Abstract
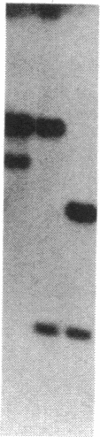
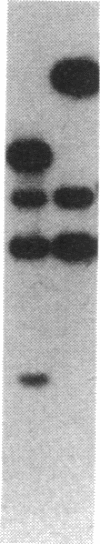
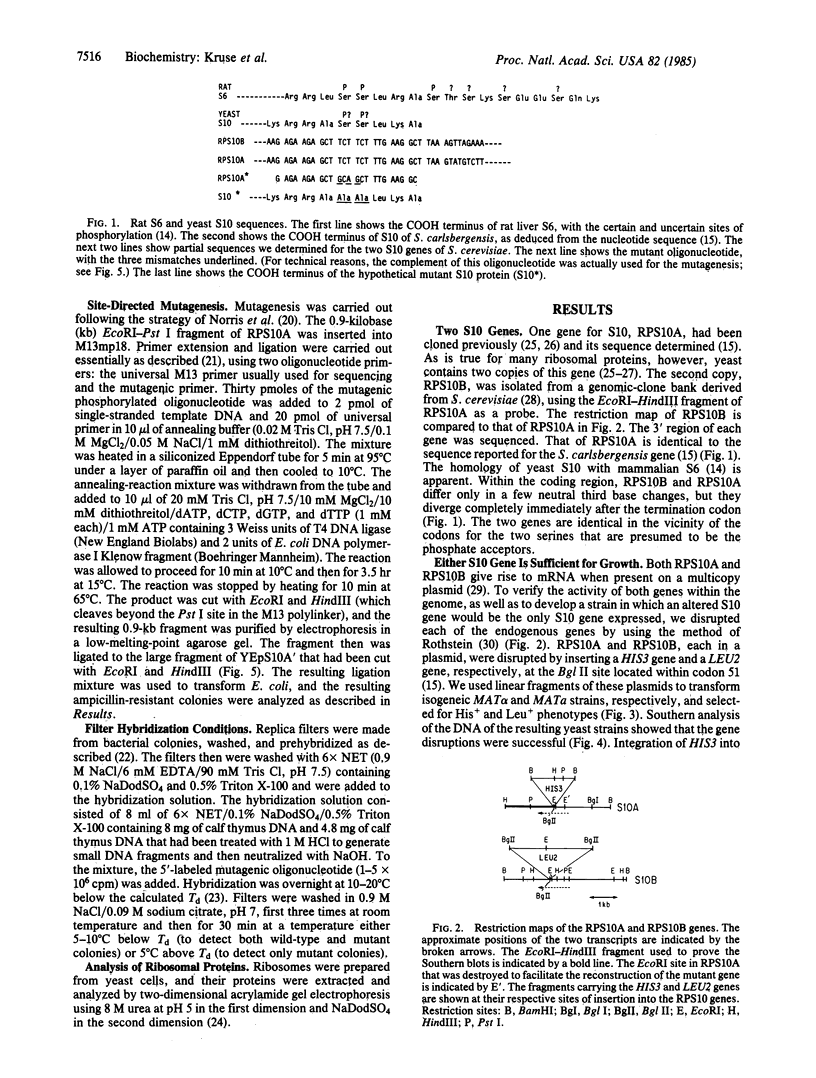
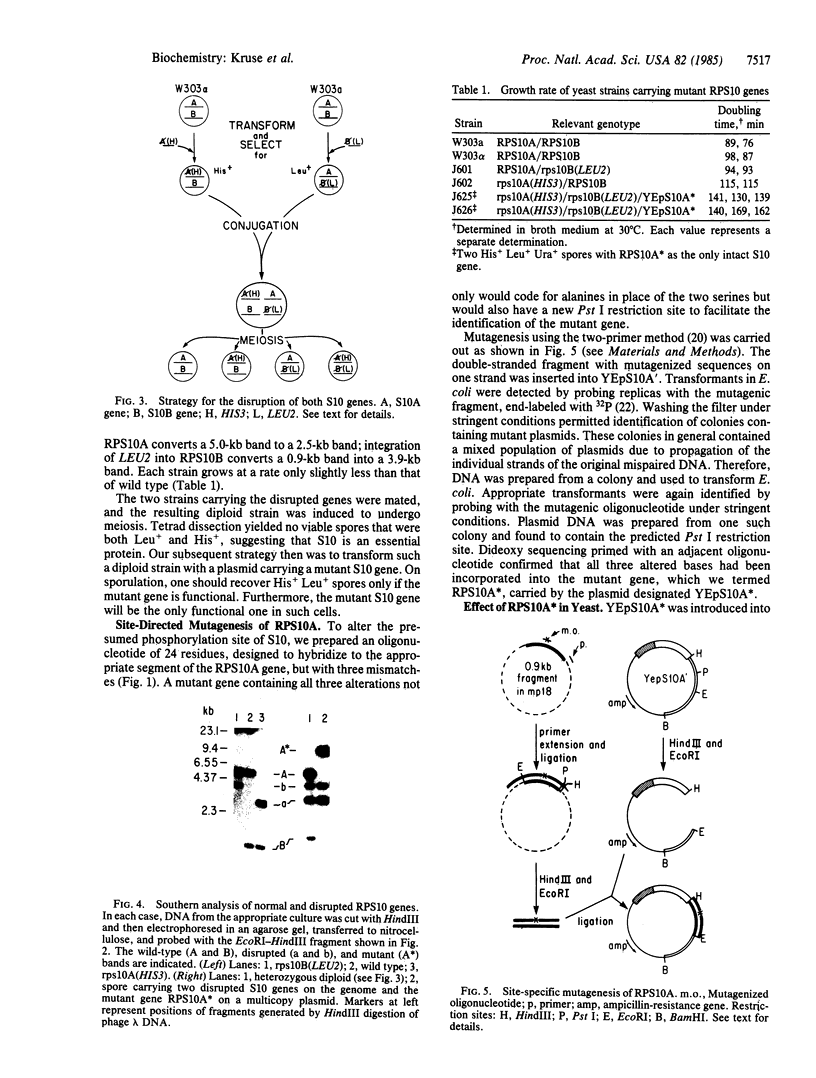
The yeast equivalent of ribosomal protein S6, known as S10, can be modified by the addition of two phosphates. The two adjacent serines that are likely to be subject to phosphorylation were deduced by comparison with the known sites of phosphorylation on rat liver S6. Using oligonucleotide mutagenesis, we altered the gene for S10 to replace these two serines with alanines. This mutant gene was introduced into a diploid yeast cell heterozygous for each of the two S10 genes. After sporulation, we obtained colonies in which the mutant gene was the only intact S10 gene. Although the ribosomes of these cells contained a full complement of S10, no phosphorylation of S10 was detected. These cells grow exponentially with a doubling time about 50% greater than that of control cells. We conclude that the phosphorylation of S10 is not essential for growth. However, the mutant gene in such cells is very unstable, frequently reverting to wild type, presumably by interaction with the disrupted host genes. We suggest that at some stage of the growth cycle there is strong selection for S10 that can be phosphorylated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abovich N., Rosbash M. Two genes for ribosomal protein 51 of Saccharomyces cerevisiae complement and contribute to the ribosomes. Mol Cell Biol. 1984 Sep;4(9):1871–1879. doi: 10.1128/mcb.4.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Phosphorylation of the ribosomal protein S6 is elevated in cells transformed by a variety of tumor viruses. J Virol. 1984 Jun;50(3):966–969. doi: 10.1128/jvi.50.3.966-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Spivack J. G., Erikson R. L. Phorbol ester, serum, and rous sarcoma virus transforming gene product induce similar phosphorylations of ribosomal protein S6. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6408–6412. doi: 10.1073/pnas.81.20.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen G. H., Cohen L. H., Mager W. H., Klaassen A. W., Planta R. J. Isolation of cloned ribosomal protein genes from the yeast Saccharomyces carlsbergensis. Gene. 1981 Sep;14(4):279–287. doi: 10.1016/0378-1119(81)90160-8. [DOI] [PubMed] [Google Scholar]
- Burkhard S. J., Traugh J. A. Changes in ribosome function by cAMP-dependent and cAMP-independent phosphorylation of ribosomal protein S6. J Biol Chem. 1983 Nov 25;258(22):14003–14008. [PubMed] [Google Scholar]
- Burkhard S. J., Traugh J. A. Changes in ribosome function by cAMP-dependent and cAMP-independent phosphorylation of ribosomal protein S6. J Biol Chem. 1983 Nov 25;258(22):14003–14008. [PubMed] [Google Scholar]
