Abstract
Objective:
To determine whether behavioral dissociations and interactions occur between the attentional functions—alerting, orienting, and conflict resolution—depending upon stroke location and to determine the approximate proportion of patients who can be classified into 1 of these 3 anatomical networks.
Methods:
We recruited 110 anatomically unselected acute stroke patients and 62 age-matched controls. Subjects underwent the attention network test (ANT), which provides a measure of each attention type. Their performance was related to lesion anatomy on MRI using a voxel-lesion mapping approach.
Results:
Patients as a whole performed poorer than controls, but there were no group differences in the size of attentional effects. Specific deficits in 1 of the 3 ANT-tested functions were found in the following lesion locations: alerting deficiency with bilateral anteromedial thalamus and upper brainstem (17% of patients); orienting impairment with right pulvinar and right temporoparietal cortex (15%); conflict resolution with bilateral prefrontal and premotor areas (23%). Lesions to right frontoparietal regions also modified interactions among the 3 types of attention.
Conclusions:
More than half of all stroke patients can be expected to have a lesion location classifiable into 1 of the 3 principal attention networks. Our results have potential implications for therapy personalization in focal brain diseases including stroke.
Attention is critical for normal behavior, and recovery from brain injury,1 but its functional localization is debated.2 One influential, and unifying, theoretical framework proposes 3 separable attention networks: alerting, orienting, and conflict resolution,3 supported by behavioral, functional, and structural imaging evidence in healthy subjects.4–6
The attention network model has important predictions and implications for clinical neurology. First, given the wide number of regions associated with each of the attention networks—not only within right hemisphere, but also left hemisphere, striatum, thalamus, brainstem, and cerebellum7–9—it would be expected that a large proportion of patients with focal brain lesions are characterizable into of 1 of 3 profiles of attention impairment, depending upon lesion location. Second, evidence that each attention network has a distinct neuropharmacology3 points to the possibility of personalized neurotherapeutics, if focal lesions are resolvable into separate networks. While previous clinical studies3 indicate that specific attention impairments tend to be associated with different lesion locations, mounting evidence exists for certain areas being implicated in more than one type of attention,10–12 and for interdependencies between attention types.13 Moreover, studies that select and group patients by prespecified regions of interest may be insensitive to critical anatomical–functional associations, and are unable to ascertain how often focal lesions fall within attention-determining areas. In this study, we tested a large unselected series of stroke patients, and used a voxel-lesion method of analysis,14 to 1) assess the strongest anatomical associations with each type of attention; 2) determine whether these locations show behavioral dissociations, commonalities, or interactions; and 3) estimate the proportion of strokes classifiable into 1 of the 3 attention networks.
METHODS
Participants.
A total of 110 patients with acute stroke were recruited from a single center, and tested 3–10 days after presentation. A further 22 patients were enrolled, but their performance was too poor to be included (see below). There was no anatomical preponderance of lesion location in this excluded set. Additionally, 62 age-matched controls were tested, comprising 1) neurologic controls: i.e., acute focal neurologic disturbance, but normal MRI, and judged not to have a stroke; and 2) healthy adults with no history of brain disease. Other inclusion criteria were 1) right-handed; 2) able to comprehend and perform attention network test (ANT) task reliably. Exclusion criteria were 1) preexisting organic brain disease; 2) old focal brain lesion (>10 mm) or significant cerebral small-vessel disease (>1 on Age-Related White Matter Change score15); 3) bilateral strokes.
Behavioral test.
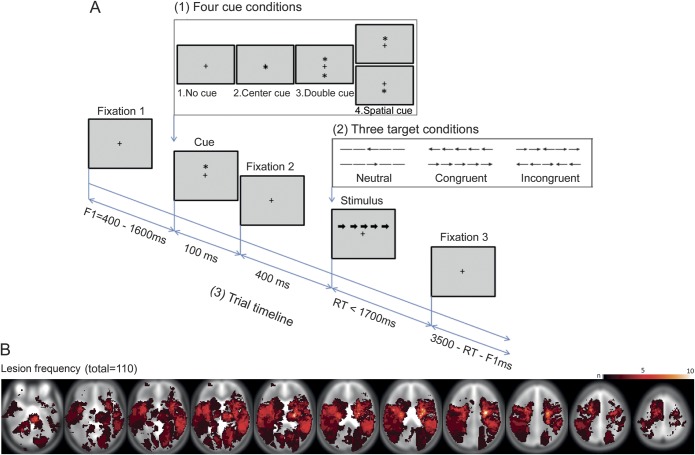
The ANT (figure 1A) measures 3 attention components—alerting, orienting, and conflict—by manipulating stimulus properties, for a constant set of instructions. Subjects are instructed to indicate the direction (right/left) of a target arrow in the upper or lower visual hemifield. Targets are preceded by 1 of 4 possible cue types: none, double, central, or spatial. Additionally, on each trial, the target is flanked by 4 arrows, pointing in the same (congruent), opposite (incongruent), or no (neutral) direction. Attention measures are derived as follows: 1) conflict = reaction time (RT) (incongruent) − RT (congruent); 2) orienting = RT (central cue) − RT (spatial cue); 3) alerting = RT (no cue) − RT (double cue); or vice versa using accuracy.
