Abstract
Objective:
To determine the risk factors associated with dementia with Lewy bodies (DLB).
Methods:
We identified 147 subjects with DLB and sampled 2 sex- and age-matched cognitively normal control subjects for each case. We also identified an unmatched comparison group of 236 subjects with Alzheimer disease (AD). We evaluated 19 candidate risk factors in the study cohort.
Results:
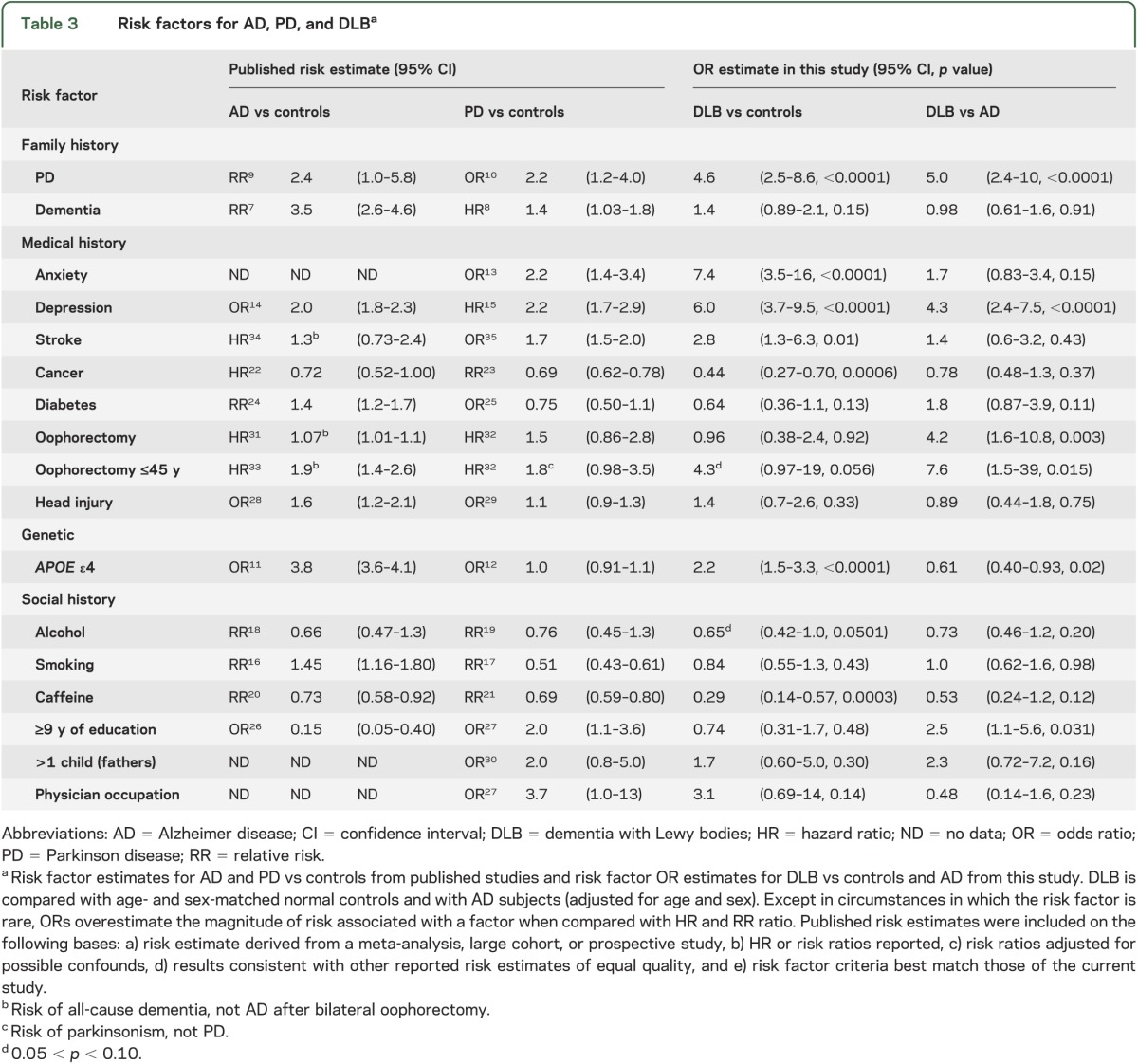
Compared with controls, subjects with DLB were more likely to have a history of anxiety (odds ratio; 95% confidence interval) (7.4; 3.5–16; p < 0.0001), depression (6.0; 3.7–9.5; p < 0.0001), stroke (2.8; 1.3–6.3; p = 0.01), a family history of Parkinson disease (PD) (4.6; 2.5–8.6; p < 0.0001), and carry APOE ε4 alleles (2.2; 1.5–3.3; p < 0.0001), but less likely to have had cancer (0.44; 0.27–0.70; p = 0.0006) or use caffeine (0.29; 0.14–0.57; p < 0.0001) with a similar trend for alcohol (0.65; 0.42–1.0; p = 0.0501). Compared with subjects with AD, subjects with DLB were younger (72.5 vs 74.9 years, p = 0.021) and more likely to be male (odds ratio; 95% confidence interval) (5.3; 3.3–8.5; p < 0.0001), have a history of depression (4.3; 2.4–7.5; p < 0.0001), be more educated (2.5; 1.1–5.6; p = 0.031), have a positive family history of PD (5.0; 2.4–10; p < 0.0001), have no APOE ε4 alleles (0.61; 0.40–0.93; p = 0.02), and to have had an oophorectomy before age 45 years (7.6; 1.5–39; p = 0.015).
