Abstract
Objective:
To evaluate the effects of onabotulinumtoxinA on patient-reported outcomes including health-related quality of life (HRQOL), treatment satisfaction, and treatment goal attainment in patients with urinary incontinence (UI) due to neurogenic detrusor overactivity (NDO).
Methods:
In this multicenter, double-blind, randomized, placebo-controlled, phase III, 52-week study (ClinicalTrials.gov NCT00311376), patients with UI due to NDO who were not adequately managed with anticholinergic therapy were treated with intradetrusor injections of onabotulinumtoxinA (200 or 300 U) or placebo (0.9% saline). HRQOL measures included the Incontinence Quality of Life (I-QOL) Questionnaire total score, and the 3 domain scores (avoidance and limiting behavior, psychosocial, and social embarrassment), the modified Overactive Bladder Patient Satisfaction with Treatment Questionnaire (OAB-PSTQ), and Patient Global Assessment. Assessments were made at baseline, posttreatment week 6 (primary time point), week 12, and at 12-week intervals.
Results:
Patients (mean age of 46 years with 30.5 weekly UI episodes at baseline) were randomized to receive placebo (n = 149) or onabotulinumtoxinA (200 U [n = 135] or 300 U [n = 132]). At week 6, improvements from baseline in I-QOL Questionnaire total score were greater (p < 0.001) in both onabotulinumtoxinA-treated groups vs placebo. Responses to the OAB-PSTQ also demonstrated greater mean improvements from baseline (p < 0.001) in both onabotulinumtoxinA-treated groups vs placebo at week 6. Patients who received onabotulinumtoxinA also reported greater improvement in the Patient Global Assessment than those in the placebo group (p ≤ 0.001 vs placebo).
Conclusions:
Patients with UI due to NDO reported greater improvement in HRQOL and treatment satisfaction with onabotulinumtoxinA than with placebo consistently across several patient-reported outcome instruments.
Classification of evidence:
This study provides Class I evidence that onabotulinumtoxinA intradetrusor injections (200 or 300 U) can improve quality of life measures in patients with NDO not adequately managed with anticholinergic therapy.
Neurogenic detrusor overactivity (NDO) frequently occurs as a consequence of spinal cord injury (SCI) or other neurologic conditions such as multiple sclerosis (MS). Urinary symptoms associated with NDO, such as urinary incontinence (UI), are detrimental to patients' health-related quality of life (HRQOL).1–3 These patients require lifelong, intensive medical care to maintain their physical health.4 Anticholinergic drugs, frequently combined with clean intermittent catheterization (CIC), are considered first-line therapy for UI in these patients. However, lack of efficacy and side effects, including dry mouth, constipation, and blurred vision,5 often lead patients with NDO to discontinue treatment.6
OnabotulinumtoxinA can reduce the number of UI episodes, and improve urodynamic parameters and HRQOL measures.7–9 Herein, we present the health outcomes analyses of a phase III study of onabotulinumtoxinA in patients with NDO8 using disease-specific, patient-reported outcomes (PROs) that focus on HRQOL, treatment satisfaction, and treatment goal attainment.
METHODS
Study design.
This was a global, multicenter, double-blind, randomized, placebo-controlled, phase III study conducted in North America, Europe, and the Asia-Pacific region (part of the Dignity [Double-blind Investigation of Purified Neurotoxin-complex in Neurogenic Detrusor Overactivity] Study Program) from September 2006 to May 2010.
Standard protocol approvals, registrations, and patient consents.
This study (ClinicalTrials.gov identifier NCT00311376) was performed in accordance with independent ethics committee regulations in each country, with approval obtained before study initiation in accordance with country and local regulatory requirements. Written informed consent was obtained from all patients.
Study population.
