 45. In comparison with the sham rats, MCT-PAH rats treated with aerosolized saline or AAV1.βGal demonstrated increased pulmonary artery systolic, pulmonary artery diastolic, and mean pulmonary artery pressures consistent with PAH. By contrast, pulmonary artery systolic, pulmonary artery diastolic, and mean pulmonary artery pressures were decreased in MCT-PAH rats treated with aerosolized AAV1.SERCA2a (Figure 3D). In addition, although there was an increase in pulmonary vascular resistance in the saline- and AAV1.βGal-treated MCT rats in comparison with the sham controls (0.03±0.01 versus 0.24±0.01 versus 0.18±0.03 mm Hg×min/ mL, P<0.001), treatment with AAV1.SERCA2a decreased pulmonary vascular resistance (0.07±0.01 mm Hg×min/mL, P<0.001 versus saline, AAV1.βGal) to levels observed in sham rats. These findings indicate that a reduction in pulmonary pressures in AAV1.SERCA2a-treated MCT-PAH rats is an effect of SERCA2a and not the viral gene transduction process, because pulmonary pressures remained elevated in AAV1.βGal-treated MCT-PAH rats.
SERCA2a Gene Transfer Limits Pulmonary Artery Remodeling in PAH
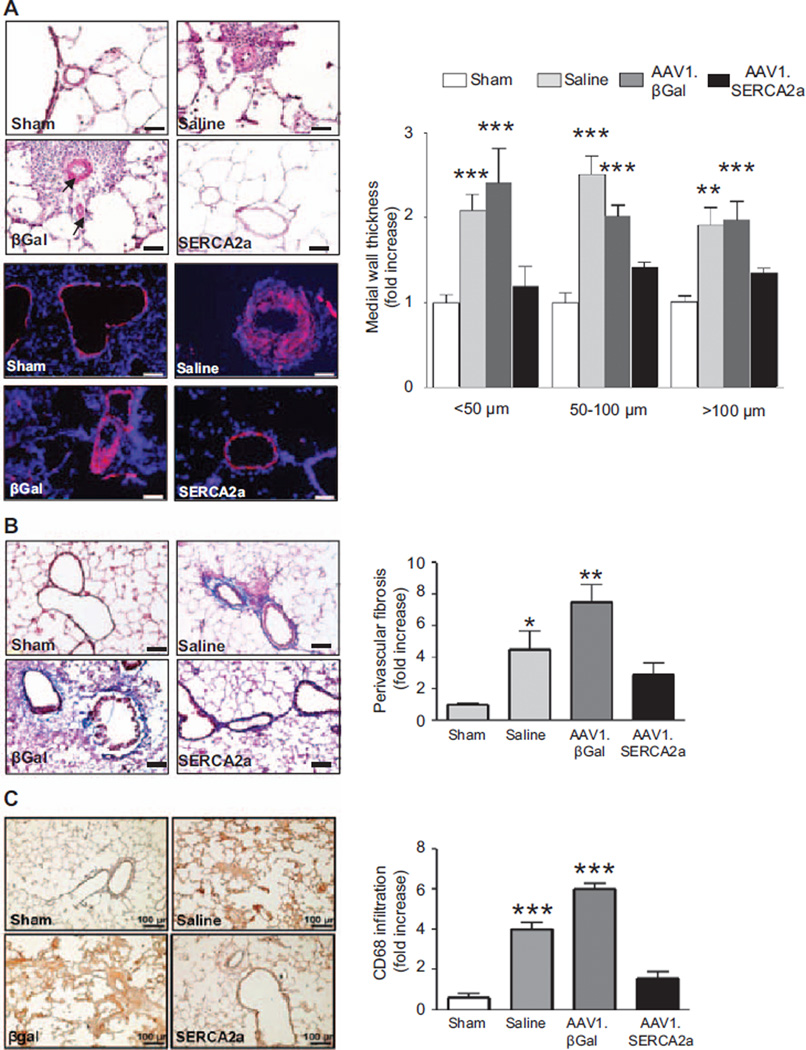
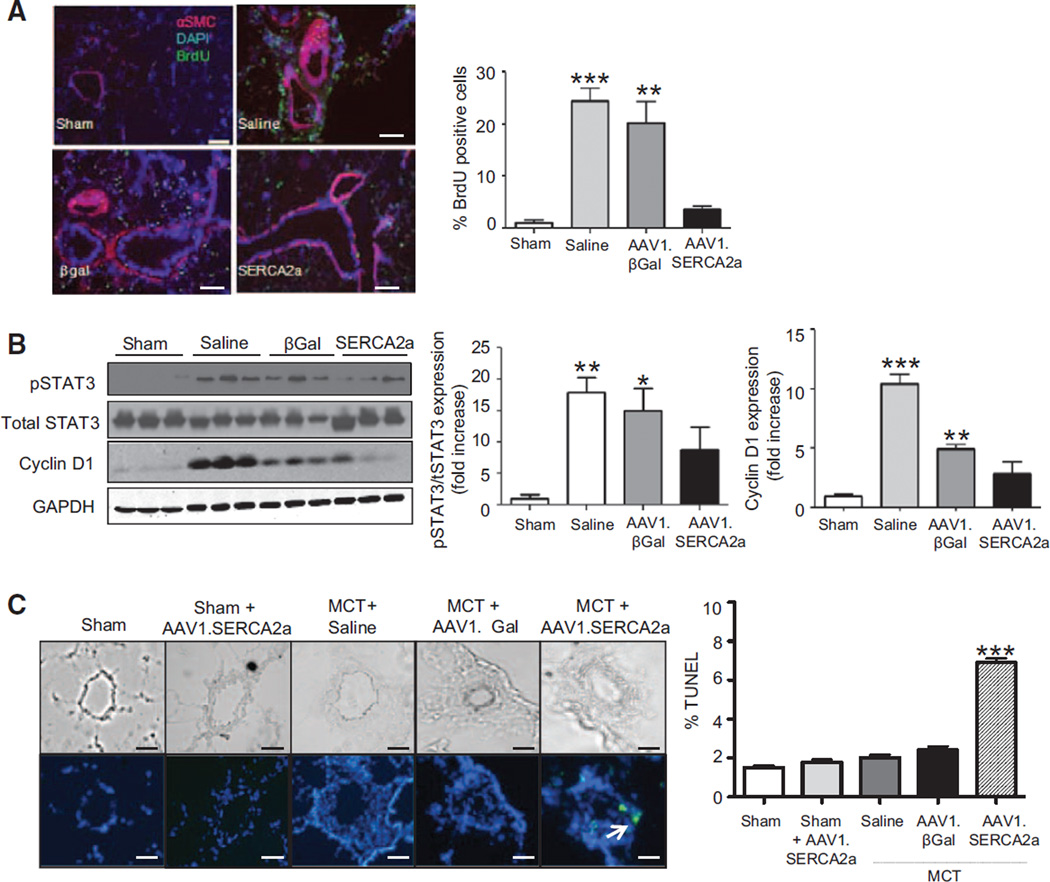
Vascular gene transfer of SERCA2 in MCT-PAH rats also reversed or limited pulmonary vascular remodeling in comparison with the saline- or AAV1.βGal-treated groups. Morphometric analysis of distal pulmonary arteries demonstrated a significant increase in medial thickness in both saline-and AAV1.βGal-treated MCT-PAH rats in comparison with sham controls, regardless of vessel diameter (small <50 µm, medium 51–100 µm, or large >100 µm). By contrast, vessel hypertrophy was attenuated significantly in the SERCA2a-treated animals, resulting in a decrease in the number of muscularized small vessels of all sizes in comparison with the control groups. This finding was confirmed by demonstrating a decrease in the presence of α-smooth muscle actin–positive cells in the medial layer of vessels from AAV1.SERCA2a-treated MCT-PAH rats in comparison with the other treatment groups (Figure 4A).