- Cobb M. H., Rosen O. M. Description of a protein kinase derived from insulin-treated 3T3-L1 cells that catalyzes the phosphorylation of ribosomal protein S6 and casein. J Biol Chem. 1983 Oct 25;258(20):12472–12481. [PubMed] [Google Scholar]
- Decker S. Phosphorylation of ribosomal protein S6 in avian sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4112–4115. doi: 10.1073/pnas.78.7.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue M. J., Masaracchia R. A. Phosphorylation of ribosomal protein S6 at multiple sites by a cyclic AMP-independent protein kinase from lymphoid cells. J Biol Chem. 1984 Jan 10;259(1):435–440. [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. S6 phosphorylation accompanies recruitment of ribosomes and mRNA into polysomes in response to dichlororibofuranosyl benzimidazole. Exp Cell Res. 1984 Jun;152(2):520–527. doi: 10.1016/0014-4827(84)90654-2. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Pearson N. J., Kim C. H., Warner J. R. The genes for fifteen ribosomal proteins of Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):10176–10183. [PubMed] [Google Scholar]
- Fried H. M., Warner J. R. Cloning of yeast gene for trichodermin resistance and ribosomal protein L3. Proc Natl Acad Sci U S A. 1981 Jan;78(1):238–242. doi: 10.1073/pnas.78.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974 Nov 10;249(21):6917–6925. [PubMed] [Google Scholar]
- Holland J. P., Holland M. J. Structural comparison of two nontandemly repeated yeast glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1980 Mar 25;255(6):2596–2605. [PubMed] [Google Scholar]
- Kruiswijk T., de Hey J. T., Planta R. J. Modification of yeast ribosomal proteins. Phosphorylation. Biochem J. 1978 Oct 1;175(1):213–219. doi: 10.1042/bj1750213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. P., Thomas A., Voorma H. O. The protein synthetic activity in vitro of ribosomes differing in the extent of phosphorylation of their ribosomal proteins. Biochim Biophys Acta. 1981 Nov 27;656(1):69–75. doi: 10.1016/0005-2787(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Leer R. J., van Raamsdonk-Duin M. M., Molenaar C. M., Cohen L. H., Mager W. H., Planta R. J. The structure of the gene coding for the phosphorylated ribosomal protein S10 in yeast. Nucleic Acids Res. 1982 Oct 11;10(19):5869–5878. doi: 10.1093/nar/10.19.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L., Foulkes J. G., Erikson E., Baltimore D. Phosphorylation of ribosomal protein S6 on serine after microinjection of the Abelson murine leukemia virus tyrosine-specific protein kinase into Xenopus oocytes. Proc Natl Acad Sci U S A. 1985 Jan;82(2):272–276. doi: 10.1073/pnas.82.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Pérez J., Siegmann M., Thomas G. EGF, PGF2 alpha and insulin induce the phosphorylation of identical S6 peptides in swiss mouse 3T3 cells: effect of cAMP on early sites of phosphorylation. Cell. 1984 Feb;36(2):287–294. doi: 10.1016/0092-8674(84)90222-8. [DOI] [PubMed] [Google Scholar]