Figure 1. Schematic of attention network test (A) and lesion histogram (B) superimposed upon Montreal Neurological Institute template brain.
The task was run on a laptop using Cogent 2000 graphics (www.vislab.ucl.ac.uk), with identical visual and timing parameters to those previously described,4 and 2 blocks of 96 trials. Participants pressed 1 of 2 keys with their right hand, unless this was paralyzed, in which case they used their nonparetic hand. Subjects first undertook a practice session for 5 minutes with auditory feedback, with those achieving more than two-thirds correct proceeding to test session. Error trials (incorrect, or RT >2,000 ms) were excluded from the RT analysis. Subjects had to respond to >60% of trials, and achieve >80% accuracy on responded trials to be included in the final analysis.
Imaging and lesion delineation.
Patients underwent MRI 2–7 days from stroke onset on a Siemens 1.5 T scanner, providing T2, fluid-attenuated inversion recovery, diffusion-weighted imaging (DWI), and susceptibility-weighted acquisitions. DWI dimensions were 192 × 192 × 19. Delineation of acute ischemic lesions was performed by an intensity-based, lesion-growing technique using MATLAB (v7.10.0), with the option to manually edit, e.g., for hemorrhage, or overrunning artefact. Lesions were subsequently normalized in SPM8 (www.fil.ion.ucl.ac.uk/spm) using a coregistered T2 image, and matching to a canonical T2 in Montreal Neurological Institute space (voxel size 2 × 2 × 2 mm), with cost-function lesion masking.16
Statistics and voxel lesion mapping.
Differences between groups in terms of subject characteristics were tested for with one-way analyses of variance (ANOVAs), χ2, or Fisher Exact tests in SPSS (v 19.0). The effect of group on attention type was assessed by a 3-way mixed-design ANOVA, with factors group (lesions, neurologic controls, healthy controls), flanker (congruent, neutral, incongruent), and cue (none, double, central, spatial). Since there were no differences in performance between neurologic and healthy controls, nor interactions comparing these 2 control groups with cue or flanker effects, we subsequently pooled all controls into one group.
The effect of lesion location on attention type was similarly assessed using group × flanker (or/and) × cue ANOVAs, now conducted at each voxel where at least 2 lesions occurred.14 The group comparison refers to patients with lesions in the interrogated voxel (Lesion+) vs all controls, or vs patients with lesions in other voxels (Lesion−). The analysis was run iteratively within MATLAB and statistical parameters for each voxel depicted as a map in Montreal Neurological Institute space. Correction for multiple comparisons at p < 0.05 threshold was made using a false discovery rate procedure.17 Significant interactions were subsequently characterized by masking with post hoc t tests that tested for group differences in any of the 3 attention contrasts of interest (thresholded at p < 0.05, corrected for the respective ANOVA search volume; and for exploratory purposes, at p < 0.001, uncorrected). Regions showing such effects were subsequently probed for group effects in the remaining attention contrasts down to a threshold of p < 0.05, uncorrected. To correct for age and lesion size, the Lesion+ vs Lesion− t test reflected the regression coefficient of B1 in the regression equation: Network effect = B0 + (B1 × lesion presence/absence) + (B2 × age) + (B3 × lesion size). Tests for parametric assumptions confirmed suitability of ANOVAs where reported.
