Abstract
Objective:
To investigate predictors of outcome in patients with all-cause encephalitis receiving care in the intensive care unit.
Methods:
A retrospective analysis of encephalitis cases at The Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center was performed. Using multivariate logistic regression analysis, we examined mortality and predictors of good outcome (defined as modified Rankin Scale scores of 1–3) and poor outcome (scores 4 and 5) in those surviving to hospital discharge.
Results:
In our cohort of 103 patients, the median age was 52 years (interquartile range 26), 52 patients (50.49%) were male, 28 patients (27.18%) had viral encephalitis, 19 (18.45%) developed status epilepticus (SE), 15 (14.56%) had cerebral edema, and 19 (18.45%) died. In our multivariate logistic regression analysis, death was associated with cerebral edema (odds ratio [OR] 18.06, 95% confidence interval [CI] 3.14–103.92), SE (OR 8.16, 95% CI 1.55–43.10), and thrombocytopenia (OR 6.28, 95% CI 1.41–28.03). Endotracheal intubation requirement with ventilator support was highly correlated with death (95%). In addition, in those patients who survived, viral, nonviral, and unknown causes of encephalitis were less likely to have a poor outcome at hospital discharge compared with an autoimmune etiology (viral encephalitis: OR 0.09, 95% CI 0.01–0.57; nonviral encephalitis: OR 0.02, 95% CI 0.01–0.31; unknown etiology: OR 0.18, 95% CI 0.04–0.91).
Conclusions:
Our study suggests that predictors of death in patients with encephalitis comprise potentially reversible conditions including cerebral edema, SE, and thrombocytopenia. Further prospective studies are needed to determine whether aggressive management of these complications in patients with encephalitis improves outcome.
Encephalitis is challenging to manage given the diversity of clinical and epidemiologic features. More than 100 infectious species have been identified as causative agents of meningoencephalitis, with a burgeoning of new infectious and autoimmune etiologies in the last decade. Despite advances in diagnosis, more than 50% of encephalitis cases remain cryptogenic, posing additional management challenges.1–3
Guidelines for management of encephalitis emphasize the role of targeted disease treatment with antimicrobial agents and anti-inflammatory treatment, as well as supportive care.4,5 Little is known of the contribution of supportive measures, nor of the adverse consequences of medical and neurologic complications, in those with encephalitis. Many patients with encephalitis are critically ill and require care in intensive care units (ICUs) for prolonged periods of time, and we therefore focused on this population to investigate predictors of death and outcome at hospital discharge in those who survived. To ensure broad applicability of our findings, we examined predictors of outcome in patients with encephalitis of all causes.
METHODS
Standard protocol approvals, registrations, and patient consents.
The Johns Hopkins University Institutional Review Board approved this study.
Study design.
We conducted a retrospective review of all patients with acute encephalitis presenting to The Johns Hopkins Hospital (JHH) and Johns Hopkins Bayview Medical Center (JHBMC), 2 medical centers in Baltimore, MD, between January 1997 and July 2011. We identified encephalitis cases within our database using ICD-9 diagnosis codes corresponding to encephalitis. Diagnoses were confirmed by neurologists' review of patient charts including physicians' notes, laboratory results, neuroimaging studies, and other supporting data.
Definitions.
Encephalitis was defined as a patient hospitalized with encephalopathy (defined by depressed or altered level of consciousness lasting 24 hours or more, lethargy, or personality change) with at least 2 of the following characteristics: fever, seizure, focal neurologic deficit, CSF pleocytosis (white blood cell [WBC] count >5 cells/mm3), and EEG or neuroimaging findings consistent with encephalitis.6 Active malignancy, HIV infection/AIDS, and use of chronic immunosuppressants defined immunocompromised state. Leukopenia was defined as WBC count <4,000/mm3 and thrombocytopenia by platelet count <100,000/mm3. Seizure activity was defined clinically or through EEG. Status epilepticus (SE) was defined as continuous seizure activity lasting longer than 5 minutes or recurrent seizures without regaining consciousness between seizures for more than 5 minutes.7
Inclusion/exclusion criteria.
Patients were included in this study if they met the definition of encephalitis, with a length of stay in an ICU of at least 48 hours during their hospital stay, and were older than 16 years. A minimum length of stay in the ICU of 48 hours was determined in order to exclude those who had only transient critical care needs. We included patients admitted to the JHH and JHBMC neurosciences critical care unit (NCCU). Patients with hospital stays in the medical ICU, coronary care unit, and surgical ICU with acute encephalitis were also included and designated as being in “other” ICUs. Patients were excluded if they had a diagnosis of delirium or encephalopathy secondary to sepsis, toxins, or metabolic causes (hypoglycemia, electrolyte disturbances).
Clinical categories.
Patients were categorized as having viral encephalitis, nonviral infectious (including bacterial and fungal) encephalitis, autoimmune encephalitis, or encephalitis of unknown etiology. Viral and nonviral infectious encephalitides were defined by serology, positive PCR, culture, or histopathology. Cases of presumed herpes simplex virus (HSV) encephalitis with acute presentation and brain MRI revealing hyperintensity and/or hemorrhage in the bilateral mesial temporal lobes were also included.8 Autoimmune encephalitis was defined by the presence of antigen-specific antibodies in the serum and/or CSF or cases with histopathologic evidence of autoimmune encephalitis. Cases of acute disseminated encephalomyelitis were categorized as autoimmune etiology and defined by clinical features and imaging characteristic of acute disseminated encephalomyelitis or histology-proven cases.9
Clinicoradiographic parameters.