Figure 4.
AAV1.SERCA2a gene therapy decreases pulmonary artery hypertrophy. A, Lung sections from sham (n=6) and MCT-PAH rats treated with aerosolized saline (n=5), AAV1.βGal (n=5), or AAV1. SERCA2a (n=7) were stained with hematoxylin and eosin and distal pulmonary arterioles were examined by microscopy for morphometric analysis and assessment of medial thickness (top left). Sections were also immunostained with α-smooth muscle cell actin to confirm medial hypertrophy (bottom left). Representative images are shown (n=5 sections per rat). Scale bar, 100 µm. Morphometric analysis was used to categorize vessels as small (<50 µm), medium (51–100 µm), or large (>100 µm; right). **P<0.005 vs sham, AAV1.SERCA2a, ***P<0.001 vs sham, AAV1.SERCA2a. B, Perivascular fibrosis was examined in lung sections stained with Masson trichrome to visualize collagen deposition. Representative images are shown (n=3 sections per rat). Scale bar, 100 µm. *P<0.02 vs sham, AAV1.SERCA2a, **P<0.005 vs sham, AAV1.SERCA2a. C, Inflammatory cell infiltrate was visualized by CD68 immunostaining to identify monocytes/macrophage infiltrates in lung tissue from sham (n=3) or MCT-PAH rats treated with saline (n=3), AAV1-βGal (n=3), or AAV1-SERCA2a (n=4). Representative photomicrographs are shown (n=3). Scale bar, 100 µm. ***P<0.0001 vs sham, AAV1.SERCA2a. MCT indicates monocrotaline; and PAH, pulmonary arterial hypertension.
Pulmonary arteriole perivascular collagen deposition and fibrosis was increased in saline- and AAV1.βGal-treated MCT rats, whereas treatment with AAV1.SERCA2a in MCT-PAH rats decreased the profibrotic response (Figure 4B). SERCA2a gene transfer was also associated with a decrease in the infiltration of inflammatory cells in the MCT-injured lungs. Immunohistochemistry for the macrophage/monocyte marker CD68 revealed a marked infiltration of macrophages/monocytes in lung samples from the saline- and AAV1.βGal-treated groups in comparison with sham rats, with decreased recruitment of macrophages in AAV1.SERCA2a-treated MCT-PAH rats (Figure 4C).