To exclude possible RT accuracy tradeoffs, and to test for network specificity, we recalculated the 3 attentional contrasts substituting an efficiency metric (accuracy/RT) for each condition18 (contrast expressed as a % of efficiency for no cue, neutral condition); and then performed group × attention-type ANOVAs on these contrast sizes at each peak identified for the group × flanker × cue interactions. For this, we used negative conflict, rather than conflict, so that generalized attentional impairments would be indicated by decreases across all 3 measures. Behavioral dissociations were ascertained with location × attention-type ANOVAs that compared the 3 attentional contrasts for pairwise combinations of regions taken from separate categories (e.g., lesions in region A1 showing raised conflict, vs lesions in region B1 showing reduced orienting).
Statistically thresholded 3D maps were superimposed upon normalized, reference atlases of Brodmann areas, cortical and subcortical structures, white matter tracts, and thalamic nuclei.19–21 Calculation of volume of significant voxels overlying each atlas-defined region and the proportion of each region occupied by significant voxels were determined by matrix multiplication, i.e., thresholded results (row vector) × atlas (column vector).
Standard protocol approvals, registrations, and patient consents.
Ethical approval was granted by the Charing Cross Hospital Research Ethics Committee and all participants gave informed consent.
RESULTS
Group comparisons.
Patients and controls were matched for age and sex (for further group characteristics, see table e-1A on the Neurology® Web site at www.neurology.org). The ANT was performed more slowly and less accurately in the lesion group than controls (p ≤ 0.001), but there were no overall group × cue, group × flanker, or group × cue × flanker interactions (p > 0.05; table e-1B; figure e-1); nor were there correlations between any of the ANT measures and lesion size or age (|r| < 0.19; p > 0.05). Lesions were distributed among right hemisphere, left hemisphere, and brainstem-cerebellum in the ratio 41:47:22 (figure 1B).
Locations associated with heightened conflict.
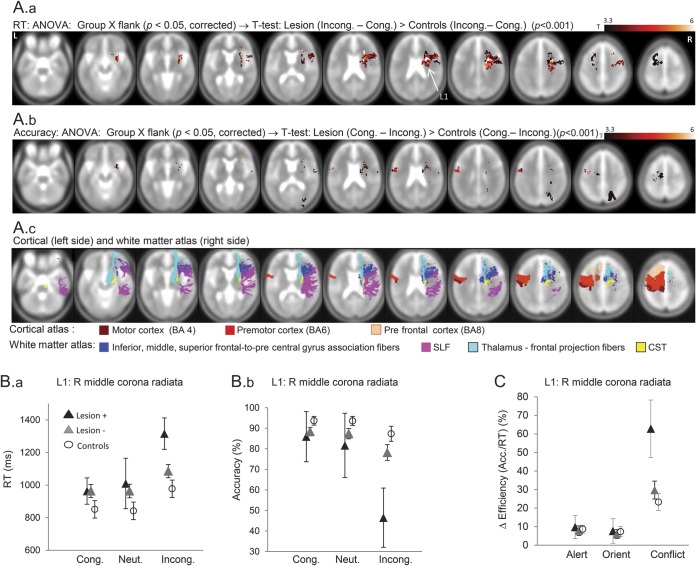
Lesion locations that influenced conflict processing were located predominantly in bilateral frontal white matter, and reflected exclusively Lesion+ showing greater conflict than controls or Lesion− (n = 25; figure 2; table e-2; figure e-2). The largest cluster to show this effect was in right middle corona radiata (RMCR), anterior to corticospinal tract, in white matter connecting prefrontal with premotor cortices. Smaller clusters also occurred in right inferior prefrontal, anterior corona radiata, anterior insula, and left superior frontal cortex. There were no differences in effect size comparing lesions to RMCR alone vs right prefrontal ±RMCR overlap and no differences in stroke severity or lesion size between patients with lesions in the main RMCR cluster vs lesions elsewhere (p > 0.1). Lesions to these frontal regions increased conflict specifically (group × attention-type ANOVA; p < 0.05; figure 2C), although in a small proportion of the above regions, orienting was also reduced at a more liberal statistical threshold (p < 0.01, uncorrected).
Figure 2. Lesion sites that enhance conflict costs.