Conclusion:
DLB risk factors are an amalgam of those for AD and PD. Smoking and education, which have opposing risk effects on AD and PD, are not risk factors for DLB; however, depression and low caffeine intake, both risk factors for AD and PD, increase risk of DLB more strongly than in either.
Dementia with Lewy bodies (DLB) is the second most common dementia syndrome, representing 10% to 15% of cases. Knowledge of risk factors for DLB may provide clues to the underlying pathophysiology, yet the only known risk factors are advanced age, male sex,1 and a family history of dementia.2 We compared the frequency of Alzheimer disease (AD) and Parkinson disease (PD) risk factors among subjects with DLB to age- and sex-matched controls, and to subjects with AD. Risk factors driving amyloid pathology should be present in AD and, to a lesser extent, in DLB. In contrast, risk factors driving Lewy body pathology should be found in DLB, but not AD or control subjects.
METHODS
Standard protocol approvals, registrations, and patient consents.
All protocols were approved by our institutional review board, and consent was obtained from subjects and carers.
Subjects.
Subjects were recruited into 3 longitudinal studies at Mayo Clinic, Rochester, MN. The Alzheimer Disease Patient Registry (1985–2004)3 and Alzheimer Disease Research Center Study (1999–present) recruited patients with incident dementia and age- and sex-matched controls. The Mayo Clinic Study of Aging (2004–present) follows all community-dwelling persons aged 70 to 89 years.4 No major recoding was required to harmonize the data between the studies.
Cases were diagnosed as clinically probable DLB or AD by experienced behavioral neurologists on the basis of published criteria.5,6 DLB was defined as dementia with 2 or more core features (fluctuations, parkinsonism, or visual hallucinations), or by one core feature plus one or more suggestive features (neuroleptic sensitivity, reduced dopamine uptake on functional imaging, or REM sleep behavior disorder).5 These features, and risk factor exposure, were ascertained as part of a standardized enrollment protocol; cases were then followed prospectively. At enrollment, a clinician obtains a medical history and performs a neurologic examination, a study coordinator interviews the subject and their informant, and a neuropsychologist administers a comprehensive test battery. The clinician, study coordinator, and neuropsychologist independently evaluate or diagnose the subject before a weekly consensus meeting. Further details on recruitment, subjects, procedures, and data definitions are provided elsewhere.3,4 Individuals with structural brain lesions were excluded. Each subject with DLB was matched to 2 age-matched (±5 years) and sex-matched controls from the cohort studies. The control subjects underwent an extensive cognitive and medical evaluation, did not have evidence of cognitive impairment, movement disorder, stroke, head injury, or other neurologic disease, and saw the physician in the same month as a clinical subject was diagnosed. Control subjects who were subsequently diagnosed with DLB or AD remained in the control group for the purposes of the analysis. Pathologic confirmation of the diagnosis was obtained when possible.
Candidate risk factors.