- Martin-Pérez J., Thomas G. Ordered phosphorylation of 40S ribosomal protein S6 after serum stimulation of quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):926–930. doi: 10.1073/pnas.80.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K., Norris F., Christiansen L., Fiil N. Efficient site-directed mutagenesis by simultaneous use of two primers. Nucleic Acids Res. 1983 Aug 11;11(15):5103–5112. doi: 10.1093/nar/11.15.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A. S., Triemer D. F., Sanders M. M. Dephosphorylation of S6 and expression of the heat shock response in Drosophila melanogaster. Mol Cell Biol. 1983 Nov;3(11):2017–2027. doi: 10.1128/mcb.3.11.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson N. J., Fried H. M., Warner J. R. Yeast use translational control to compensate for extra copies of a ribosomal protein gene. Cell. 1982 Jun;29(2):347–355. doi: 10.1016/0092-8674(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J. W., Hereford L., Grunstein M. Histone H2B genes of yeast encode two different proteins. Cell. 1980 Dec;22(3):799–805. doi: 10.1016/0092-8674(80)90556-5. [DOI] [PubMed] [Google Scholar]
- Wallis J. W., Rykowski M., Grunstein M. Yeast histone H2B containing large amino terminus deletions can function in vivo. Cell. 1983 Dec;35(3 Pt 2):711–719. doi: 10.1016/0092-8674(83)90104-6. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Gorenstein C. The ribosomal proteins of Saccharomyces cerevisiae. Methods Cell Biol. 1978;20:45–60. doi: 10.1016/s0091-679x(08)62008-7. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Mitra G., Schwindinger W. F., Studeny M., Fried H. M. Saccharomyces cerevisiae coordinates accumulation of yeast ribosomal proteins by modulating mRNA splicing, translational initiation, and protein turnover. Mol Cell Biol. 1985 Jun;5(6):1512–1521. doi: 10.1128/mcb.5.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettenhall R. E., Morgan F. J. Phosphorylation of hepatic ribosomal protein S6 on 80 and 40 S ribosomes. Primary structure of S6 in the region of the major phosphorylation sites for cAMP-dependent protein kinases. J Biol Chem. 1984 Feb 25;259(4):2084–2091. [PubMed] [Google Scholar]
- Woolford J. L., Jr, Hereford L. M., Rosbash M. Isolation of cloned DNA sequences containing ribosomal protein genes from Saccharomyces cerevisiae. Cell. 1979 Dec;18(4):1247–1259. doi: 10.1016/0092-8674(79)90236-8. [DOI] [PubMed] [Google Scholar]
- Zinker S., Warner J. R. The ribosomal proteins of Saccharomyces cerevisiae. Phosphorylated and exchangeable proteins. J Biol Chem. 1976 Mar 25;251(6):1799–1807. [PubMed] [Google Scholar]