SERCA2a Overexpression Inhibits PASMC Proliferation and Apoptosis Resistance In Vivo
Our in vitro studies demonstrated that forced expression of SERCA2a inhibited PASMC proliferation. To determine if SERCA2a-mediated inhibition of proliferation limited pulmonary artery remodeling in PAH, we next examined proliferation in vivo. Vascular gene transfer of SERCA2a decreased the number of proliferating PASMCs identified as bromodeoxyuridine-positive cells in the medial layer of remodeled pulmonary arteries (Figure 5A). Consistent with the decrease in PASMC proliferation, treatment with AAV1.SERCA2a in MCT-PAH rats decreased phosphorylation and activation of STAT3 and downregulated expression of Cyclin D1 in comparison with what was observed in saline- and AAV1.βGal-treated MCT-PAH rats (Figure 5B). Treatment with AAV1.SERCA2a also restored eNOS expression levels to those observed in sham controls (Figure IV in the online-only Data Supplement) suggesting that improved endothelial function may also participate in inhibiting PASMC proliferation in vivo.
Figure 5.
AAV1.SERCA2a decreases proliferation and increases apoptosis in pulmonary arterioles. A, Lung sections from sham (n=6) and MCT-PAH rats treated with aerosolized saline (n=5), AAV1.βGal (n=5), or AAV1.SERCA2a (n=7) were immunostained for bromodeoxyuridine (BrdU; green) in the vessel wall of pulmonary arterioles. Sections were counterstained with anti–smooth muscle cell α-actin (αSMC; red) and DAPI (blue). Representative images are shown (n=3 per rat). Scale bar, 100 µm. **P<0.005 vs sham, AAV1.SERCA2a, ***P<0.001 vs sham, AAV1.SERCA2a. B, Cyclin D1, phosphorylated STAT3, and total STAT3 levels were examined in lung homogenates by Western immunoblotting. GAPDH was used as a loading control. Representative blots are shown (n=3). *P<0.02 vs sham, AAV1.SERCA2a, **P=0.003 vs sham, AAV1.SERCA2a, ***P<0.001 vs sham, AAV1.SERCA2a. C, Apoptosis was evaluated by TUNEL staining of pulmonary arterioles. TUNEL-positive nuclei are green. Nuclei are counterstained with DAPI (blue). Representative images are shown (n=3). Scale bar, 100 µm. The % TUNEL positive cells was determined for 10 vessels per section, 12 sections per animal. ***P<0.001 vs sham, sham+AAV1.SERCA2a, saline, AAV1.βGal. DAPI indicates 4',6-diamidino-2-phenylindole; MCT, monocrotaline; PAH, pulmonary arterial hypertension; and TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
In PAH, pulmonary vascular remodeling is characterized by apoptosis-resistant smooth muscle cells that contribute to vessel hypertrophy.20 We therefore next sought to determine if SERCA2a overexpression decreased the number of medial smooth muscle cells by promoting apoptosis. Using an in situ terminal deoxynucleotidyl transferase dUTP nick end labeling assay, we observed that the number of terminal deoxynucleotidyl transferase dUTP nick end labeling–positive cells was significantly higher in AAV1.SERCA2a-treated MCT-PAH rats than in animals treated with saline, AAV1.βGal, and the sham control group. Interestingly, SERCA2a overexpression in sham rats by delivery of AAV1.SERCA2a did not induce apoptosis in normal distal pulmonary arteries (Figure 5C). This indicates that SERCA2a overexpression modified pulmonary vascular remodeling by increasing apoptosis in a limited number of proliferating cells in addition to directly inhibiting proliferation of pulmonary vascular cells.
Right Ventricular Remodeling in PAH Is Improved by Vascular SERCA2a Gene Transfer
In PAH, elevated pulmonary vascular pressures result in right ventricular (RV) hypertrophy and ultimately the development of right heart failure. Because it is known that SERCA2a expression is downregulated in the left ventricle (LV) in heart failure,18,19 we first examined SERCA2a protein expression in RV tissue homogenates. Immunoblot analysis showed a significant downregulation of SERCA2a in the RV of saline- or AAV1.βGal-treated rats in comparison with the sham control group. By contrast, SERCA2a expression in the RV of MCT rats treated with AAV1.SERCA2a was not downregulated and was similar to that observed in sham controls (Figure 6A). SERCA2a levels in the LV were similar in the sham control and MCT-PAH rats treated with saline, AAV1.βGal, or AAV1.SERCA2a (Figure VA in the online-only Data Supplement).
Figure 6.
SERCA2a expression in the RV in PAH. A, The RV was harvested from sham (n=6) and MCT-PAH rats treated with aerosolized saline (n=5), AAV1.βGal (n=5), or AAV1.SERCA2a (n=7), and homogenates were used to examine SERCA2a expression by Western blotting. A representative blot is shown (n=3). *P<0.02 vs sham, AAV1.SERCA2a, **P=0.003 vs sham, AAV1.SERCA2a. B, Levels of human SERCA2a and viral genome copies were assessed in the rat RV, LV, and lungs to determine the specificity of AAV1.SERCA2a gene transfer to lungs with aerosol delivery (n=3). ***P<0.001 vs RV, LV, and AAV1.βGal-injected lung. LV indicates left ventricle; MCT, monocrotaline; PAH, pulmonary arterial hypertension; and RV, right ventricle.
Next, we assessed the distribution of SERCA2a gene transduction by aerosolized AAV1.SERCA2a to determine if RV SERCA2a expression was altered following AAV1.SERCA2a treatment by off-target gene transfer. We analyzed viral genome copies in tissue samples from the lung, RV, and LV in MCT-PAH rats treated with aerosolized AAV1.SERCA2a; AAV1.βGal-treated MCT-PAH rats served as the control. The number of exogenous SERCA2a genome copies (specific to the SERCA2a in the AAV1 vector) in the lung tissue samples was increased in AAV1.SERCA2a-treated MCT-PAH rats in comparison with the AAV1.βGal group. There was no viral genome detected in the RV or LV of the AAV1.SERCA2a-treated group, demonstrating the specificity of the intratracheal aerosol delivery method for the lung and that RV SERCA2a expression in AAV1.SERCA2a-treated rats did not result from transduction of RV cardiomyocytes (Figure 6B).