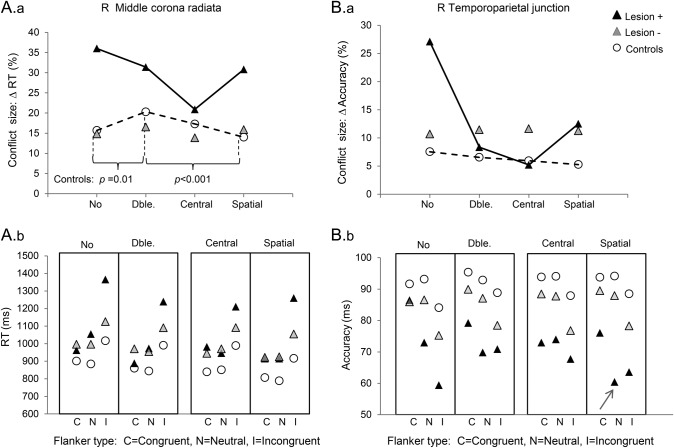
(A) Results of a group × flanker analysis of variance (ANOVA) (p < 0.05, corrected) were masked by a t test comparing Lesion+ vs controls for conflict (i.e., incongruent–congruent flanker), thresholded at p < 0.001, uncorrected. These are shown separately for reaction time (RT) (top panel) and accuracy (center panel). The bottom panel shows cortical and white matter anatomical landmarks relevant to the results, derived from coregistered standard atlases. (B) Plots of RT (left panel) and accuracy (center panel) for the 3 flanker conditions in Lesion+, Lesion−, and control groups, where Lesion+ refers to lesions affecting right middle corona radiata (L1). The 95% confidence intervals are shown (in this plot and elsewhere). (C) Plot of individual network performance at L1 shows selective conflict heightening. Contrasts are as defined in Methods for Accuracy, e.g., Conflict = Congruent – Incongruent, but now using efficiency values, i.e., Accuracy/RT, for each condition; values are plotted as percentage of each subject's efficiency in no cue, neutral condition. BA = Brodmann area; CST = corticospinal tract; SLF = superior longitudinal fasciculus.
Locations associated with reduced orienting.
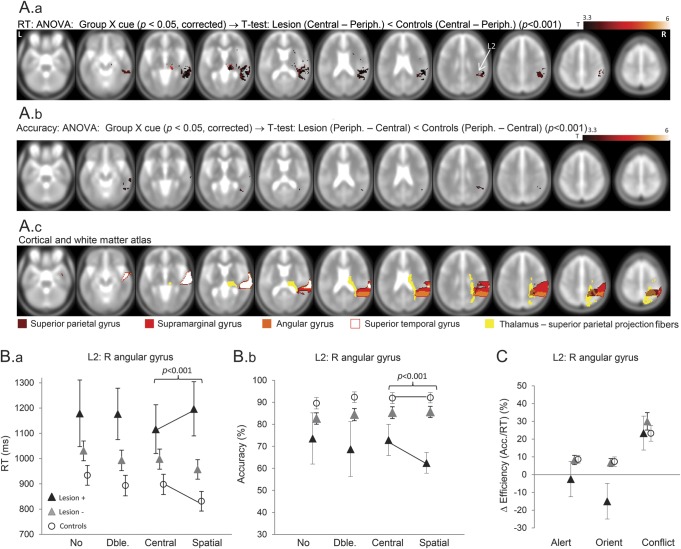
Regions where lesions reduced spatial orienting were in right pulvinar, right temporoparietal junction (RTPJ), and right posterior insula (n = 16; figure 3; table e-2; figure e-2). These interactions were accountable by Lesion+, failing to show speeding to spatial (relative to central) cues as seen with Lesion− and controls. Lesions to a smaller subset of RTPJ regions also demonstrated less orienting due to reduced accuracy to spatial cues (explored in the Lesion × cue × flanker interactions section below). The reduction in orienting due to lesions in right pulvinar and RTPJ was specific, given nonsignificant effects of lesions here on conflict and alerting; and significant group × attention-type interactions (p < 0.05; figure 3C). However, a small focus in anterior insula was associated with both reduced orienting and heightened conflict (p < 0.05, uncorrected).
Figure 3. Lesion sites that reduce orienting.
(A) Results of a group × cue analysis of variance (ANOVA) (p < 0.05, corrected) were masked by a t test comparing Lesion+ vs controls for orienting (i.e., central − spatial cue), thresholded at p < 0.001, uncorrected. These are shown separately for reaction time (RT) (A.a) and accuracy (A.b). The bottom panel (A.c) shows cortical and white matter anatomical landmarks relevant to the results. (B) Plots of RT (B.a) and accuracy (B.b) for the 4 cue conditions in Lesion+, Lesion−, and control groups, where Lesion+ refers to lesions affecting right angular gyrus (L2). (C) Plot of individual network performance at L2 shows selective orienting reduction (p < 0.001).