Data collected included demographic information (age, sex, race), presence of comorbid conditions calculated by the Charlson comorbidity scale, and immunocompromised state.10 Hospitalization data included outside hospital length of stay, total length of stay at JHH/JHBMC, ICU location (NCCU or other), and ICU length of stay. Clinical information gathered on admission to JHH/JHBMC included Glasgow Coma Scale (GCS) score as well as laboratory data including CSF profile (WBC count, red blood cell count, glucose, protein, culture/PCR data), and presence of leukopenia or thrombocytopenia. Medication administration of antimicrobial treatment, IV steroids, and treatment with hyperosmolar agents was recorded. The presence of seizure activity, SE, and pharmacologic burst suppression was examined. We identified patients who were intubated with ventilator support and those that had intracranial pressure (ICP) monitoring. Radiographic data based on noncontrast head CT and/or brain MRI were assessed for evidence of cerebral edema.
Clinical outcome.
At discharge, all patients underwent a neurologic examination performed by a neurologist, and the outcome was graded according to the modified Rankin Scale (mRS). In those who survived, good outcome was defined as mRS scores 0 to 3 and poor outcome as mRS score 4 or 5.11
Statistical analysis.
We calculated the mean, median, and SD on all continuous variables. Parametric and nonparametric tests were used to identify differences between groups in continuous outcomes, and χ2 tests were used to compare categorical outcomes.
Univariate analysis examined clinical and demographic features to determine whether there was a statistically significant relationship with our outcomes of interest. We assessed all potential variables including etiology of encephalitis, host-related factors, clinical course, ICU care, and complications for their association with outcomes of interest. We performed multivariate logistic regression to examine the association between potential predictors and the likelihood of an unfavorable outcome. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to quantify the strength of these associations. Variables included in the multivariate logistic analysis were those found to be significant in our univariate analysis as well as those determined a priori based on clinical relevance. Our multivariable logistic model for mortality included age, sex, immunocompromised state, thrombocytopenia, Charlson comorbidity index, SE, and cerebral edema. Our final regression model for good and poor outcome in survivors included age, sex, etiology of encephalitis, intubation requirement with ventilator support, thrombocytopenia, Charlson comorbidity index, SE, and cerebral edema. The Hosmer-Lemeshow goodness-of-fit statistic was used to assess all models for final model fit. There were no missing data fields in the variables analyzed. To determine the contribution of each variable to outcome prediction, we also performed marginal probability analysis. Coefficients from the logistic regression analysis were used in computing average marginal coefficients for each variable of interest.