SERCA2a gene transfer also had beneficial effects on RV hemodynamics. In comparison with sham rats, there was a significant increase in RV systolic pressure in the saline- (24.5±1 versus 58±6.8 mm Hg, P<0.001) and AAV1.βGal-treated MCT-PAH rats (50.4±3.6 mm Hg, P<0.001) in comparison with sham controls. In AAV1.SERCA2a-treated rats, RV systolic pressure was not significantly different from that observed in the sham controls (Figure 7A, online-only Data Supplement Figure VA). There were no differences between treatment groups in the RV diastolic pressure or the LV systolic and diastolic pressures (Figure 7A, @@Figure VB through VE in the online-only Data Supplement). These findings support our hypothesis that intratracheal delivery of aerosolized AAV1.SERCA2a restores vascular SERCA2a expression to limit pulmonary vascular remodeling and pulmonary hypertension to decrease the pressure load on the RV and prevent RV remodeling.
Figure 7.
AAV1.SERCA2a improves RV hemodynamics and decreases RV hypertrophy and fibrosis. A, Cardiac hemodynamics were measured invasively in sham (n=6) and MCT-PAH rats treated with aerosolized saline (n=5), AAV1.βGal (n=5), or AAV1.SERCA2a (n=7) at day 45. ***P<0.001 vs sham, AAV1.SERCA2a. **P<0.005 vs AAV1.βGal. B, RV hypertrophy was determined by the RV/LV+septum weight ratio (Fulton index). *P<0.04 vs sham, AAV1.SERCA2a, **P=0.006 vs AAV1.βGal. C, RV sections were stained with fluorescence-tagged wheat germ agglutinin to examine RV cardiomyocyte cross-sectional area (CSA; n=3 per animal). ***P<0.0001 vs sham, AAV1.SERCA2a. D, Collagen deposition and fibrosis was examined in RV tissue sections by using Masson trichrome stain (n=3 per animal). ***P<0.001 vs sham, AAV1.SERCA2a. E, Infiltration of the RV by macrophages/monocytes was evaluated by CD68-immunostaining (n=3 per animal). Representative images are shown. Scale bar, 100 µm. *P=0.04 vs sham, AAV1.SERCA2a **P=0.002. sham, AAV1.SERCA2a. LV indicates left ventricle; MCT, monocrotaline, PAH, pulmonary arterial hypertension; and RV, right ventricle.
Right ventricular hypertrophy, assessed by RV/LV + septum weight, was present in MCT-PAH rats treated with saline or AAV1.βGal in comparison with sham control rats. MCT-PAH rats treated with AAV1.SERCA2a, however, had a significant reduction in RV hypertrophy (Figure 7B). Similarly, RV cardiomyocyte cross-sectional area was enlarged in saline- or AAV1.βGal-treated rats in comparison with sham controls, a finding that was not observed in AAV1.SERCA2a-treated rats (Figure 7C). RV hypertrophy was associated with an increase in RV collagen content in saline- and AAV1.βGal-treated MCT-PAH rats in comparison with sham rats but not in the AAV1.SERCA2a-treated group (Figure 7D). There was also evidence of increased RV perivascular and interstitial inflammatory cell infiltrates and CD68+ cells in the saline- and AAV1.βGal-treated groups, as well, in comparison with sham rats or the AAV1.SERCA2a-treated group (Figure 7E).