Details of patient selection have been published.8 In brief, eligible patients were men or women aged 18 to 80 years with confirmed MS or SCI, who reported ≥14 UI episodes/week due to NDO, and whose symptoms were not adequately managed by anticholinergics. Not adequately managed was defined as an inadequate response to or intolerable side effects after at least 1 month of anticholinergic therapy on an optimized dose. Despite the fact that patients had either inadequately responded to or experienced intolerable side effects with anticholinergic therapy, they were allowed to remain on anticholinergics as long as they continued at a stable dose throughout the study. Conversely, patients who were not on anticholinergics at study entry were not allowed to start anticholinergics during the study. The study included patients using CIC, spontaneous/voluntary voiding, or both. Patients were required to maintain established frequency if already using CIC or be willing to initiate CIC if necessary. The study excluded patients with pelvic or urologic abnormalities or those who had previous botulinum toxin therapy for urologic conditions.
Methodology and treatment.
Patients were randomized (1:1:1) to receive 30 intradetrusor injections (1 mL each) of onabotulinumtoxinA (200 or 300 U; BOTOX, Allergan, Inc., Irvine, CA) or placebo (0.9% saline), administered via cystoscopy, avoiding the bladder trigone.8 It must be noted that the biological activity of onabotulinumtoxinA cannot be compared with or converted into units of any other botulinum toxin product.10 Patients were followed for 52 weeks and could request a second treatment from 12 weeks onward.
Each patient who qualified for entry was assigned a randomization number through an automated interactive voice response system/interactive web response system, which determined the treatment group assignment. Sites dispensed medication according to interactive voice response system/interactive web response system instructions. To prevent possible unblinding, access to study medication kits was prohibited for staff involved in the care and evaluation of study patients or the handling of study data. An independent reconstitutor, not affiliated with the care of the patient, was required to prepare the study medication for administration for each study patient. The independent reconstitutor could have been a pharmacist at the study site or someone at the study clinic qualified to prepare study medication (e.g., nurse).
Study hypotheses/classification of evidence.
The overall hypotheses tested in this trial were that onabotulinumtoxinA treatment would provide a reduction in UI episodes, improvement in urodynamic parameters, and improvement in HRQOL in patients with NDO (Incontinence Quality of Life [I-QOL] Questionnaire total score). The primary efficacy endpoint for this trial was defined at 6 weeks postinjection by the change from baseline in the weekly number of UI episodes, as measured using a 7-day patient bladder diary. Secondary efficacy endpoints included maximum cystometric capacity and maximum detrusor pressure during the first involuntary detrusor contraction. Efficacy and tolerability endpoints have been reported elsewhere.8 The secondary PRO endpoints described here included disease-specific HRQOL measures. This study provides Class I evidence that onabotulinumtoxinA intradetrusor injections (200 or 300 U) can improve quality-of-life (QOL) measures in patients with NDO not adequately managed with anticholinergic therapy.
Assessments.
Incontinence Quality of Life Questionnaire.
The I-QOL is a self-administered, validated, disease-specific, 22-item questionnaire designed to measure the impact of UI on patients' lives.3 It provides a total score ranging from 0 to 100 with higher scores reflecting better QOL (sum of all 22 individual items) plus 3 domain scores: avoidance and limiting behavior (items 1, 2, 3, 4, 10, 11, 13, and 20); psychosocial impact (items 5, 6, 7, 9, 15, 16, 17, 21, and 22); and social embarrassment (items 8, 12, 14, 18, and 19). The I-QOL Questionnaire was administered at baseline, weeks 6 and 12, and at clinic visits every 12 weeks thereafter. A responder analysis was based on a ≥11-point increase from baseline, previously shown to be at the upper range of the minimally important difference (MID) (defined as the smallest difference in score in which patients perceive as beneficial and would mandate a change in the patient's management) for the I-QOL Questionnaire in patients with NDO.3,11
Modified Overactive Bladder Patient Satisfaction with Treatment Questionnaire.