Further subset analysis was performed on patients admitted directly to JHH/JHBMC or transferred from an outside hospital within 24 hours of presentation. This was done to restrict the analysis to those patients managed primarily at JHH/JHBMC. All statistical tests were 2-tailed, and p values <0.05 were considered statistically significant. All statistical analyses were performed using STATA version 11 software (StataCorp, College Station, TX).
RESULTS
Patient characteristics.
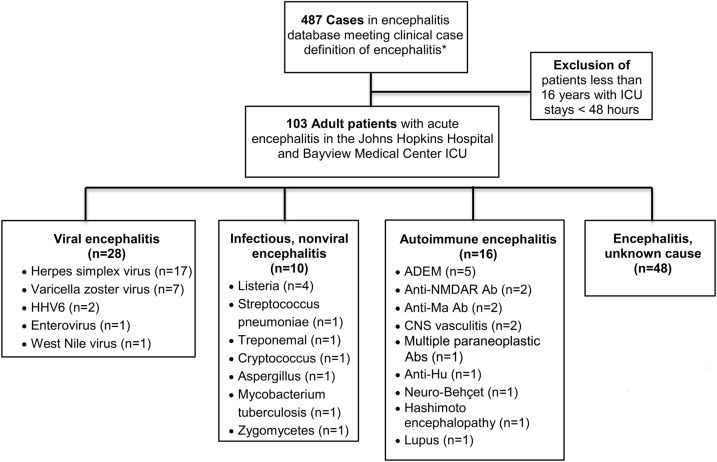
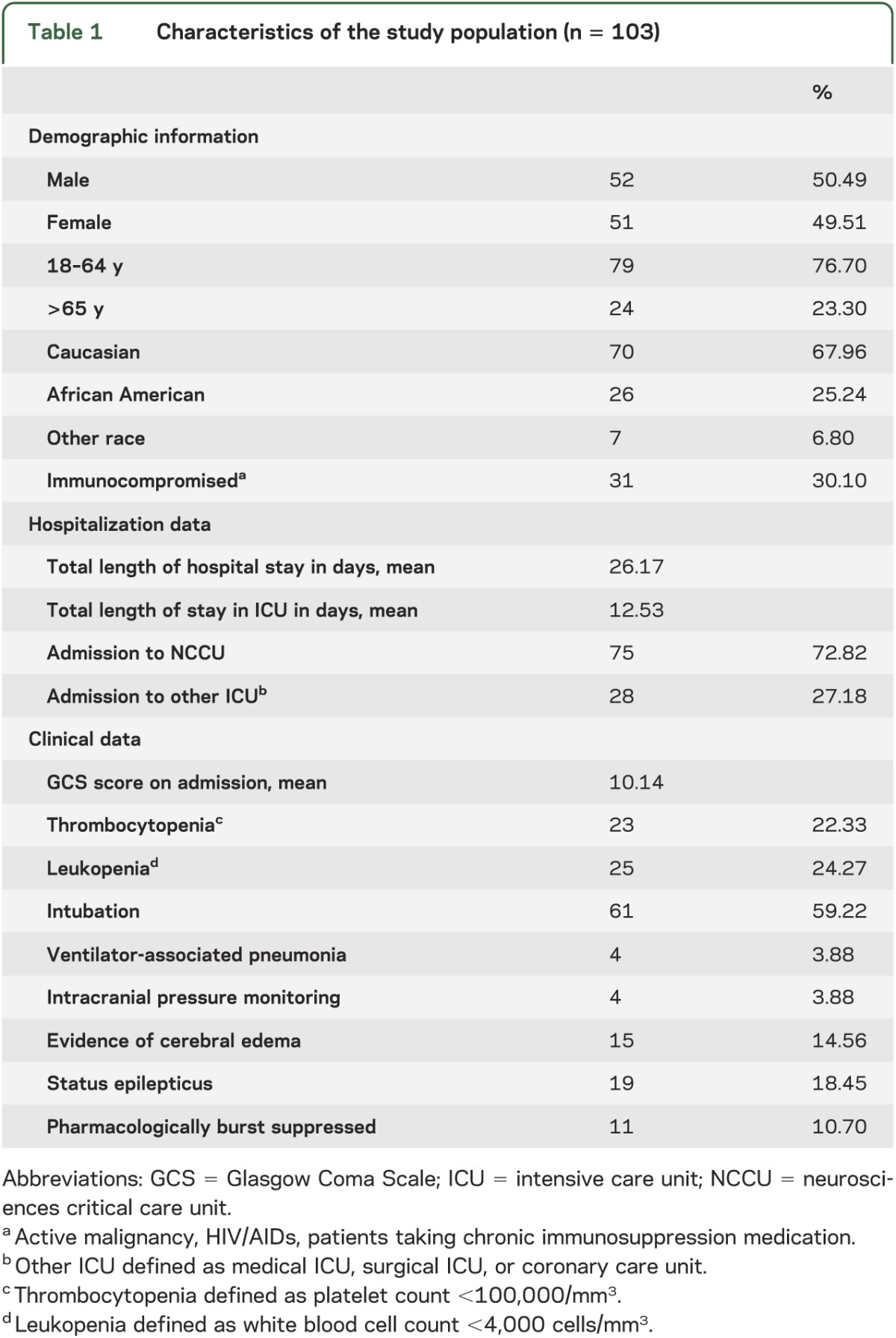
From the encephalitis databases at JHH and JHBMC, 103 of a total of 487 patients with encephalitis met our inclusion criteria. The median age was 52 years (interquartile range 26), 52 patients (50.49%) were male, 24 (23.30%) were 65 years and older, 70 (67.96%) Caucasian, 26 (25.24%) African American, and 31 (30.10%) immunocompromised (see table 1). Charlson comorbidity scores were used as a surrogate marker of degree of comorbidity.
Table 1.
Characteristics of the study population (n = 103)
The etiologies of encephalitis included 28 patients (27.18%) with viral encephalitis, 10 patients (9.71%) with bacterial or fungal encephalitis, 17 (16.50%) with autoimmune encephalitis, and 48 (46.60%) with encephalitis of unknown cause (see figure 1). The most common specific etiology of encephalitis was HSV, with 17 cases (16.50%) in our cohort.
Figure 1. Study population and etiologies of encephalitis.
One hundred three of the 487 patients in the encephalitis database at The Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center met inclusion criteria. Patients were categorized as viral, infectious nonviral (including bacterial and fungal), autoimmune, and unknown causes of encephalitis. *Case definition of encephalitis: admitted to hospital with encephalopathy and ≥2 of the following: fever (≥38°C), seizures, and/or focal neurologic findings (with evidence of brain parenchyma involvement), CSF pleocytosis (>5 WBCs/mm3), EEG findings compatible with encephalitis, and abnormal neuroimaging in keeping with encephalitis. Exclusion criteria included delirium or encephalopathy secondary to sepsis, toxic or metabolic causes (hypoglycemia, electrolyte disturbances), or primary psychiatric illness. Ab = antibody; ADEM = acute disseminated encephalomyelitis; HHV6 = human herpesvirus 6; ICU = intensive care unit; NMDAR = NMDA receptor; WBC = white blood cell.
Clinical course.
The mean GCS score on admission was 10.14 (SD 4.33) with 39 patients (37.86%) having a GCS score <8. Mean total length of hospital stay was 26.17 days (SD 26.17) and mean ICU length of stay was 12.53 days (SD 15.05). Overall, 75 patients (72.82%) were cared for in the NCCU whereas 28 patients (27.18%) were treated in other ICUs.