We confined our risk factor assessment to 19 demographic, genetic, or disease characteristics associated with DLB, AD, or PD and for which data were available from the studies: age,5 sex,5 family history of dementia2,7,8 or PD,9,10 APOE ε4 status (any ε4 alleles vs none),11,12 history of anxiety13 or depression,14,15 smoking (ever vs never in lifetime),16,17 alcohol (ever vs never)18,19 and caffeine consumption (ever vs never),20,21 cancer (excluding nonmelanoma skin cancer),22,23 diabetes mellitus,24,25 education (9 or more years vs less than 9),26,27 head injury,28,29 number of children in men (more than one vs zero or one child),30 occupation as a physician (vs all other occupations),27 oophorectomy (uni- or bilateral, with or without hysterectomy),31,32 oophorectomy at or before age 45,32,33 and stroke.34,35 Data definitions are in table e-1 on the Neurology® Web site at www.neurology.org. We also verified risk factor exposure interview responses by reviewing the relevant patient data in the unique resource medical records linkage system of the Rochester Epidemiology Project.36 If the data sources conflicted, the risk factor was marked as present on the presumption that errors of omission are more likely than errors of commission. To minimize recall bias, anxiety and depression data were generated solely from the medical history section of the medical record. Missing values were excluded case-wise from analyses except in the case of oophorectomy, whereby missing values were coded as not present.
Statistical analyses.
Associations for potential risk factors were assessed with univariate conditional logistic regression analyses comparing DLB and control subjects; these were powered (β = 90%) to detect an odds ratio (OR) of 2.5 if the risk factor was present in 10% of cases. We adjusted for age and sex in unconditional logistic regression analyses of DLB vs AD. The number of children was considered only in men because a previous study in PD identified a link only among men.30 This is the first analysis of multiple risk factors for DLB; we therefore chose to report results without correcting for multiple comparisons. However, we did repeat the analyses controlling for false discovery rate, and list the findings that did not survive correction.
In addition to the single-variable models, we examined all candidate risk factors found to be significant in the univariate analyses, and for which we had data on at least 90% of subjects, in multivariable logistic regression models adjusted for age and sex differences. We did this in order to identify the collection of features that displayed differences between study groups while controlling for the effects of the others. We omitted oophorectomy and number of children data from these multivariate models.
Sensitivity analyses.
We analyzed the data to determine whether the DLB diagnostic criteria, changes in diagnosis during follow-up, or changing diagnostic practices over time affected our findings. Details can be found in the e-Methods.
RESULTS
There were 147 subjects with DLB, 294 controls, and 236 subjects with AD (table 1). The DLB group contained more males and was younger at diagnosis than the AD group. Risk factor frequencies are shown in table 2. To facilitate a comparison of risk factor profiles for each disease, the risk estimates from published studies on AD and PD are compared with the OR estimates from this study in table 3. Comparing DLB and age- and sex-matched normal controls, subjects with DLB were more likely to have anxiety, depression, stroke, APOE ε4 alleles, and a family history of PD. Cancer and caffeine provided estimates of association that were suggestive of protective effects. Comparing DLB with AD, male sex, long education, depression, family history of PD, oophorectomy, and no APOE ε4 alleles were observed significantly more often in DLB than in AD.
Table 1.
Demographic data
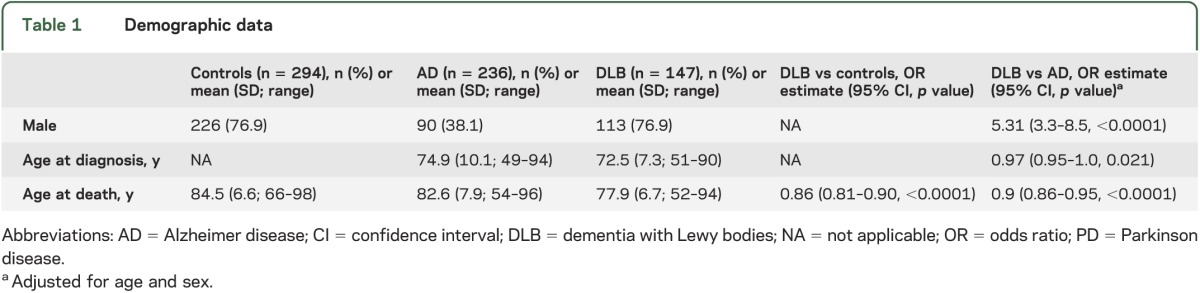
Table 2.