The Overactive Bladder Patient Satisfaction with Treatment Questionnaire (OAB-PSTQ) is a validated measure consisting of 12 questions (2–13 in the study questionnaire; see table e-1 on the Neurology® Web site at www.neurology.org) that assess the respondent's satisfaction with the impact of medication on 3 separate concepts: 1) various symptoms of overactive bladder (OAB) including incontinence; 2) the individual's ability to interact more freely in social situations, activities, and relationships; and 3) on OAB/incontinence-related costs.12–15 The OAB-PSTQ was administered at baseline, weeks 6 and 12, and at clinic visits every 12 weeks thereafter, and questions were scored on a 6-point scale (1 = very satisfied, 2 = somewhat satisfied, 3 = neutral, 4 = somewhat dissatisfied, 5 = very dissatisfied, 6 = does not apply to me). Lower scores reflected greater satisfaction (a score of 6 for a specific question was scored as missing in the calculations and not included in the total score). The OAB-PSTQ total score ranged from 0 to 100 (greatest satisfaction) and was calculated using the following formula: {[(sum of item scores/number of nonmissing items) – 1]/4} × 100 for all observations with ≥50% of the 12 items completed; missing values were imputed using the mean of the items answered.
The modified OAB-PSTQ14,15 included 4 additional questions (table e-1) that addressed treatment satisfaction (question 1, “In the past 4 weeks, how satisfied have you been overall with your current or recent treatment(s)?” scored as questions 2–13), a subjective (self-reported) assessment of severity of side effects (question 14, “In the past 4 weeks, how would you rate the side effects due to your treatment(s)?” scored as 1 = no, 2 = mild, 3 = moderate, 4 = severe), achievement of primary treatment goal (question 15, “Looking back at your primary goal(s) for treatment, how would you rate how effectively the treatment helped you achieve your stated goal(s)” scored as 1 = no progress, 2 = some progress, 3 = moderate progress, 4 = significant progress, 5 = complete achievement), and fulfillment of treatment expectations (question 16, “Looking back at your primary expectation(s) for treatment, how would you rate how effectively the treatment met your stated expectation(s)?” scored as 1 = did not meet, 2 = somewhat met, 3 = moderately met, 4 = significantly met, 5 = exceeded expectations). Questions 1, 14, 15, and 16 were scored as individual items. Individual statements of treatment goals and expectations were obtained at baseline and used to assess subsequent progress.
Patient Global Assessment.
The Patient Global Assessment (PGA) included 4 single-item, nonvalidated scales that assessed the patient's symptoms, QOL, activity limitations, and overall emotions related to UI secondary to NDO.14,15 Each item was scored on a scale ranging from −7 to +7 (−7 = a very great deal worse; −6 = a great deal worse; −5 = a good deal worse; −4 = moderately worse; −3 = somewhat worse; −2 = a little worse; −1 = almost the same, hardly any worse at all; 0 = no change; +1 = almost the same, hardly any better at all; +2 = a little better; +3 = somewhat better; +4 = moderately better; +5 = a good deal better; +6 = a great deal better; +7 = a very great deal better). At each follow-up visit, the mean group change was calculated by summing the scores (−7 to +7) and dividing by the number of complete responses. A separate analysis was performed by reporting the number and proportion of patients reporting a score of −2 or below indicating deterioration; a score of −1, 0, or 1 indicating the condition is unchanged; and a score of +2 and higher indicating improvement. The PGA evaluated change since the last visit to the clinic and was therefore not completed at baseline.
Statistical analyses.
Analyses were performed using the intention-to-treat population and focused on the 6- and 12-week time points after initial treatment. Differences between the onabotulinumtoxinA-treated groups and placebo groups in the change from baseline I-QOL Questionnaire total and domain scores and OAB-PSTQ total scores were analyzed using an analysis of covariance model with baseline value as a covariate and treatment group, etiology at entry, concurrent use/nonuse of anticholinergics at screening, and investigative site as factors. A 2-sided test with p value ≤0.05 (unadjusted for multiplicity) was considered statistically significant, except for the I-QOL Questionnaire total score at week 6, which was analyzed as 1 of 3 key secondary efficacy endpoints using a hierarchical approach.8 The proportion of patients in each active treatment group achieving a ≥11-point increase from baseline in I-QOL Questionnaire total score (MID) compared with placebo was evaluated using Fisher exact test or Pearson χ2 test, as appropriate.