Locations associated with reduced alerting.
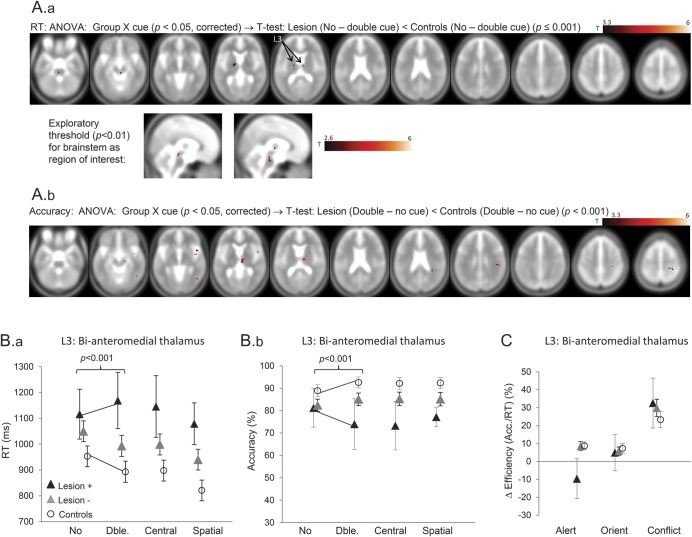
Alerting was reduced with lesions to bilateral anteromedial thalamus, upper brainstem, and right cerebral peduncle (n = 19; figure 4; table e-2; figure e-2), as well as in several small areas across right hemisphere. Significant thalamic voxels mostly overlay those nuclei projecting to prefrontal regions. These interactions were driven by controls and Lesion− showing speedier and more accurate responses to double (vs no) cues, while Lesion+ showed neither. The thalamic and brainstem peaks where lesions reduced alerting did not show effects on other attention types (group × attention-type interaction; p < 0.01; figure 4C). However, 2 of the right frontoparietal regions showing reduced alerting (including frontal operculum–anterior insula), also heightened conflict at lower statistical threshold (p < 0.05, uncorrected).
Figure 4. Lesion sites that reduce alerting.
(A) Results of a group × cue analysis of variance (ANOVA) (p < 0.05, corrected) were masked by a t test comparing Lesion+ vs controls for orienting (i.e., double − no cue), thresholded at p < 0.001, uncorrected. These are shown separately for reaction time (RT) (A.a and A.b) and accuracy (A.c). The second panel shows these effects within upper brainstem at an exploratory threshold of p < 0.01. (B) Plots of RT (B.a) and accuracy (B.b) for the 4 cue conditions in Lesion+, Lesion−, and control groups, where Lesion+ refers to bilateral anteromedial thalamus (L3). Note that the apparent numerical reversal of alerting effects in Lesion+ (i.e., negative alerting) is not significantly different from 0. (C) Plot of individual network performance at L3 shows selective alerting reduction (p < 0.001).
In order to establish whether the stereotypical attentional profiles outlined above differed between regions—i.e., double dissociations—we performed pairwise location × attention-type ANOVAs, e.g., comparing RMCR (raising conflict) vs right pulvinar (reducing orienting) or right pulvinar vs anteromedial thalamus (lowering alerting) (final column table e-2A). Interactions (p < 0.05) occurred contrasting peaks from each of the 3 attention network categories pairwise with peaks from either of the other 2 categories. The sensitivity and specificity of the ANT at predicting lesion location with respect to the appropriate anatomical network, as identified here, averaged 84% and 69%, respectively (table e-2C).
Lesion × cue × flanker interactions.
In controls, conflict increased with alerting, but decreased with orienting (RT data; table e-1B; see also reference 13). To ascertain whether certain lesion sites altered this profile, we conducted group × cue × flanker ANOVAs in regions showing group × flanker, or group × cue, interactions (figure 5; table e-3). Lesions to RMCR, inferior prefrontal, and RTPJ reversed the control profile, in that alerting decreased conflict; while orienting heightened conflict. In the case of RTPJ, one interpretation of increased conflict with orienting—and of decreased accuracy with orienting, noted earlier—is that spatial, but not central, cues misdirected attention towards flanker, rather than target, locations.22 This is supported by the observation that, with RTPJ Lesion+ specifically, spatial cues worsened accuracy similarly for neutral and incongruent targets, relative to congruent targets (cue × group: p < 0.001, and p < 0.05, respectively; arrow in lower panes figure 5).