Risk factor frequencies for DLB compared with controls and unmatched subjects with AD
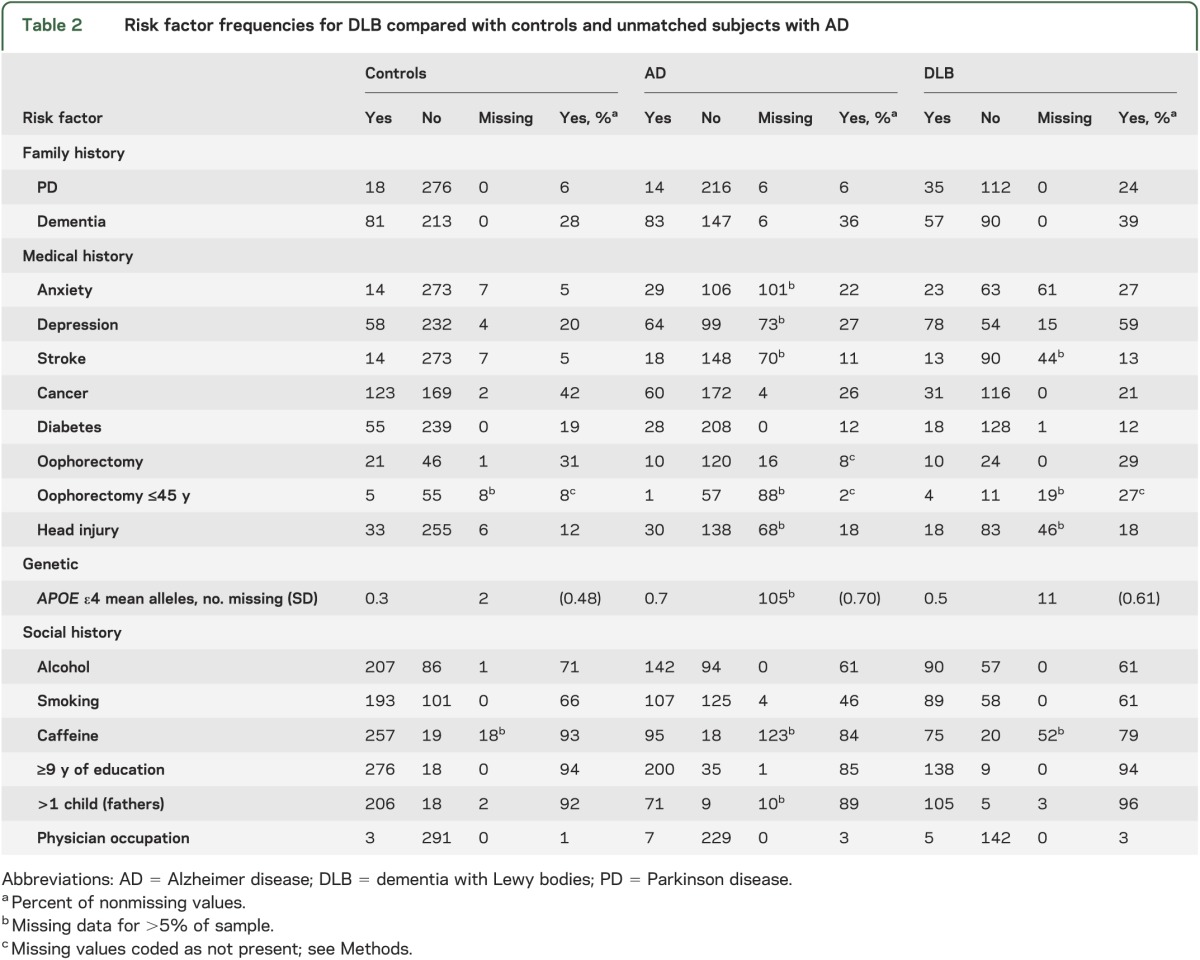
Table 3.
Risk factors for AD, PD, and DLBa
When cases were defined with more specific criteria37 or by final diagnosis, the patterns of association were unchanged, except that alcohol use became statistically significant in the DLB vs control comparison (table e-2). In the DLB vs AD comparison, alcohol and caffeine reached statistical significance, but oophorectomy before age 45 lost significance, when the specific, but not final, diagnosis was used (table e-3). Only 7 of 677 subjects’ diagnosis was changed to or from DLB during follow-up or at autopsy. Education in the DLB vs AD comparison was the only factor that did not retain statistical significance when included in a multivariate model. Only one variable interacted with enrollment date: in the DLB vs control comparison, the OR for cancer was greater among subjects enrolled later in the study (interaction p = 0.03). Redefining missing values of anxiety and depression as the absence of the feature did not alter significance of any comparison (data not shown).