Pairwise comparisons between each of the onabotulinumtoxinA-treated groups with the placebo group were performed using a χ2 test for questions 1 and 14 to 16 on the modified OAB-PSTQ at weeks 6 and 12. For question 1, the percentage of patients reporting satisfaction with treatment (somewhat satisfied and very satisfied) was determined, and pairwise comparisons between each of the onabotulinumtoxinA-treated groups and placebo group were performed. The same types of analyses were performed for question 15 (combining the percentages of patients reporting significant progress and complete achievement of goal) and question 16 (combining the percentages of patients reporting having met or exceeded treatment expectations).
Two separate analyses of the 4 PGAs were conducted. At each follow-up visit, the mean group change was calculated by summing the scores (−7 to +7) and dividing by the number of complete responses to obtain an overall mean change for the group. Pairwise comparison among treatment groups was performed using a χ2 test.
RESULTS
Patient disposition.
A total of 416 patients (227 with MS and 189 with SCI) were randomized and 407 received treatment. At week 12, 399 remained in the study. There were no differences between groups at baseline (table 1).
Table 1.
Baseline demographic and clinical characteristics (intention-to-treat population)

Efficacy.
As reported previously,8 at 6 weeks, onabotulinumtoxinA reduced mean weekly UI episodes from baseline (30.5 UI episodes/week) by 21 episodes/week (67% reduction) in the 200 U group, and by 23 episodes/week (74% reduction) in the 300 U group, compared with 9 episodes/week (30% reduction) in the placebo group. Efficacy plateaued at 200 U with no extra benefit accruing from an increase in dose up to 300 U. Maximum cystometric capacity increased from baseline (255 mL) by more than 150 mL in both treated groups vs only 16 mL in the placebo group. Maximum detrusor pressure during first involuntary detrusor contraction was reduced from baseline (50 cm·H2O) by more than 33 cm·H2O in both treated groups vs only 2.4 cm·H2O in the placebo group. There were no differences in any efficacy measure between the 2 onabotulinumtoxinA doses.
Patient-reported outcomes.
Incontinence Quality of Life Questionnaire.
At weeks 6 and 12, improvements from baseline in I-QOL Questionnaire total score were greater after onabotulinumtoxinA treatment compared with placebo (all p values <0.001 vs placebo; figure 1A). A larger proportion of patients who received either dose of onabotulinumtoxinA had a ≥11-point increase from baseline in I-QOL Questionnaire total score compared with placebo (all p values <0.001 vs placebo; figure 1B). Improvements from baseline in the avoidance/limiting behavior, psychosocial impact, and social embarrassment domains of the I-QOL Questionnaire also were greater after active treatment compared with placebo (all p values <0.001 vs placebo; table 2).
Figure 1. Effects of onabotulinumtoxinA on I-QOL Questionnaire score (intention-to-treat population).

(A) Change from baseline in I-QOL Questionnaire total score at weeks 6 and 12. (B) Percentage of patients with increase from baseline in I-QOL Questionnaire total score ≥11 points at weeks 6 and 12. *p < 0.001 vs placebo. I-QOL = Incontinence Quality of Life; MID = minimally important difference; OnabotA = onabotulinumtoxinA.
Table 2.