Prevention of MCT-Induced PAH by Intratracheal Delivery of AAV1.SERCA2a
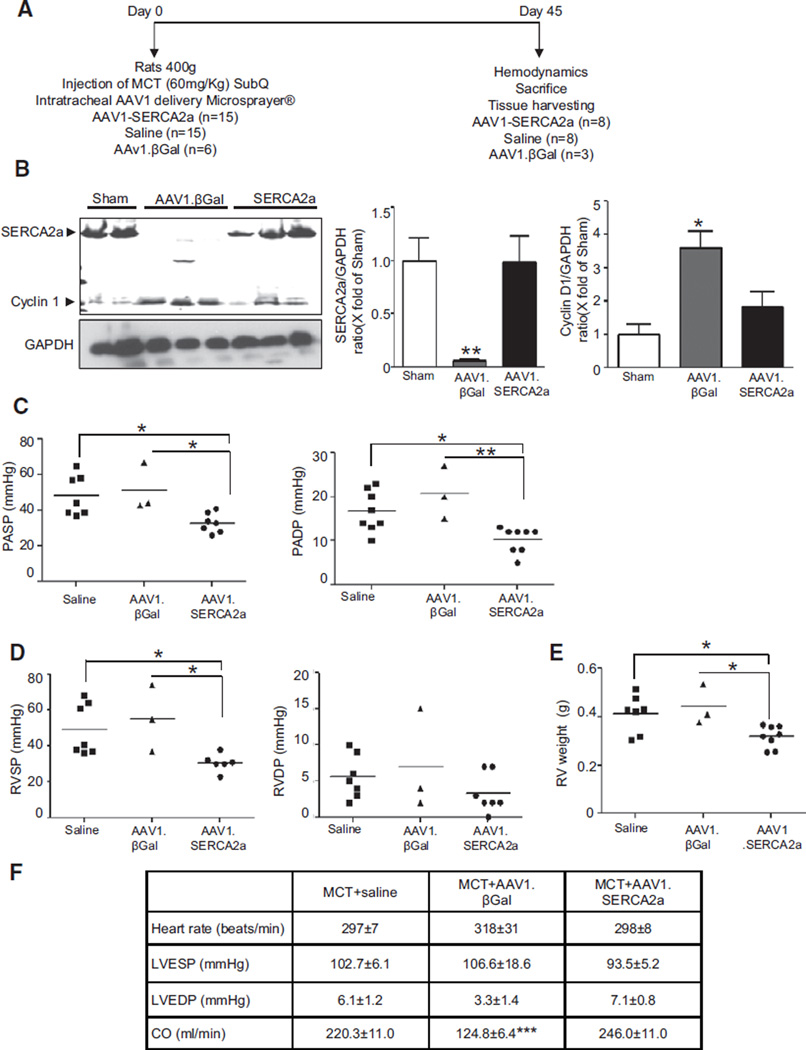
Given that AAV1.SERCA2a had therapeutic efficacy in established PAH, we also sought to determine whether gene transfer of SERCA2a via AAV1.SERCA2a could prevent the development of PAH. To examine this hypothesis, we administered aerosolized saline, AAV1.βGal, or AAV1.SERCA2a immediately following injection with MCT (Figure 8A). After 45 days, as expected, pulmonary SERCA2a expression was decreased in MCT-PAH rats treated with AAV1.βGal in comparison with sham rats; however, SERCA2a was not downregulated in MCT-PAH rats treated with AAV1. SERCA2a. This was associated with a concomitant decrease in pulmonary Cyclin D1 expression consistent with a decrease in cell proliferation (Figure 8B). Hemodynamic assessment demonstrated that treatment with AAV1.SERCA2a prevented an increase in pulmonary artery systolic and diastolic filling pressures (Figure 8C). Treatment with AAV1.SERCA2a also prevented the increase in pulmonary vascular resistance observed in AAV1.βGal-treated rats (0.25±0.05 versus 0.08±0.01 mm Hg×min/mL, P<0.001). Similarly, treatment with AAV1.SERCA2a prevented RVH as evidenced by a decrease in RV weight and RV systolic pressure in comparison with the saline- or AAV1.βGal-treated groups (Figure 8D and 8E). As noted in the treatment protocol, there was no difference between treatment groups with respect to LV filling pressures or weight (Figure VI in the online-only Data Supplement). Thus, a single intratracheal injection of aerosolized AAV1.SERCA2a can prevent PAH in the MCT-PAH rat model.
Figure 8.
AAV1.SERCA2a prevents the development of PAH. A, Schematic of the protocol to assess the role of AAV1.SERCA2a gene therapy in the prevention of MCT-induced PAH. On day 0, rats were injected with MCT and coadministered aerosolized saline (n=15), AAV1.βGal (n=6), or AAV1.SERCA2a (n=15). B, SERCA2a and Cyclin D1 protein expression was examined by Western blotting in lung homogenates (n=3). Protein expression was normalized to GAPDH. Representative blots are shown. *P<0.04 vs saline, AAV1.SERCA2a, **P=0.008 vs saline, AAV1.SERCA2a. C, AAV1.SERCA2a limited the increase in pulmonary artery systolic (PASP) and diastolic (PADP) pressures observed in control group rats. Hemodynamics were measured invasively at day 45. *P<0.04 vs saline, AAV1.SERCA2a, **P=0.002 vs AAV1.SERCA2a. D, SERCA2a overexpression also limited the increase in RV systolic (RVSP) and diastolic (RVDP) pressures at day 45. *P<0.03 vs saline, AAV1. SERCA2a. E, RV hypertrophy was determined by the RV/LV+septum weight ratio (Fulton index). *P<0.02 vs saline, AAV1.SERCA2a. F, SERCA2a overexpression had no effect on LV systolic (LVSP) and diastolic (LVEDP) pressures or cardiac output (CO). ***P<0.001 vs MCT+saline, MCT+AAV1.SERCA2a. LV indicates left ventricle; MCT, monocrotaline, PAH, pulmonary arterial hypertension; and RV, right ventricle.
Discussion
This study provides the first evidence that SERCA2a protein expression levels are downregulated in lung samples from patients with PAH and in cultured proliferating human PASMCs and PAECs. Similarly, in the rat MCT model of PAH, we observed a decrease in pulmonary vascular SERCA2a protein expression 45 days after MCT injection. Impaired SERCA2a activity resulting from a decrease in SERCA2a expression may therefore be implicated in the pathogenesis of PAH. Herein, we show that a single intratracheal delivery of aerosolized AAV1.SERCA2a has therapeutic efficacy in PAH. Four weeks after gene transfer, the SERCA2a mRNA transcript was still detectable in the pulmonary tissue of animals transduced with SERCA2a, demonstrating persistent transgene expression. In addition, AAV1.SERCA2a gene transfer reduced the severity of pulmonary vascular remodeling accompanied by anti-inflammatory and antifibrotic effects. Pulmonary vascular gene transfer of AAV1.SERCA2a also diminished RV hypertrophy and fibrosis and prevented the decrease in RV SERCA2a expression seen in control animals with RVH and failure, likely as a result of the reduction in right heart load.