None of the findings in the DLB vs control comparison lost significance after controlling the false discovery rate. In the DLB vs AD comparison, education and APOE ε4 lost significance after this adjustment. Neuropathologic confirmation was obtained in 27 of 94 DLB cases (18%) and 28 of 236 AD cases (12%). There were no significant risk factor differences between these groups, likely reflecting the lack of statistical power (table e-3). There were insufficient control subjects with autopsy data (7/294; 2%) for meaningful statistical comparisons.
DISCUSSION
In this retrospective study, we examined the frequency of 19 possible risk factors associated with the likelihood of DLB, AD, and normal aging. Our findings were consistent with risk factors for DLB being similar to those already identified for AD and PD, with additive effects seen in risk factors common to both (e.g., depression, cancer risk) and reduced effects on opposing risk factors such as smoking and education. A clinical diagnosis of DLB was associated with a greater likelihood of having a history of depression or anxiety, a family history of PD, history of stroke, and having APOE ε4 alleles. Prior caffeine use and history of cancer were associated with a reduced likelihood of an eventual DLB diagnosis. When considering whether a patient has DLB or AD, the presence of the following features are suggestive of a diagnosis of DLB: male sex, early oophorectomy, a history of depression, family history of PD, longer education, and zero APOE ε4 alleles.
Prior diagnoses of anxiety and depression were more likely to be present in subjects diagnosed with DLB compared with controls. Prior diagnosis of depression was also more common in subjects with DLB than in those with AD. In DLB, anxiety and depression likely have many interacting causes that blur the distinction between risk factor and premorbid symptom. First, they may be caused by atrophy and/or dysfunction of pontomesencephalic-limbic emotional circuitry. In PD, premorbid anxiety disorders and depression are more common than in matched controls, but only the association with anxiety holds true for more than 5 years before diagnosis.13 In AD, lifetime history of depression and especially late-life depression is a risk factor.38 Second, anxiety and depression may be direct risk factors for DLB, may mediate behaviors that place a subject at risk, or reflect recall bias, referral bias, or natural emotional responses to incipient cognitive decline. Finally, premorbid anxiety and depression may unmask symptoms of DLB earlier than in subjects without such personality traits. The inverse of this ascertainment bias may explain why a history of anxiety and depression is less common in subjects with incidental Lewy body pathology at autopsy.39 In some instances, late-life anxiety and depression may reflect preclinical α-synucleinopathy: further research is needed to determine the duration of anxiety and depression before DLB diagnosis, and to identify features that could facilitate predementia diagnosis of DLB. For example, some subjects with bradykinesia, hypomimia, and abulia may be misdiagnosed as depressed by family members and physicians.
Our DLB cohort contained a majority of males, as have numerous autopsy series.1 PD is 1.5 times more common in men.19 The sex effect may be driven by differential toxicant and head trauma exposure, mitochondrial dysfunction, X-linkage of genetic risk factors, or neuroprotection from α-synucleinopathy by estrogen. Although initial studies of postmenopausal estrogen replacement indicated a reduced risk of dementia, follow-up investigations revealed that the risk is dependent on the woman’s age. Consistent with this, we observed a trend suggesting that oophorectomy increased the likelihood of DLB compared with control subjects, but the effect disappeared if oophorectomy occurred after 45 years of age. Notably, this is a pattern that has also been shown in parkinsonism, dementia, and cognitive impairment.40 Compared with AD subjects, DLB subjects were 2.4 years younger at diagnosis and 4.7 years younger at death (table 1); earlier death may be attributable to earlier disease onset, more rapid progression, or reflect an interaction with male sex.
A family history of parkinsonism is a well-known risk factor for PD but not for AD.10 In our study, a first-degree family history of PD was more common in DLB compared with AD and normal control subjects. It is not known whether these relatives also had accompanying dementia. Obtaining this information will determine whether the presence of dementia accompanying parkinsonism, or PD alone, is a risk factor for DLB.