Baseline and mean change from baseline in I-QOL Questionnaire domain scores at weeks 6 and 12 (intention-to-treat population)
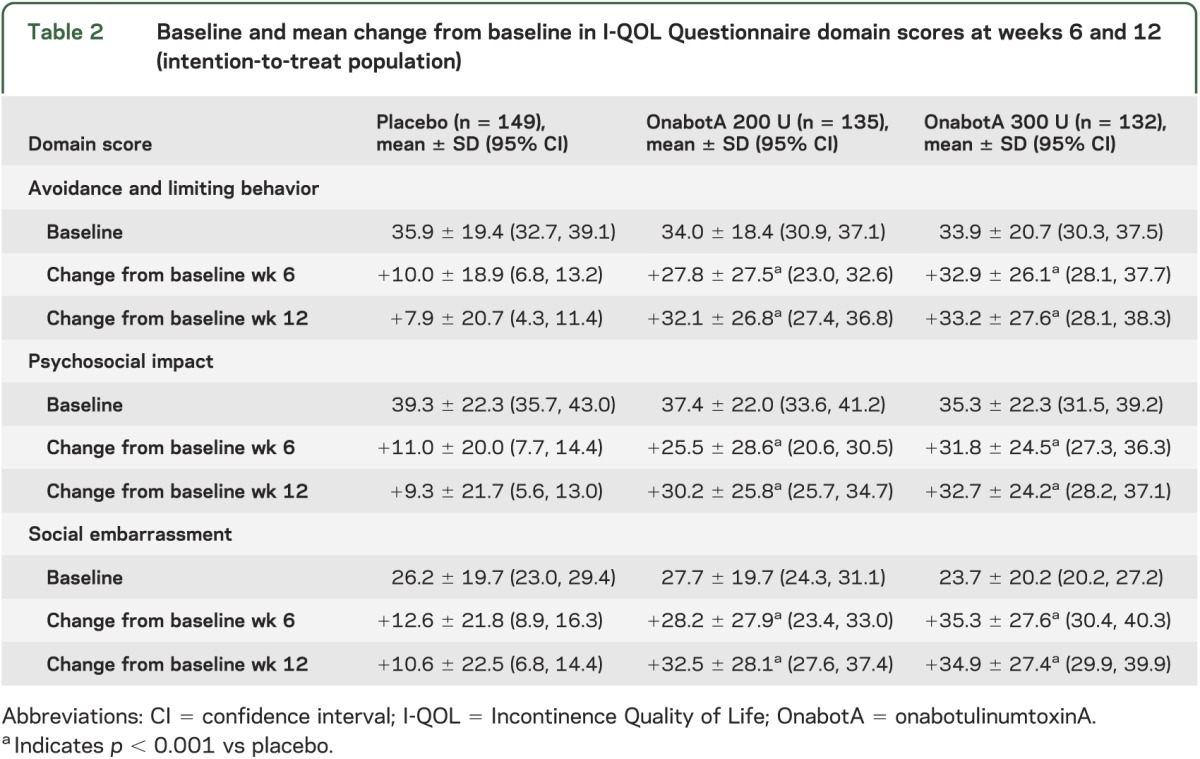
Modified OAB-PSTQ.
OAB-PSTQ total score (Q2–Q13) improved in the onabotulinumtoxinA-treated groups compared with the placebo group (all p values <0.001 vs placebo; figure 2A). These results indicated that onabotulinumtoxinA treatment improved overall satisfaction with treatment effects in a number of areas including urinary frequency, ability to engage in social activities, sleep, travel, relationships, money spent on treatments, and embarrassment.
Figure 2. Effects of onabotulinumtoxinA on modified OAB-PSTQ score at weeks 6 and 12 (intention-to-treat population).
(A) Change from baseline in OAB-PSTQ total score (questions 2–13). (B) Percentage of patients reporting satisfaction with treatment (somewhat satisfied or very satisfied, modified OAB-PSTQ question 1). (C) Percentage of patients with self-report of no side effects (modified OAB-PSTQ question 14). (D) Percentage of patients reporting achievement of primary treatment goal (significant progress or complete achievement, modified OAB-PSTQ question 15). (E) Percentage of patients reporting having met or exceeded treatment expectations (modified OAB-PSTQ question 16). Percentages are based on the numbers of patients who answered at each time point. *p < 0.001 vs placebo. OAB-PSTQ = Overactive Bladder Patient Satisfaction with Treatment Questionnaire; OnabotA = onabotulinumtoxinA.