Figure 5. Group × cue × flanker interactions.
Plots of conflict size (incongruent − congruent flanker, expressed as % of mean) against cue type, for each subject group, demonstrate significant group × cue × flanker interactions (p < 0.001) in (A) a right middle corona radiata (for reaction times [RTs]) and (B) a right temporoparietal junction (for accuracy). For RTs, controls (dashed line) showed a significant cue × flanker interaction due to greater conflict with alerting, but less conflict with orienting—both of which patterns were reversed in these lesion groups. Panels A.b and B.b indicate the raw values of RT and accuracy, for each cue type, and for each flanker. Note that for right temporoparietal lesions, spatial cues resulted in poorer accuracy for both neutral and incongruent targets (arrowed), a pattern not seen elsewhere, and possibly indicating that such subjects were misdirected by spatial cues towards flanker locations.
Interactions of group × flanker (or cue) × target location × target direction, in each of the regions presented, are summarized in table e-3.
DISCUSSION
We have identified 3 sets of lesion locations that result in selective impairments to 1 each of the 3 attention types proposed by the “attention network” model3,4: namely, conflict resolution with bilateral prefrontal and premotor areas; orienting with right pulvinar and temporoparietal regions; and alerting with anteromedial bithalamic nuclei and upper brainstem. To the degree that these anatomical pairings are nonoverlapping, and that behavioral dissociations are manifest among the 3 sets of regions, our data provide strong support for the model's taxonomy, and prediction of functional–anatomical network independence. Whereas previous evidence for tri-network separability comes from behavioral,4,13 functional imaging,5 and structural imaging6,23 studies, our results demonstrate causal dependence among the 3 attention types and specific neural substrates. Furthermore, unlike previous lesion studies that investigate different attentional types, in different groups of subjects, or using prespecified regions of interest,24 we compare the 3 attentional functions within a single test, in the same patients, while employing an assumption-free, pan-brain, voxelwise analysis.14 The latter point allowed us to appraise the relative importance of a large number of brain regions (not just those in right hemisphere) implicated in attention8,9 and to establish the strongest anatomical associations, with projections onto white matter connections.25 Other strengths of our study were 1) a homogeneous population; 2) small lesion volumes, so enhancing spatial precision; and 3) testing 3–10 days after presentation, thereby lessening possibilities of functional or maladaptive plasticity.
The strongest effect of anatomy on ANT performance was found in premotor areas (RMCR), where lesions increased conflict. This area is more posterior than frontal areas, e.g., anterior cingulate most consistently identified with conflict,6,26 which tend to be uncommon locations for stroke, and so are poorly represented in our sample (figure 1B). However, the fact that there was no difference in conflict size between lesions affecting RMCR alone, compared to those overlapping prefrontal regions, implies that RMCR lesions are sufficient to impair conflict processing, rather than that RMCR lesions tend to extend anteriorly. According to a “hierarchical” model of motor control, the position along an anteroposterior frontal axis at which units becomes activated depends upon the number and complexity of rule inputs.27 By this account, premotor lesions can be sufficient to impede conflict processing, when the task components that generate conflict are relatively low order,28 as is the case for the ANT. Moreover, our finding that conflict processing was impaired most strongly with lesions to prefrontal–premotor white matter projections supports functional imaging findings of prefrontal–premotor coactivation during conflict.29 An additional explanation for the RMCR conflict effect we found is that disruption to right superior longitudinal fasciculus (SLF) and thalamo-prefrontal projections contributed. Right SLF, in particular, has been shown to correlate with executive function,30 possibly by potentiating signal gain from posterior to anterior areas.31