Family history of dementia was not more common in DLB compared with control or AD subjects, in contrast to positive findings in an autopsy-confirmed DLB series.2 Recall and selection bias in that series may have overestimated risk. More work is needed to clarify whether a family history of dementia poses a greater risk of the development of DLB. Taken together, these findings support the view that positive family history for PD reflects risk of α-synucleinopathy. Further investigation is needed to determine whether a family history of dementia is associated with a greater risk of amyloid deposition or mixed pathology.
Similarly, APOE ε4 was more frequent in our subjects with DLB compared with controls. It was less frequent when DLB was compared with AD dementia, although this result lost significance when we controlled the false discovery rate. The results likely reflect the mixed synuclein/AD pathology seen in most DLB cases. The presence of one or more APOE ε4 alleles is a well-known risk factor for AD10 but it is unrelated to PD.19
Studies indicate that the risk of PD,19 and possibly AD,20 is reduced among coffee and caffeine users. In this study, caffeine use was associated with a reduced likelihood of DLB when compared with normal controls. Similar sized, significant associations with a dose effect are seen in PD after adjusting for education, smoking, and alcohol with almost all of the effect in that study attributed to men and younger-onset PD.19
Some studies show a protective effect of moderate alcohol intake on risk of AD but others do not.18 There has been no consistent effect on PD risk.19 In our study, there was a trend for reduced risk of DLB in alcohol users compared with normal controls (p = 0.0501), but not compared with AD.
Smoking was not associated with DLB in our study. In contrast, studies have demonstrated a 30% to 60% smoking-related risk reduction with a dose effect for PD17 but a risk increase, again with a dose effect, for AD.16 Many DLB cases have dual α-synuclein and AD pathology, therefore the lack of association of smoking with DLB may reflect opposing influences on these pathologies canceling out. Alternatively, it may reflect a unique difference between PD and DLB, or an unknown secondary or confounding association. Secondary associations may be responsible for the links between caffeine, alcohol, and smoking and many neurodegenerative syndromes. For example, socioeconomic or personality features, or prodromal manifestations of disease, might curtail substance use.
Longer duration of education reduces risk of AD26 but increases risk of PD.27 In our study, more than 9 years of education was more common in subjects with DLB compared with subjects with AD, but this distinction did not survive adjustment for the false discovery rate. Education level did not distinguish DLB from controls.
Education level and occupation are highly correlated, and physicians have 6.8 times increased risk of incidental Lewy body disease39 and 3.7 times increased risk of PD.27 Higher complexity of work has been inversely associated with AD risk, or at least time to the development of the dementia. Current results show that physician occupation was not associated with a greater likelihood of developing DLB, although this may reflect a restricted sample. In this and previous studies, there have been only 4 to 11 affected physicians per group. The data are particularly subject to ascertainment and surveillance bias: physicians may be more prone to see a specialist and participate in research.27
AD22 and PD23 have been associated with reduced risk of cancer. We also demonstrated that a history of cancer was less likely among those with DLB compared with normal controls, but not compared with AD subjects. This adds to the growing body of evidence supporting an inverse correlation between cancer and neurodegenerative disease, perhaps mediated by inherited immune protective factors.
In our study, history of stroke was more common in subjects with DLB vs controls, but this effect was not seen in the AD comparison. Drawing inferences is difficult because symptoms due to stroke are listed as exclusionary criteria for DLB, AD, and PD. Stroke, a risk factor for PD35 and dementia, is not itself an established risk factor for AD,34 although vascular risk factors such as midlife hypertension, diabetes, and hypercholesterolemia increase risk of AD. Their relationship to PD is unclear, although premorbid blood pressure can be affected by prodromal α-synuclein–induced dysautonomia. Diabetes did not alter risk in this study. Head injury has been associated with AD28; its association with PD is likely attributable to reverse causation.19 It was not a risk factor for DLB. Fathering more than one child, a PD but not AD risk factor,30 did not affect DLB risk.
We minimized selection and incidence/prevalence bias with stringent recruitment methods, and minimized recall and surveillance bias by verifying patient and carer reports in the medical record. Misclassification bias was reduced by use of more specific diagnostic criteria, but this remains an important limitation of our study. Pathologic confirmation of the clinical diagnoses supported the significance of associations suggested by the study; however, this was available for only a small subset of subjects.