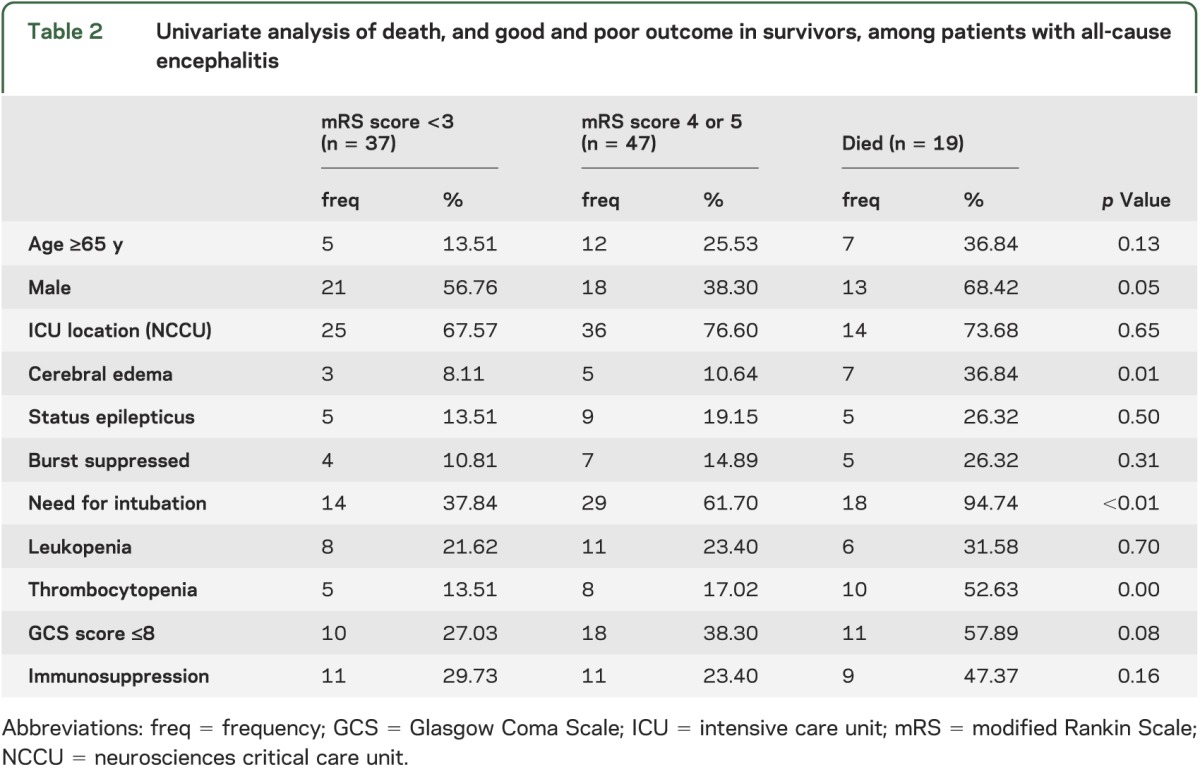
Twenty-three patients (22.33%) had thrombocytopenia and 25 (24.27%) were leukopenic. Of the 19 patients (18.45%) who developed SE, 11 (10.70% of the overall population) were pharmacologically treated to induce EEG burst suppression. Radiographic evidence of cerebral edema was seen in 15 patients (14.56%), of whom 4 (3.96% of the overall population) underwent ICP monitoring and 9 (8.74% of the overall population) received hyperosmolar therapy. Univariate analysis was performed to examine clinical and demographic features associated with outcome (table 2).
Table 2.
Univariate analysis of death, and good and poor outcome in survivors, among patients with all-cause encephalitis
Predictors of mortality.