At baseline, approximately 45% of patients in all treatment groups reported being somewhat or very satisfied with their current or recent therapy (table 1). After onabotulinumtoxinA treatment, patient satisfaction was increased (question 1), with more than 75% of onabotulinumtoxinA-treated patients reporting that they were “somewhat satisfied” or “very satisfied” at weeks 6 and 12 compared with only 45% (week 6) and 30% (week 12) of placebo-treated patients (p < 0.001 for both comparisons; figure 2B). At week 6, a majority of patients reported “no side effects” in the onabotulinumtoxinA-treated groups (figure 2C). Greater percentages of patients randomized to onabotulinumtoxinA (both dose groups) reported “significant progress” or “complete achievement” of primary treatment goal (question 15) at weeks 6 and 12 (p < 0.001 for both comparisons; figure 2D) and reported having “significantly met” or “exceeded expectations” for treatment (question 16) compared with those who received placebo (p < 0.001 for both comparisons; figure 2E).
Patient Global Assessment.
PGAs performed at week 6 revealed greater proportions of patients in the onabotulinumtoxinA-treated groups who reported improvement in overall symptoms, QOL, activity limitations, and overall emotions related to their bladder problem than those in the placebo group (all p values ≤0.001 vs placebo; figure 3, A–D). Because the PGA evaluates change from the last visit, the percentage of patients reporting improvement at week 12 (relative to week 6) was lower than week 6 (relative to baseline). Even so, from week 6 to week 12, the majority of patients receiving onabotulinumtoxinA 200 or 300 U either improved or maintained the improvement obtained at week 6 (figure 3, A–D). Patients who received placebo treatment most often reported no change for all 4 scales.
Figure 3. Effects of onabotulinumtoxinA on Patient Global Assessment in the intention-to-treat population.
Percentage of patients reporting deterioration (worse), no change (NC), or improvement (Impr) in (A) overall symptoms; (B) quality of life; (C) activity limitations; and (D) overall emotion. Percentages are based on the numbers of patients who answered at each time point. *p < 0.001 vs placebo. OnabotA = onabotulinumtoxinA.
DISCUSSION
One of the primary goals in the treatment of patients with NDO is to improve patient QOL and support independent living and rehabilitation.16 For this reason, disease-specific PROs are an important adjunct to diary and urodynamic measures of clinical efficacy for any NDO treatment, as they reflect the patient-perceived impact of treatment on daily life. In this study, patients with NDO who received onabotulinumtoxinA demonstrated important improvements vs placebo in I-QOL Questionnaire total score, modified OAB-PSTQ, and PGA. Indeed, in this study, most onabotulinumtoxinA-treated patients achieved improvement in I-QOL Questionnaire total scores of ≥11 points at both 6 and 12 weeks, greater than the MID of 4 to 11 points estimated by Schurch et al.3
The improvements in I-QOL Questionnaire scores observed in this study were consistent with previous reports in patients treated with onabotulinumtoxinA. In a parallel study,15 onabotulinumtoxinA improved I-QOL Questionnaire total and domain scores at 6 and 12 weeks. Improvements in I-QOL Questionnaire total and domain scores at 24 weeks were also noted in a smaller study of 59 patients with NDO who received a single treatment with onabotulinumtoxinA.2
One important consideration for patients with NDO is that many of them may need to initiate CIC because treatment with onabotulinumtoxinA may result in compromised bladder emptying. Indeed, willingness to initiate CIC may be intimidating to some patients and it must be part of the physician/patient conversation before initiating this therapy. To evaluate whether initiating CIC might negatively affect HRQOL, changes from baseline in total I-QOL Questionnaire score in patients using CIC at baseline and in those who did not use CIC at baseline, but initiated CIC after treatment, were examined and found to be similar.8 This finding suggested that the need to begin CIC after onabotulinumtoxinA treatment did not have a negative effect on this parameter.