We were unable to include several factors in the multivariate model because of missing data. Missing data are disproportionately found in the AD group because data for some risk factors began to be collected partway through the AD cohort study. Only one of 19 risk factor associations interacted with date of enrollment, suggesting that this historical fact should not affect our conclusions. Our findings on education and APOE ε4 status in the DLB vs AD comparison did not survive correction for multiple comparisons: they may represent type I errors. For some factors (e.g., caffeine, smoking, and alcohol), we have only binary data and thus cannot determine the degree of exposure. Two of the 3 cohorts that contributed subjects to the study were convenience samples, and ascertainment bias might have affected the results, particularly in the AD vs DLB comparison. Other suspected risk factors for DLB were not assessed in this study, including toxin exposure, well-water consumption, non-APOE genetic factors (e.g., GBA), hypercholesterolemia, obesity, cognitive and physical inactivity, and attention deficit and hyperactivity disorder, as information on these was not captured in the 3 Mayo Clinic studies on which this analysis was based. We excluded others because of mixed evidence in α-synucleinopathy: nonphysician occupation19,27 and hypertension. Nor did we include many prodromal α-synucleinopathy symptoms, including REM sleep behavior disorder, olfactory dysfunction, dysautonomia, and repeated falls.
Supplementary Material
ACKNOWLEDGMENT
The authors are thankful to the subjects and their families for participating in this research.
GLOSSARY
- AD
Alzheimer disease
- DLB
dementia with Lewy bodies
- OR
odds ratio
- PD
Parkinson disease
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Boot and Dr. Orr: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, acquisition of data, and statistical analysis. Dr. Ahlskog, Dr. Knopman, and Dr. Boeve: drafting/revising the manuscript for content, including medical writing for content, study concept or design, and study supervision or coordination. Dr. Pankratz and J.A. Aakre: analysis or interpretation of data, and statistical analysis. Dr. Ferman, Dr. Roberts, Dr. Geda, Dr. Dickson, Dr. Parisi, and Dr. Petersen: drafting/revising the manuscript for content.
STUDY FUNDING
Supported by grants U01 AG06786, P50 AG16574, and P01 AG07216 from the National Institute on Aging (NIA), by the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program, and R01 AG15866.
DISCLOSURE
B. Boot, C. Orr, J. Ahlskog, and T. Ferman report no disclosures. R. Roberts receives research support from Abbott Research Labs and the Driskill Foundation. V. Pankratz reports no disclosures. D. Dickson serves on the editorial boards of the American Journal of Pathology, Journal of Neuropathology and Experimental Neurology, Brain Pathology, Neurobiology of Aging, Journal of Neurology, Neurosurgery & Psychiatry, Annals of Neurology, and Neuropathology; and receives research support from the NIH and Cure PSP/Society for PSP. J. Parisi serves on scientific advisory boards for the US Government Defense Health Board and the Subcommittee for Laboratory Services and Pathology; serves as a Section Editor for Neurology®; receives royalties from the publication of Principles & Practice of Neuropathology, 2nd ed. (Oxford University Press, 2003); and receives research support from the NIH. J. Aakre and Y. Geda report no disclosures. D. Knopman serves as Deputy Editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; served as a consultant to TauRx; was an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals, and Forest Pharmaceuticals; and receives research support from the NIH. R. Petersen serves on scientific advisory boards for the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc. (Wyeth), and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA. B. Boeve has served as an investigator for clinical trials sponsored by Cephalon, Inc., Allon Pharmaceuticals, and GE Healthcare; receives royalties from the publication of a book titled Behavioral Neurology of Dementia (Cambridge Medicine, 2009); has received honoraria from the American Academy of Neurology; serves on the Scientific Advisory Board of the Tau Consortium; and receives research support from the NIA (P50 AG016574, U01 AG006786, RO1 AG032306, RO1 AG041797) and the Mangurian Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 2010;257:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodruff BK, Graff-Radford NR, Ferman TJ, et al. Family history of dementia is a risk factor for Lewy body disease. Neurology 2006;66:1949–1950 [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Kokmen E, Tangalos E, Ivnik RJ, Kurland LT. Mayo Clinic Alzheimer's Disease Patient Registry. Aging 1990;2:408–415 [DOI] [PubMed] [Google Scholar]