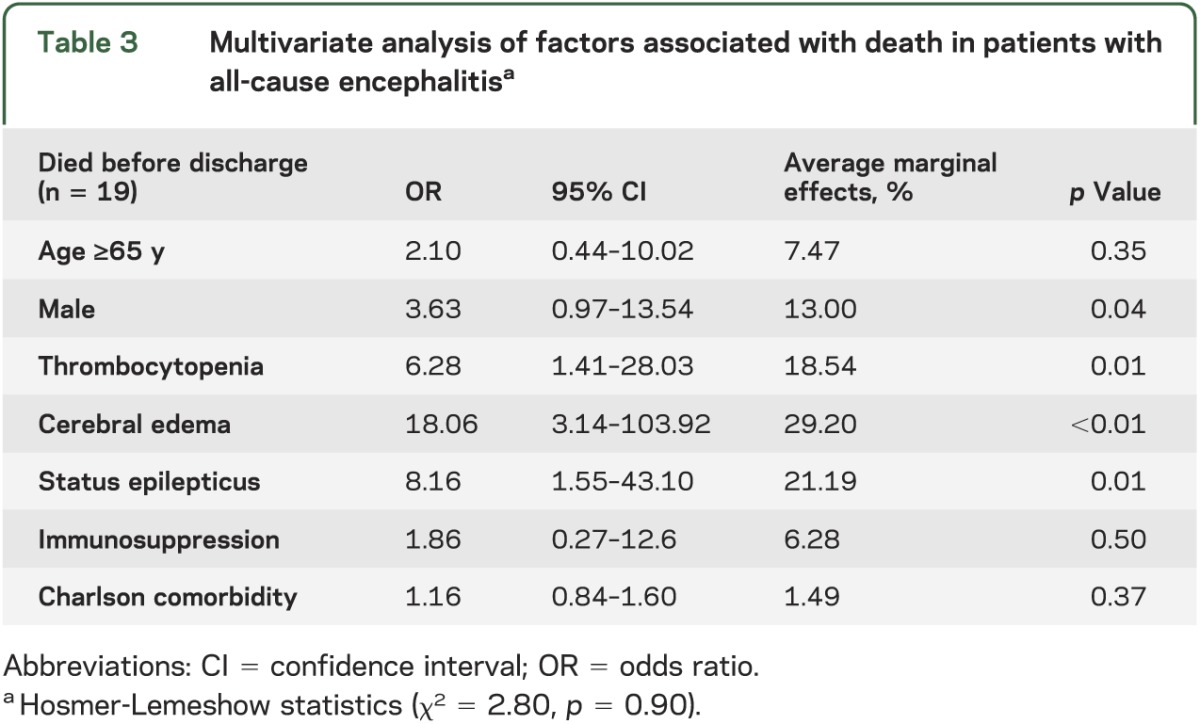
In our patient cohort, 19 patients (18.45%) died. Multivariate logistic regression analysis demonstrated that the presence of cerebral edema (OR 18.06, 95% CI 3.14–103.92), SE (OR 8.16, 95% CI 1.55–43.10), and thrombocytopenia (OR 6.28, 95% CI 1.41–28.03) were all associated with mortality (see table 3). Mortality was associated with a marginal probability 29% higher with radiologic evidence of cerebral edema (p < 0.01), 21% higher with SE (p = 0.01), and 19% higher for thrombocytopenic patients compared to those with normal platelet counts (p = 0.01). Although there were trends toward slightly increased probability of death among those who were aged 65 years and older (7%; p = 0.36), immunocompromised patients (6%; p = 0.54) and those with significant comorbid conditions (2%; p = 0.37), these findings were not statistically significant. In a separate subset analysis performed comparing infectious (both viral and nonviral) vs autoimmune and unknown causes of encephalitis, an increased likelihood of mortality from infectious causes was observed, but these findings were not significant. In further subset analysis, a trend toward reduced odds of death was seen among patients with cerebral edema receiving ICP monitoring and hyperosmolar therapy.
Table 3.
Multivariate analysis of factors associated with death in patients with all-cause encephalitisa
Furthermore, in our patient cohort, 95% of the patients who died during hospitalization required endotracheal intubation with ventilator support. In our final model predicting mortality, the need for intubation with ventilator support was therefore excluded because it was strongly associated with mortality. However, sensitivity analysis performed with inclusion of endotracheal intubation in our model and addition of other variables including etiology of encephalitis did not differ from our findings using a more parsimonious model.
Predictors of outcome among survivors.
Of the surviving patients, 37 (35.92%) had favorable outcome (mRS scores 0–3) and 47 (55.95%) had poor outcome (mRS scores 4 and 5). Twenty-one patients (25% of the surviving patients) were discharged home, 49 (58.33%) discharged to rehabilitation, 10 (11.9%) to a nursing home, and 4 (4.67%) to another hospital (see figure e-1 on the Neurology® Web site at www.neurology.org).
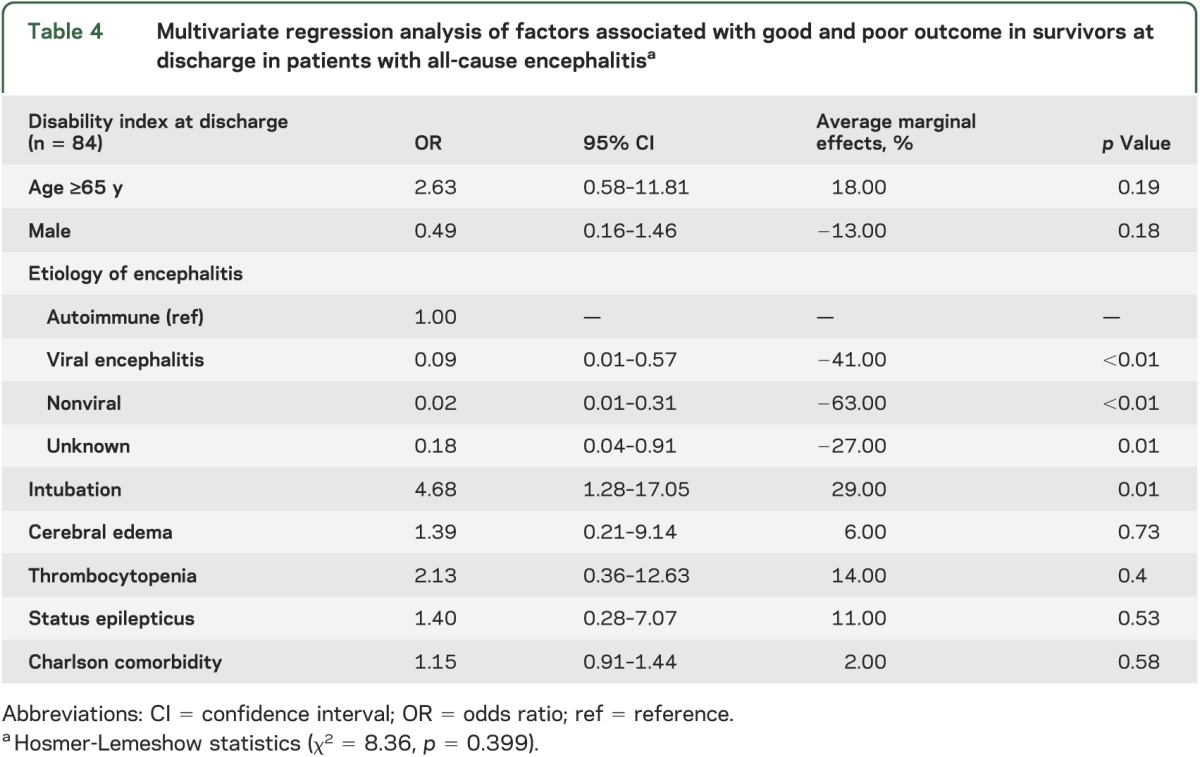
Results of the multivariate regression analysis in those patients who survived showed that intubation requirement with ventilator support was associated with poor outcome (p < 0.001). In addition, patients with viral (OR 0.09, 95% CI 0.01–0.57), nonviral (OR 0.02, 95% CI 0.01–0.31), and unknown (OR 0.18, 95% CI 0.04–0.91) causes of encephalitis were all less likely to have a poor outcome compared with those patients with an autoimmune etiology (see table 4).
Table 4.
Multivariate regression analysis of factors associated with good and poor outcome in survivors at discharge in patients with all-cause encephalitisa
There were 49 patients (47.57%) directly admitted to JHH/JHBMC or with outside hospital stays <24 hours. When analysis was restricted to this subset of patients, our findings were similar to those obtained from the overall study population.
DISCUSSION