This study utilized the OAB-PSTQ, which includes specific treatment satisfaction items derived from patients with OAB.3,14,15,17 Changes in this composite measure reflect the comparative beneficial effect of onabotulinumtoxinA on symptoms as well as patients' lives. Across the domains, patients were more satisfied with the benefits from onabotulinumtoxinA treatment compared with placebo. In addition, patients responding to the additional questions (questions 1 and 14–16) were more likely to report being satisfied with treatment, reaching their self-defined treatment goals, and meeting treatment expectations. Thus, it is reasonable to conclude that the reductions in weekly UI episodes and improved urodynamics reported in the primary analysis of this study8 were associated with improved QOL, as reported here.
The PGA is a well-researched scale used to assess patient-reported changes in many disease states.18 Although the PGA is not a validated measure for patients with NDO, it has been used both in patients with OAB14 and NDO,15 and the results provide further evidence of the benefit of onabotulinumtoxinA treatment from the patient's perspective.
The primary efficacy analysis showed no benefits of onabotulinumtoxinA 300 U over the 200-U dose regarding efficacy or duration of effect.8 In the current PRO analysis, there also appears to be no additional benefit of onabotulinumtoxinA 300 U over 200 U regarding the I-QOL or OAB-PSTQ measures, as was found in a similar study.15 Overall, there was no clinically relevant difference in PROs between the onabotulinumtoxinA 200- and 300-U doses.
This study had a number of limitations, including the lack of validation of the PGA for use in patients with NDO. The PRO instruments used in this trial were the best tools available to address the specific concepts at the time that the trial protocol was developed. Another limitation was that results from this study cannot be generalized or extrapolated to patients with idiopathic OAB. Finally, this study was not adequately powered to allow statistical comparisons between active treatment groups.
In patients with NDO and UI, treatment with onabotulinumtoxinA improved HRQOL, treatment satisfaction, and goal attainment. This study supports the use of onabotulinumtoxinA in patients with NDO and UI who are not adequately managed by anticholinergic medications.
Supplementary Material
GLOSSARY
- CIC
clean intermittent catheterization
- HRQOL
health-related quality of life
- I-QOL
Incontinence Quality of Life
- MID
minimally important difference
- MS
multiple sclerosis
- NDO
neurogenic detrusor overactivity
- OAB
Overactive bladder
- OAB-PSTQ
Overactive Bladder Patient Satisfaction with Treatment Questionnaire
- PGA
Patient Global Assessment
- PRO
patient-reported outcome
- QOL
Quality of life
- SCI
spinal cord injury
- UI
urinary incontinence
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Chancellor: drafting/revising the study manuscript, analysis and interpretation of data, study investigator. Dr. Patel: study design, drafting/revising the study manuscript, data analysis, interpretation of data. Dr. Leng and Dr. Shenot: drafting/revising the study manuscript, analysis and interpretation of data, study investigator. Dr. Lam: study design, drafting/revising the study manuscript, data analysis, interpretation of data, biostatistical analysis. Dr. Globe: drafting/revising the study manuscript, data analysis, data interpretation. Dr. Loeb: writing/editorial assistance, preparation of figures and tables. Dr. Chapple: drafting/revising the study manuscript, analysis and interpretation of data, study investigator.
STUDY FUNDING
This study and its analysis were sponsored by Allergan, Inc., Irvine, CA.