- 4.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872 [DOI] [PubMed] [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 7.Breteler MM, van Duijn CM, Chandra V, et al. Medical history and the risk of Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 1991;20(suppl 2):S36–S42 [DOI] [PubMed] [Google Scholar]
- 8.Rocca WA, Bower JH, Ahlskog JE, et al. Risk of cognitive impairment or dementia in relatives of patients with Parkinson disease. Arch Neurol 2007;64:1458–1464 [DOI] [PubMed] [Google Scholar]
- 9.van Duijn CM, Stijnen T, Hofman A. Risk factors for Alzheimer's disease: overview of the EURODEM collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 1991;20(suppl 2):S4–S12 [DOI] [PubMed] [Google Scholar]
- 10.Rosen AR, Steenland NK, Hanfelt J, Factor SA, Lah JJ, Levey AI. Evidence of shared risk for Alzheimer's disease and Parkinson's disease using family history. Neurogenetics 2007;8:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet 2011;43:436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson's disease. J Neurol 2009;256:493–498 [DOI] [PubMed] [Google Scholar]
- 13.Shiba M, Bower JH, Maraganore DM, et al. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Mov Disord 2000;15:669–677 [DOI] [PubMed] [Google Scholar]
- 14.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006;63:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson's disease for patients with major affective disorder: a register study. Acta Psychiatr Scand 2001;104:380–386 [DOI] [PubMed] [Google Scholar]
- 16.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer's Disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis 2010;19:465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernandez-Crehuet Navajas R. Smoking and Parkinson's disease: systematic review of prospective studies. Mov Disord 2004;19:614–621 [DOI] [PubMed] [Google Scholar]
- 18.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry 2009;17:542–555 [DOI] [PubMed] [Google Scholar]
- 19.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol 2011;26(suppl 1):S1–S58 [DOI] [PubMed] [Google Scholar]
- 20.Barranco Quintana JL, Allam MF, Serrano Del Castillo A, Fernandez-Crehuet Navajas R. Alzheimer's disease and coffee: a quantitative review. Neurol Res 2007;29:91–95 [DOI] [PubMed] [Google Scholar]
- 21.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002;52:276–284 [DOI] [PubMed] [Google Scholar]
- 22.Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010;74:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajaj A, Driver JA, Schernhammer ES. Parkinson's disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control 2010;21:697–707 [DOI] [PubMed] [Google Scholar]
- 24.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One 2009;4:e4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cereda E, Barichella M, Pedrolli C, et al. Diabetes and risk of Parkinson's disease: a systematic review and meta-analysis. Diabetes Care 2011;34:2614–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngandu T, von Strauss E, Helkala EL, et al. Education and dementia: what lies behind the association? Neurology 2007;69:1442–1450 [DOI] [PubMed] [Google Scholar]
- 27.Frigerio R, Elbaz A, Sanft KR, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology 2005;65:1575–1583 [DOI] [PubMed] [Google Scholar]
- 28.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on—a partial replication. J Neurol Neurosurg Psychiatry 2003;74:857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. BMJ 2008;337:a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frigerio R, Breteler MM, de Lau LM, et al. Number of children and risk of Parkinson's disease. Mov Disord 2007;22:632–639 [DOI] [PubMed] [Google Scholar]
- 31.Phung TK, Waltoft BL, Laursen TM, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 2010;30:43–50 [DOI] [PubMed] [Google Scholar]
- 32.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology 2008;70:200–209 [DOI] [PubMed] [Google Scholar]
- 33.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083 [DOI] [PubMed] [Google Scholar]
- 34.Jin YP, Ostbye T, Feightner JW, Di Legge S, Hachinski V. Joint effect of stroke and APOE 4 on dementia risk: the Canadian Study of Health and Aging. Neurology 2008;70:9–16 [DOI] [PubMed] [Google Scholar]
- 35.Becker C, Jick SS, Meier CR. Risk of stroke in patients with idiopathic Parkinson disease. Parkinsonism Relat Disord 2010;16:31–35 [DOI] [PubMed] [Google Scholar]
- 36.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–274 [DOI] [PubMed] [Google Scholar]
- 37.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 2011;77:875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuno N, Homma A. What is the association between depression and Alzheimer's disease? Expert Rev Neurother 2009;9:1667–1676 [DOI] [PubMed] [Google Scholar]
- 39.Frigerio R, Fujishiro H, Maraganore DM, et al. Comparison of risk factor profiles in incidental Lewy body disease and Parkinson disease. Arch Neurol 2009;66:1114–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phung T, Waltoft B, Laursen T, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 2010;30:43–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