We assessed outcome at hospital discharge of patients with acute encephalitis who received treatment and supportive care in the ICU. Our goal was to examine patients with acute encephalitis broadly and determine which factors in this critically ill population were predictive of outcome at hospital discharge. Our results show that factors associated with mortality, regardless of etiology, include the potentially reversible conditions of cerebral edema, thrombocytopenia, and SE. In addition, we found that in those patients who survived to hospital discharge, patients with viral, nonviral, and unknown causes of encephalitis were all less likely to have a poor outcome compared with those patients with an autoimmune etiology.
We found that cerebral edema was strongly predictive of mortality in our patient cohort. A nonsignificant trend toward reduced death was seen among patients with cerebral edema receiving ICP monitoring and hyperosmolar therapy, but the number of patients receiving these interventions was too small to draw any definitive conclusions. There have been few previous studies examining the utility of ICP monitoring and management of cerebral edema with hyperosmolar therapy and decompressive surgery in patients with meningoencephalitis.12–18 Our results suggest that clinicians must maintain vigilance for any change in pupil reactivity, development of focal neurologic deficits, and changes in level of consciousness. Given evidence that herniation may be reversible with aggressive management, use of hyperosmolar therapy should be initiated emergently, and neurosurgery should be considered if there are signs of mass effect.19 Further studies in larger cohorts are needed to determine whether there is survival benefit in those critically ill patients with encephalitis who have ICP monitoring, along with aggressive medical and surgical management of cerebral edema.
In addition to the increased mortality seen with cerebral edema, patients in SE were found to have an increased risk of death. Several studies have found a high 30-day mortality risk in patients who develop SE.20–23 In a previous study evaluating SE in patients with encephalitis and response to antiepileptic drugs and mortality, 36.7% remained refractory to the second antiepileptic drug and approximately one-third of patients died.24 Studies have shown that encephalitis is a common cause of refractory SE (RSE).25,26 In our study, 11 patients (57.89% of those patients in SE) had RSE and required pharmacologic burst suppression. The increased likelihood of patients with encephalitis to develop RSE is probably related to the predilection for infections such as HSV and autoimmune encephalitides to target epileptogenic limbic structures and other cortical regions. Ongoing studies of early EEG features may shed further light on prognostication in patients with encephalitis.
The presence of thrombocytopenia was also significantly associated with mortality. The overall incidence of thrombocytopenia among the critically ill is 35% to 44% and may be attributable to a variety of causes including decreased platelet production as a result of bone marrow suppression, increased platelet destruction due to immune and nonimmune causes, hemodilutional effects due to blood loss, or splenic sequestration.27 In previous studies of critically ill patients, thrombocytopenia was found to be a stronger independent predictor for ICU mortality than were composite scoring systems used in the ICU, such as the APACHE (Acute Physiology and Chronic Health Evaluation) II score or the Multiple Organ Dysfunction Score.28–30 Our study suggests that this association between thrombocytopenia and mortality in patients who are critically ill extends to those with encephalitis.