In contrast to the anatomical pairings we found for conflict, the associations we found for orienting and alerting matched more closely those reported from functional imaging of the ANT,5 and from lesion studies testing these functions in isolation. Hence our finding that lesions to RTPJ and pulvinar interfered most strongly with orientation concord well with anatomical profiling of hemispatial neglect,12 and, more relevantly for the ANT, vertical orienting deficits.32 The fact that RTPJ lesions impaired both upwards and downwards orienting supports a conception of this region in terms of nonlateralized spatial orienting processes,33 although it is possible that our RTPJ lesion set straddled both upper and lower hemifield representations.34 Meanwhile, our anatomical results for alerting-deficit trace well the projections among locus ceruleus, reticular thalamic nuclei, and (right > left) cerebral hemispheres, proposed as the circuit of an ascending norepinephrinergic activation system.7,12
While our principal results confirmed separable anatomical networks for the 3 attention types, we additionally found evidence for anatomical dependency of internetwork interactions and, to a lesser extent, overlap (e.g., in anterior insula). Lesion locations in right hemisphere that had shown strong conflict- or orienting-specific deficits showed a reversal of the normal profile by which alerting and orienting influence conflict.13 The first finding—that with right inferior frontal, RMCR, and RTPJ lesions, alerting ameliorates conflict processing—supports studies showing that alerting may enhance other attention types when they are suboptimal (e.g., due to disease)35 and that RTPJ registers uncued events.12 The second finding—that with RMCR-prefrontal and RTPJ lesions, orienting worsens conflict processing—may be because right hemisphere lesions result in impaired processing at the same locations as preceding spatial cues, (i.e., “attentional blink”36). Alternatively, lesions here may have engendered inappropriately vectored orientation22 (similar to “optic apraxia” seen with biparietal lesions), with spatial rather than central cues. Finally, the fact that lesions to RTPJ and anteromedial thalamus impaired performance across all conditions (figures 2–4, B) underlines the importance of these regions in mediating general responsiveness or sustained attention7,11 as well as orienting and phasic alerting, respectively. By contrast, lesions to RMCR engendered a deficit specifically under high-conflict conditions, matching results of previous studies of frontal lesions,37 especially to ventrolateral rather than superomedial areas (the latter of which can retard motor responses generally, but are infrequent sites for stroke).
As well as consolidating an influential neurobiological theory of attention, our results strike significant clinical resonance. More than half of our unselected sample of stroke patients contributed to 1 of the 3 principal anatomical networks showing associations with 1 each of the 3 attention network functions while a far smaller proportion actually had neglect or inattention. These results support observations showing that clinically relevant attentional deficits may be missed by bedside examination, but detectable by computer-based tests,38 and indicate those lesion locations most at risk of attentional deficits, for which more thorough assessment might be worthwhile. Furthermore, because each of the 3 attention networks may be influenced by different pharmacologic,3 electrical,39 or cognitive-behavioral40 therapies, and because certain attention deficits can impact other functional impairments, e.g., weakness,1 our results might help in the anatomical stratification of patients for rehabilitation.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the late Jon Driver for comments.
GLOSSARY
- ANOVA
analysis of variance
- ANT
attention network test
- DWI
diffusion-weighted imaging
- RMCR
right middle corona radiata
- RT
reaction time
- RTPJ
right temporoparietal junction
- SLF
superior longitudinal fasciculus
Footnotes
Supplemental data at www.neurology.org
Editorial, page 782
AUTHOR CONTRIBUTIONS
Paul Rinne: acquisition of data, analysis, write-up. Mursyida Hassan: acquisition of data, analysis, write-up. Despina Goniotakis: acquisition of data, analysis, write-up. Kiran Chohan: acquisition of data. Pankaj Sharma: study supervision. Dawn Langdon: study supervision. David Soto: interpretation and critical revision of the manuscript for important intellectual content. Paul Bentley: study concept and design, analysis, interpretation, write-up.