In PAH, hypertensive pulmonary vascular disease and pulmonary artery hypertrophy is characterized by proliferation and migration of both PAECs and PASMCs, which is associated with downregulation of SERCA2a in the systemic vasculature.21 In our study, gene transfer of SERCA2a to restore or maintain SERCA2a levels in proliferating cells exerted beneficial effects by inhibiting the activation of the STAT3-NFAT pathway and, thereby, cell proliferation that resulted in near-normal vessel morphology. This is consistent with our previous reports that indicated that SERCA2a overexpression inhibits human coronary artery smooth muscle cell proliferation and migration by restoring Ca2+ homeostasis and increasing the rate of Ca2+-store refilling to inhibit store-operated channel stimulation of protein phosphatase 2B/NFAT signaling.7 This phenomenon may be supported by the observation that SERCA2a is localized near the nucleus in PASMCs, suggesting that its location may favor a regulatory role for nuclear trafficking of transcription factors such as STAT3 and NFAT.22 Other studies have found that the SERCA2 expression level was diminished in the airway smooth muscle cells from patients with moderately severe asthma, and that SERCA2a downregulation contributed to the airway secretory and hyperproliferative phenotype.23
Dysregulated proliferation of PASMCs may also be driven by a decrease in PAEC eNOS expression and activation. Our findings confirm previous reports that described a decrease in eNOS expression in MCT-PAH rats and are in accordance with observations in the lung tissue of PAH patients in comparison with control subjects.24,25 It is plausible that decreased pulmonary vascular eNOS expression may contribute to pulmonary vasoconstriction and to the excessive medial hypertrophy observed in PAH. In this study, SERCA2a gene transfer restored eNOS expression in pulmonary arteries in comparison with what was observed in controls. This finding is also consistent with our previous study that demonstrated that intracoronary AAV1.SERCA2a gene transfer increased endothelial cell eNOS protein expression and activity and improved coronary blood flow in a preclinical porcine model of heart failure.26 Taken together, these observations suggest that SERCA2a gene transfer may play an important role in the control of pulmonary vascular cell proliferation and vessel remodeling.
Recombinant AAV vectors have attracted much interest for clinical gene therapy owing to their safety profile (no known pathology has been found to be associated with AAV in humans), broad tissue tropism, and, more importantly, prolonged gene expression without the integration of their DNA into the host chromosome.27–30 These vectors have been shown to exhibit fewer inflammatory and immune reactions than the adenoviruses. In this study, we used an AAV1 vector based on our previous work that demonstrated effective and efficient transduction of endothelial and smooth muscle cells of coronary arteries in vitro and in vivo and long-term expression of our therapeutic protein in heart tissue in human failing hearts.26,28,31 This feature of AAV vectors may be advantageous in investigating the effects of transgenes on chronic disease processes such as PAH.
Because AAV1 has tropism for the heart (cardiomyocytes), and the endothelium and vascular smooth muscle, as well, and we were interested in gene transfer only to the pulmonary vessels, we rationalized that local delivery of AAV1. SERCA2a via a single aerosolized intratracheal instillation would limit off-target transduction of the heart. The use of intratracheal aerosol delivery for pulmonary gene transfer has been described previously with the use of adenovirus.32 The use of recombinant adenovirus, however, is limited by the poor long-term expression of the transgene with a peak in expression after 7 days and loss of transgene expression by 21 days, making adenovirus an inadequate vector platform to trial therapeutic gene transfer in experimental PAH. However previous clinical studies have demonstrated that repeated aerosolized delivery of AAV1 encoding the human cystic fibrosis transmembrane regulator to the lungs of subjects with cystic fibrosis was safe and well tolerated.33 There are few experimental studies that have trialed AAV for therapeutic gene transfer in pulmonary hypertension; only 2 previous studies used an intranasal or intratracheal aerosol route for AAV delivery in PAH models.34,35 In these studies, however, the AAV serotype was not divulged, and, therefore, it is difficult to interpret the findings reported without this information. Other studies that have performed gene transfer of AAV1 or AAV2 in rodent models of PAH have used an intramuscular injection delivery approach.36–38,39,40 In these studies, there is likely off-target gene transfer throughout the cardiovascular system, and it would be difficult to determine if favorable pulmonary hemodynamics resulted from an improvement in pulmonary vascular remodeling or direct transduction of the RV.
Although gene transfer of SERCA2a to the RV was not an aim of this study, it is plausible that future therapeutic interventions in established PAH with RV failure may combine directed pulmonary vascular and RV transduction. It has been shown that, in isolated cardiomyocytes and animal models of left heart failure, restoring SERCA2a expression by gene transfer corrects contractile abnormalities, improves energetic and electric remodeling, and improves hemodynamic function along with survival in rodent and large-animal models of heart failure.41,42 The overall beneficial profile of SERCA2a targeting has led to the initiation and successful completion of a first-in-human gene therapy trial (Calcium Up-Regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease [CUPID]) to investigate the effects of AAV1-SERCA2a gene transfer in patients with heart failure.28–30
In conclusion, our study demonstrates that selective pulmonary vascular gene transfer of SERCA2a with aerosolized AAV1.SERCA2a modulates hypertrophic vascular remodeling in established PAH to lower pulmonary pressures and adverse right ventricular remodeling. Furthermore, maintaining pulmonary vascular SERCA2a expression prevents pulmonary arterial remodeling to prevent the development of PAH. Our findings have important clinical implications, as at the time of PAH diagnosis, the majority of patients have already developed some form of pathological pulmonary arterial remodeling, which, based on our findings, correlates with a marked downregulation of SERCA2a expression. Therefore, in this setting, and supported by an already successful application in patients with heart failure and clinical feasibility of aerosolized AAV1, maintaining or restoring SERCA2a expression by AAV1.SERCA2a gene transfer during the pathogenesis of PAH or once PAH is established holds promise as a therapeutic approach for the disease.