Endotracheal intubation requirement with ventilator support was found to be strongly associated with poor prognosis and risk of death. These findings are consistent with previous studies of critically ill patients including those with encephalitis and indicate that physicians must assess the need for mechanical ventilation early with ongoing evaluation of the need for respiratory support.31–33
There have been few previous studies in large cohorts on prognostic indicators of encephalitis. In HSV encephalitis, the most frequently studied etiology in adult and pediatric literature, delay in initiation of acyclovir therapy, Simplified Acute Physiology Score II score >27, older age, and GCS score <10 at initiation of therapy were associated with poor outcome.34 In a prospective study of outcome in acute infectious encephalitis, several factors, including older age, immunosuppression, and mechanical ventilation, were associated with death during hospitalization.35 In addition to the expanding array of infectious causes, novel antibody-mediated forms of encephalitis have become recognized over the last decade. There is growing interest in factors associated with outcome in this subtype of encephalitis. A recent study of 500 anti-NMDA receptor encephalitis cases showed that almost half of the patients had no improvement in the first month after initiation of immunotherapy or tumor removal, but improvements from severe to slight disability occurred within the first 24 months of treatment in 81% of patients.36 Results of our study are consistent with previous studies on prognostication in autoimmune encephalitis in that this patient population may experience substantial delays before meaningful functional recovery.
A major limitation of our study is its retrospective nature. We assessed cases of encephalitis at 2 large medical centers, with a large referral base, which may not be representative of encephalitis cases seen in other hospitals. Our study focused on acute encephalitis patients with ICU hospital stays and may not be generalizable to all patients with encephalitis. Despite an increased trend toward mortality observed for those who are immunosuppressed, older than 65 years of age, or with infectious causes of encephalitis, the small size of our study population imposes limitations on any broader conclusions that can be made from these findings. Furthermore, we are also limited in our evaluation of potentially beneficial interventions including ICP monitoring and hyperosmolar therapy on the outcome of patients with encephalitis because of the relatively few numbers of patients undergoing such interventions in our study. In addition, we did not gather long-term data and are therefore unable to comment on long-term outcome in our patient population.
Our study suggests that those patients with autoimmune encephalitis have a higher risk of short-term disability compared with other etiologies of encephalitis, but further studies are needed in larger cohorts to validate this finding. Our study also suggests that regardless of etiology of encephalitis, monitoring for ongoing seizure activity and signs of increased ICP with aggressive treatment of SE, cerebral edema, and platelet derangement in the ICU may decrease mortality and improve functional outcome at hospital discharge. Further prospective studies are needed to determine whether these measures, along with specialized care in a neurocritical care unit, improve outcome in those with acute encephalitis.
Supplementary Material
GLOSSARY
- CI
confidence interval
- GCS
Glasgow Coma Scale
- HSV
herpes simplex virus
- ICD-9
International Classification of Diseases, ninth revision
- ICP
intracranial pressure
- ICU
intensive care unit
- JHBMC
Johns Hopkins Bayview Medical Center
- JHH
The Johns Hopkins Hospital
- mRS
modified Rankin Scale
- NCCU
neurosciences critical care unit
- OR
odds ratio
- RSE
refractory status epilepticus
- SE
status epilepticus
- WBC
white blood cell
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
K.T. Thakur, MD, and M. Motta, MD, MPH: design, analysis, and writing of the manuscript. A.O. Asemota, MBBS, MPH: design, statistical analysis, and writing of the manuscript. H.L. Kirsch, BS: analysis and writing of the manuscript. D.R. Benavides, MD, PhD: design, analysis, and writing of the manuscript. E.B. Schneider, PhD: design and statistical analysis of the manuscript. J.C. McArthur, MBBS, MHP: design, review, and review of the manuscript. R.G. Geocadin, MD, FAAN: design, review, and writing of the manuscript. A. Venkatesan, MD, PhD: design, analysis, writing and review of the manuscript.
STUDY FUNDING
Supported by grant UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. The contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. This study was also supported by award 5P30MH075673-S02 from the National Institute of Mental Health (PI, J.C.M.), a T32 grant from the National Institute of Neurological Disorders and Stroke (NS069351) (PI, J.C.M.), and the Aarons Family Fund for Encephalitis (A.V.).