STUDY FUNDING
P.R. is funded by a NIHR Biomedical Research Council Project Award. D.S. is supported by a Medical Research Council Award (UK, 89631). P.B. is supported by a Department of Health Higher Education Funding Council for England Award.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: a 2-year follow-up study. Neuropsychology 1997;11:290–295 [DOI] [PubMed] [Google Scholar]
- 2.Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci 2006;7:367–379 [DOI] [PubMed] [Google Scholar]
- 3.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci 2012;35:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci 2002;14:340–347 [DOI] [PubMed] [Google Scholar]
- 5.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage 2005;26:471–479 [DOI] [PubMed] [Google Scholar]
- 6.Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb Cortex 2011;21:345–356 [DOI] [PubMed] [Google Scholar]
- 7.Clemens B, Zvyagintsev M, Sack A, Heinecke A, Willmes K, Sturm W. Revealing the functional neuroanatomy of intrinsic alertness using fMRI: methodological peculiarities. PLoS One 2011;6:e25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweizer TA, Oriet C, Meiran N, Alexander MP, Cusimano M, Stuss DT. The cerebellum mediates conflict resolution. J Cogn Neurosci 2007;19:1974–1982 [DOI] [PubMed] [Google Scholar]
- 9.Suchan J, Karnath HO. Spatial orienting by left hemisphere language areas: a relict from the past? Brain 2011;134:3059–3070 [DOI] [PubMed] [Google Scholar]
- 10.Coulthard EJ, Nachev P, Husain M. Control over conflict during movement preparation: role of posterior parietal cortex. Neuron 2008;58:144–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain 2009;132:645–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci 2011;34:569–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callejas A, Lupiàñez J, Funes MJ, Tudela P. Modulations among the alerting, orienting and executive control networks. Exp Brain Res 2005;167:27–37 [DOI] [PubMed] [Google Scholar]
- 14.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003;6:448–450 [DOI] [PubMed] [Google Scholar]
- 15.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322 [DOI] [PubMed] [Google Scholar]
- 16.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 2001;14:486–500 [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300 [Google Scholar]
- 18.Townsend J, Ashby F. Methods of Modeling Capacity in Simple Processing Systems. Hillsdale, NJ: Erlbaum; 1978 [Google Scholar]
- 19.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–757 [DOI] [PubMed] [Google Scholar]
- 20.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhang J, Oishi K, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 2010;52:1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Làdavas E, Carletti M, Gori G. Automatic and voluntary orienting of attention in patients with visual neglect: horizontal and vertical dimensions. Neuropsychologia 1994;32:1195–1208 [DOI] [PubMed] [Google Scholar]
- 23.Yin X, Han Y, Ge H, et al. Inferior frontal white matter asymmetry correlates with executive control of attention. Hum Brain Mapp 2011;34:796–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci 1984;4:1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage 2012;62:2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niogi S, Mukherjee P, Ghajar J, McCandliss BD. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Front Neuroanat 2010;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badre D, D'Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci 2009;10:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badre D, Hoffman J, Cooney JW, D'Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat Neurosci 2009;12:515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melcher T, Gruber O. Decomposing interference during Stroop performance into different conflict factors: an event-related fMRI study. Cortex 2009;45:189–200 [DOI] [PubMed] [Google Scholar]
- 30.Seiler CB, Jones KE, Shera D, Armstrong CL. Brain region white matter associations with visual selective attention. Brain Imaging Behav 2011;5:262–273 [DOI] [PubMed] [Google Scholar]
- 31.Roberts RE, Anderson EJ, Husain M. Expert cognitive control and individual differences associated with frontal and parietal white matter microstructure. J Neurosci 2010;30:17063–17067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitzalis S, Spinelli D, Zoccolotti P. Vertical neglect: behavioral and electrophysiological data. Cortex 1997;33:679–688 [DOI] [PubMed] [Google Scholar]
- 33.Nachev P, Husain M. Disorders of visual attention and the posterior parietal cortex. Cortex 2006;42:766–773 [DOI] [PubMed] [Google Scholar]
- 34.Mennemeier M, Wertman E, Heilman KM. Neglect of near peripersonal space: evidence for multidirectional attentional systems in humans. Brain 1992;115(pt 1):37–50 [DOI] [PubMed] [Google Scholar]
- 35.Chica AB, Thiebaut de Schotten M, Toba M, Malhotra P, Lupiáñez J, Bartolomeo P. Attention networks and their interactions after right-hemisphere damage. Cortex 2012;48:654–663 [DOI] [PubMed] [Google Scholar]
- 36.Correani A, Humphreys GW. An impaired attentional dwell time after parietal and frontal lesions related to impaired selective attention not unilateral neglect. Cogn Neuropsychol 2011;28:363–385 [DOI] [PubMed] [Google Scholar]
- 37.Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S. Regional frontal injuries cause distinct impairments in cognitive control. Neurology 2007;68:1515–1523 [DOI] [PubMed] [Google Scholar]
- 38.Rengachary J, d'Avossa G, Sapir A, Shulman GL, Corbetta M. Is the posner reaction time test more accurate than clinical tests in detecting left neglect in acute and chronic stroke? Arch Phys Med Rehabil 2009;90:2081–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiff S, Bardi L, Basso D, Mapelli D. Timing spatial conflict within the parietal cortex: a TMS study. J Cogn Neurosci 2011;23:3998–4007 [DOI] [PubMed] [Google Scholar]
- 40.Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci 2007;7:109–119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.