Supplementary Material
Clinical Perspective.
Pulmonary arterial hypertension (PAH) is characterized by dysregulated pulmonary vascular remodeling that leads to increased pulmonary vascular resistance, right ventricular hypertrophy, and, ultimately, uncompensated right heart failure and death. In the systemic circulation, vascular injury is associated with downregulation of the cardiac isoform of the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), which, in turn, stimulates vascular smooth muscle cell proliferation and vessel remodeling. In the present study, we examined the role of SERCA2a in vascular remodeling in PAH and the therapeutic potential of selective pulmonary SERCA2a gene transfer. We found that SERCA2a expression was decreased in hypertrophied pulmonary arterioles from patients with PAH and in the rat monocrotaline model of PAH. In pulmonary vascular smooth muscle cells, we showed that gene transfer of SERCA2a decreased proliferation by regulating nuclear factor of activated T cells and signal transducer and activator of transcription-3 signaling. We also found that selective pulmonary SERCA2a gene transfer with the use of aerosolized adeno-associated virus serotype 1 (AAV1.SERCA2a) reversed established pulmonary hypertension by improving pulmonary vascular remodeling, lowering pulmonary artery pressures, and decreasing right ventricular hypertrophy. Pulmonary SERCA2a gene transfer also prevented the development of PAH in the rat monocrotaline model. The present study suggests that selective pulmonary gene transfer of SERCA2a with the use of AAV1.SERCA2a, which is already in clinical trials for the treatment of congestive heart failure, may be a novel therapeutic approach to limit adverse pulmonary vascular and right ventricular remodeling associated with PAH. The use of aerosolized AAV1.SERCA2a represents a novel strategy for the treatment of a disease state that has few therapeutic options.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants K01HL103176 (to Dr Hadri), K08111207 (to Dr Maron), R01 HL078691, HL057263, HL071763, HL080498, and HL083156 (to Dr Hajjar), and R01 HL105301 (to Dr Leopold).
Drs Hajjar and Zsebo have ownership interest in Celladon Corporation, which is developing AAV1.SERCA2a for the treatment of heart failure. Drs Hajjar, Kawase, and Ladage hold intellectual property around SERCA2a gene transfer as a treatment modality for PAH. Dr Maron receives funding from Gilead Sciences, Inc. to study experimental pulmonary hypertension.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.113.001585/-/DC1.
Disclosures
The other authors report no conflicts.
References
- 1.McLaughlin VV. Looking to the future: a new decade of pulmonary arterial hypertension therapy. Eur Respir Rev. 2011;20:262–269. doi: 10.1183/09059180.00006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies RJ, Morrell NW. Molecular mechanisms of pulmonary arterial hypertension: role of mutations in the bone morphogenetic protein type II receptor. Chest. 2008;134:1271–1277. doi: 10.1378/chest.08-1341. [DOI] [PubMed] [Google Scholar]
- 3.Voelkel NF, Cool C. Pathology of pulmonary hypertension. Cardiol Clin. 2004;22:343–51. v. doi: 10.1016/j.ccl.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Rubin LJ, Simonneau G, Badesch D, Galiè N, Humbert M, Keogh A, Massaro J, Matucci Cerinic M, Sitbon O, Kymes S. The study of risk in pulmonary arterial hypertension. Eur Respir Rev. 2012;21:234–238. doi: 10.1183/09059180.00003712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompré AM. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 6.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I, Liang L, Limon I, Lompré AM, Hatem SN, Hajjar RJ, Lipskaia L. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol. 2011;50:621–633. doi: 10.1016/j.yjmcc.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagunas L, Clipstone NA. Deregulated NFATc1 activity transforms murine fibroblasts via an autocrine growth factor-mediated Stat3-dependent pathway. J Cell Biochem. 2009;108:237–248. doi: 10.1002/jcb.22245. [DOI] [PubMed] [Google Scholar]
- 10.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Côté J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–565. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- 12.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aramburu J, Garcia-Cózar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 14.Aramburu J, Yaffe MB, López-Rodríguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 15.Karakikes I, Hadri L, Rapti K, Ladage D, Ishikawa K, Tilemann L, Yi GH, Morel C, Gwathmey JK, Zsebo K, Weber T, Kawase Y, Hajjar RJ. Concomitant intravenous nitroglycerin with intracoronary delivery of AAV1.SERCA2a enhances gene transfer in porcine hearts. Mol Ther. 2012;20:565–571. doi: 10.1038/mt.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126:963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol. 2006;291:H2424–H2430. doi: 10.1152/ajpheart.00369.2006. [DOI] [PubMed] [Google Scholar]