DISCLOSURE
K. Thakur, M. Motta, A. Asemota, H. Kirsch, and D. Benavides report no disclosures. E. Schneider: patent pending for secondary prevention after acute CNS insult. J. McArthur: grants from NIH, Biogen-Idec, payment from lectures, speakers bureau in various universities, book royalties, stock option from GliaMed. R. Geocadin: grants from the NIH. A. Venkatesan: grants from the NIH, Howard Hughes Medical Institute, National Multiple Sclerosis Society, and the Maryland Stem Cell Research Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Glaser CA, Gilliam S, Schnurr D, et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis 2003;36:731–742 [DOI] [PubMed] [Google Scholar]
- 2.Whitley RJ, Lakeman F. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin Infect Dis 1995;20:414–420 [DOI] [PubMed] [Google Scholar]
- 3.Granerod J, Tam CC, Crowcroft NS, Davies NW, Borchert M, Thomas SL. Challenge of the unknown: a systematic review of acute encephalitis in non-outbreak situations. Neurology 2010;75:924–932 [DOI] [PubMed] [Google Scholar]
- 4.Steiner I, Budka H, Chaudhuri A, et al. Viral meningoencephalitis: a review of diagnostic methods and guidelines for management. Eur J Neurol 2010;17:999–e57 [DOI] [PubMed] [Google Scholar]
- 5.Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008;47:303–327 [DOI] [PubMed] [Google Scholar]
- 6.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 2010;10:835–844 [DOI] [PubMed] [Google Scholar]
- 7.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med 1998;338:970–976 [DOI] [PubMed] [Google Scholar]
- 8.Baskin HJ, Hedlund G. Neuroimaging of herpesvirus infections in children. Pediatr Radiol 2007;37:949–963 [DOI] [PubMed] [Google Scholar]
- 9.Tenembaum S, Chitnis T, Ness J, et al. Acute disseminated encephalomyelitis. Neurology 2007;68:S23–S36 [DOI] [PubMed] [Google Scholar]
- 10.Hall WH, Ramachandran R, Narayan S, et al. An electronic application for rapidly calculating Charlson comorbidity score. Cancer 2004;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 1957;2:200–215 [DOI] [PubMed] [Google Scholar]
- 12.Adamo MA, Deshaies EM. Emergency decompressive craniectomy for fulminating infectious encephalitis. J Neurosurg 2008;108:174–176 [DOI] [PubMed] [Google Scholar]
- 13.Yan HJ. Herpes simplex encephalitis: the role of surgical decompression. Surg Neurol 2002;57:20–24 [DOI] [PubMed] [Google Scholar]
- 14.Mellado P, Castillo L, Andresen M, et al. Decompressive craniectomy in a patient with herpetic encephalitis associated to refractory intracranial hypertension [in Spanish]. Rev Med Chil 2003;131:1434–1438 [PubMed] [Google Scholar]
- 15.Naess H, Moen G, Mahesparan R. Decompressive craniectomy in acute encephalitis [in Norwegian]. Tidsskr Nor Laegeforen 2006;126:1208–1209 [PubMed] [Google Scholar]
- 16.Raffelsieper B, Merten C, Mennel HD, Hedde HP, Menzel J, Bewermeyer H. Decompressive craniectomy for severe intracranial hypertension due to cerebral infarction or meningoencephalitis [in German]. Anasthesiol Intensivmed Notfallmed Schmerzther 2002;37:157–162 [DOI] [PubMed] [Google Scholar]
- 17.Refai D, Lee MC, Goldenberg FD, Frank JI. Decompressive hemicraniectomy for acute disseminated encephalomyelitis: case report. Neurosurgery 2005;56:E872. [DOI] [PubMed] [Google Scholar]
- 18.Barnett GH, Ropper AH, Romeo J. Intracranial pressure and outcome in adult encephalitis. J Neurosurg 1988;68:585–588 [DOI] [PubMed] [Google Scholar]
- 19.Koenig MA, Bryan M, Lewin JL, et al. Reversal of transtentorial herniation with hypertonic saline. Neurology 2008;70:1023–1029 [DOI] [PubMed] [Google Scholar]
- 20.Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia 2001;42:714–718 [DOI] [PubMed] [Google Scholar]
- 21.Vignatelli L, Tonon C, D’Alessandro R. Incidence and short-term prognosis of status epilepticus in adults in Bologna, Italy. Epilepsia 2003;44:964–968 [DOI] [PubMed] [Google Scholar]
- 22.Coeytaux A, Jallon P, Galobardes B, et al. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR). Neurology 2000;55:693–697 [DOI] [PubMed] [Google Scholar]
- 23.Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Short-term mortality after a first episode of status epilepticus. Epilepsia 1997;38:1344–1349 [DOI] [PubMed] [Google Scholar]
- 24.Kalita J, Nair PP, Misra UK. Status epilepticus in encephalitis: a study of clinical findings, magnetic resonance imaging, and response to antiepileptic drugs. J Neurovirol 2008;14:412–417 [DOI] [PubMed] [Google Scholar]
- 25.Holtkamp M, Othman J, Buchheim K, et al. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry 2005;76:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer SA, Claassen J, Lokin J, et al. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205–210 [DOI] [PubMed] [Google Scholar]
- 27.Strauss R, Wehler M, Mehler K, et al. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med 2002;30:1765–1771 [DOI] [PubMed] [Google Scholar]
- 28.Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care 2006;10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med 2000;28:1871–1876 [DOI] [PubMed] [Google Scholar]
- 30.Drews RE, Weinberger SE. Thrombocytopenic disorders in critically ill patients. Am J Respir Crit Care Med 2000;162:347–351 [DOI] [PubMed] [Google Scholar]
- 31.Dosemeci L, Yilmaz M, Celikbilek G, et al. The complications associated with mechanical ventilation. Crit Care 2003;7:P146 [Google Scholar]
- 32.Edmond K, Clark A, Korczak VS, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:317–328 [DOI] [PubMed] [Google Scholar]
- 33.Raschilas F, Wolff M, Delatour F, et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis 2002;35:254–260 [DOI] [PubMed] [Google Scholar]
- 34.Kamei S, Sekizawa T, Shiota H, et al. Evaluation of combination therapy using acyclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry 2005;76:1544–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mailles A, Stahl JP; Steering Committee and Investigators Group Infectious encephalitis in France in 2007: a national prospective study. Clin Infect Dis 2009;49:1838–1847 [DOI] [PubMed] [Google Scholar]
- 36.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.