DISCLOSURE
M. Chancellor has been an investigator, consultant, and/or meeting participant for Allergan, Astellas, Cook, Lipella, Merck, Pfizer and Targacept. V. Patel is an employee of Allergan, Inc. W. Leng has been an investigator and/or consultant for Allergan. P. Shenot has been an investigator and/or consultant for Allergan and Merck. W. Lam and D. Globe are employees of Allergan, Inc. A. Loeb provided writing assistance for manuscript development that was funded by Allergan, Inc. C. Chapple has been an investigator and/or consultant for Lilly, AMS, Astellas, Allergan, Pfizer, and Recordati. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hollingworth W, Campbell JD, Kowalski J, et al. Exploring the impact of changes in neurogenic urinary incontinence frequency and condition-specific quality of life on preference-based outcomes. Qual Life Res 2010;19:323–331 [DOI] [PubMed] [Google Scholar]
- 2.Schurch B, Denys P, Kozma CM, Reese PR, Slaton T, Barron RL. Botulinum toxin A improves the quality of life of patients with neurogenic urinary incontinence. Eur Urol 2007;52:850–858 [DOI] [PubMed] [Google Scholar]
- 3.Schurch B, Denys P, Kozma CM, Reese PR, Slaton T, Barron R. Reliability and validity of the Incontinence Quality of Life questionnaire in patients with neurogenic urinary incontinence. Arch Phys Med Rehabil 2007;88:646–652 [DOI] [PubMed] [Google Scholar]
- 4.Stohrer M, Blok B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol 2009;56:81–88 [DOI] [PubMed] [Google Scholar]
- 5.Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int 2007;100:987–1006 [DOI] [PubMed] [Google Scholar]
- 6.Campbell UB, Stang P, Barron R. Survey assessment of continuation of and satisfaction with pharmacological treatment for urinary incontinence. Value Health 2008;11:726–732 [DOI] [PubMed] [Google Scholar]
- 7.Schurch B, de Seze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005;174:196–200 [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA in patients with urinary incontinence resulting from neurogenic detrusor overactivity. J Urol 2012;187:2131–2139 [DOI] [PubMed] [Google Scholar]
- 9.Cruz F, Herschorn S, Heesakkers J, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomized, double-blind, placebo-controlled trial. Eur Urol 2011;60:742–750 [DOI] [PubMed] [Google Scholar]
- 10.Albanese A. Terminology for preparations of botulinum neurotoxins: what a difference a name makes. JAMA 2011;305:89–90 [DOI] [PubMed] [Google Scholar]
- 11.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–415 [DOI] [PubMed] [Google Scholar]
- 12.Sissins P, Barron R. Reliability and validity of the Overactive Bladder–Patient Satisfaction with Treatment Questionnaire (OAB-PSTQ). Qual Life Res 2005;14:2032 [Google Scholar]
- 13.Barron R, Sissins P. Development of a patient satisfaction with treatment questionnaire for overactive bladder (OAB-PSTQ). Qual Life Res 2005;14:2144 [Google Scholar]
- 14.Brubaker L, Gousse A, Sand P, et al. Treatment satisfaction and goal attainment with onabotulinumtoxinA in patients with incontinence due to idiopathic OAB. Int Urogynecol J 2012;23:1017–1025 [DOI] [PubMed] [Google Scholar]
- 15.Sussman D, Patel V, Del Popolo G, Lam W, Globe D, Pommerville P. Treatment satisfaction and improvement in health-related quality of life with onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity. Neurourol Urodyn 2013;32:242–249 [DOI] [PubMed] [Google Scholar]
- 16.Chancellor MB, Anderson RU, Boone TB. Pharmacotherapy for neurogenic detrusor overactivity. Am J Phys Med Rehabil 2006;85:536–545 [DOI] [PubMed] [Google Scholar]
- 17.Fowler CJ, Auerbach S, Ginsberg D, et al. Botulinum toxin A (BOTOX®) demonstrates dose-dependent improvements in health-related quality-of-life measures in idiopathic overactive bladder. J Urol 2009;181:558 Abstract LBA8. [Google Scholar]
- 18.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994;47:81–87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