- 18.Schultze AE, Roth RA. Chronic pulmonary hypertension–the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Health B Crit Rev. 1998;1:271–346. doi: 10.1080/10937409809524557. [DOI] [PubMed] [Google Scholar]
- 19.Werchan PM, Summer WR, Gerdes AM, McDonough KH. Right ventricular performance after monocrotaline-induced pulmonary hypertension. Am J Physiol. 1989;256(5 Pt 2):H1328–H1336. doi: 10.1152/ajpheart.1989.256.5.H1328. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90:1291–1335. doi: 10.1152/physrev.00032.2009. [DOI] [PubMed] [Google Scholar]
- 21.Fishman AP, Fishman MC, Freeman BA, Gimbrone MA, Rabinovitch M, Robinson D, Gail DB. Mechanisms of proliferative and obliterative vascular diseases. Insights from the pulmonary and systemic circulations. NHLBI Workshop summary. Am J Respir Crit Care Med. 1998;158:670–674. doi: 10.1164/ajrccm.158.2.9803084. [DOI] [PubMed] [Google Scholar]
- 22.Clark JH, Kinnear NP, Kalujnaia S, Cramb G, Fleischer S, Jeyakumar LH, Wuytack F, Evans AM. Identification of functionally segregated sarcoplasmic reticulum calcium stores in pulmonary arterial smooth muscle. J Biol Chem. 2010;285:13542–13549. doi: 10.1074/jbc.M110.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, Snetkov VA, O’Connor BJ, Karner C, Cousins DJ, Macedo P, Chung KF, Corrigan CJ, Ward JP, Lee TH. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci U S A. 2009;106:10775–10780. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahara M, Sata M, Morita T, Hirata Y, Nagai R. Nicorandil attenuates monocrotaline-induced vascular endothelial damage and pulmonary arterial hypertension. PLoS One. 2012;7:e33367. doi: 10.1371/journal.pone.0033367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 26.Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F, Lebeche D, Kranias EG, Leopold JA, Lompré AM, Lipskaia L, Hajjar RJ. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther. 2010;18:1284–1292. doi: 10.1038/mt.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD., Jr Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, Ly H, Kawase Y, Wagner K, Borow K, Jaski B, London B, Greenberg B, Pauly DF, Patten R, Starling R, Mancini D, Jessup M. Design of a phase ½ trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S, Kapturczak M, Loiler SA, Zolotukhin S, Glushakova OY, Madsen KM, Samulski RJ, Hauswirth WW, Campbell-Thompson M, Berns KI, Flotte TR, Atkinson MA, Tisher CC, Agarwal A. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum Gene Ther. 2005;16:235–247. doi: 10.1089/hum.2005.16.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Jones JE, Beppu H, Keaney JF, Jr, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss RB, Milla C, Colombo J, Accurso F, Zeitlin PL, Clancy JP, Spencer LT, Pilewski J, Waltz DA, Dorkin HL, Ferkol T, Pian M, Ramsey B, Carter BJ, Martin DB, Heald AE. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- 34.Angelini DJ, Su Q, Kolosova IA, Fan C, Skinner JT, Yamaji-Kegan K, Collector M, Sharkis SJ, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PLoS One. 2010;5:e11251. doi: 10.1371/journal.pone.0011251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) induces the vascular and hemodynamic changes of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L582–L593. doi: 10.1152/ajplung.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito T, Okada T, Mimuro J, Miyashita H, Uchibori R, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Sakata Y, Shimada K, Ozawa K. Adenoassociated virus-mediated prostacyclin synthase expression prevents pulmonary arterial hypertension in rats. Hypertension. 2007;50:531–536. doi: 10.1161/HYPERTENSIONAHA.107.091348. [DOI] [PubMed] [Google Scholar]
- 37.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Shimada K, Ozawa K. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res. 2007;101:734–741. doi: 10.1161/CIRCRESAHA.107.153023. [DOI] [PubMed] [Google Scholar]
- 38.Kido M, Du L, Sullivan CC, Deutsch R, Jamieson SW, Thistlethwaite PA. Gene transfer of a TIE2 receptor antagonist prevents pulmonary hyperten-sion in rodents. J Thorac Cardiovasc Surg. 2005;129:268–276. doi: 10.1016/j.jtcvs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Kataoka M, Kawakami T, Tamura Y, Yoshino H, Satoh T, Tanabe T, Fukuda K. Gene transfer therapy by either type 1 or type 2 adeno-associated virus expressing human prostaglandin I2 synthase gene is effective for treatment of pulmonary arterial hypertension. J Cardiovasc Pharmacol Ther. 2013;18:54–59. doi: 10.1177/1074248412457046. [DOI] [PubMed] [Google Scholar]
- 40.Kawakami T, Kanazawa H, Satoh T, Ieda M, Ieda Y, Kimura K, Mochizuki H, Shimada T, Yokoyama C, Ogawa S, Tanabe T, Fukuda K. AAV-PGIS gene transfer improves hypoxia-induced pulmonary hypertension in mice. Biochem Biophys Res Commun. 2007;363:656–661. doi: 10.1016/j.bbrc.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 41.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Hadri L, Hajjar RJ. Calcium cycling proteins and their association with heart failure. Clin Pharmacol Ther. 2011;90:620–624. doi: 10.1038/clpt.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.