Abstract
OBJECTIVE
Dysfunction in amygdala and orbital frontal cortex functioning has been reported in youths and adults with psychopathic traits. However, the specific nature of the computational irregularities within these brain structures remains poorly understood. The current study used the passive avoidance task to examine responsiveness of these systems to early stimulus-reinforcement exposure, when prediction errors are greatest and learning maximized, and to reward in youths with psychopathic traits and comparison youths.
METHOD
30 youths (N=15 with conduct disorder or oppositional defiant disorder plus high psychopathic traits and N=15 comparison subjects) completed a 3.0 T fMRI scan while performing a passive avoidance learning task.
RESULTS
Relative to comparison youth, youths with conduct disorder or oppositional defiant disorder plus psychopathic traits showed reduced orbitofrontal cortex responsiveness both to early stimulus-reinforcement exposure and to rewards, as well as reduced caudate response to early stimulus-reinforcement exposure. Contrary to other predictions, however, there were no group differences in amygdala responsiveness specifically to these two task parameters. However, amygdala responsiveness throughout the task was reduced in the youths with conduct disorder or oppositional defiant disorder plus psychopathic traits.
CONCLUSIONS
This study demonstrates that youths with conduct disorder or oppositional defiant disorder plus psychopathic traits are marked by a compromised sensitivity to early reinforcement information in both orbitofrontal cortex and caudate and to reward outcome information within orbitofrontal cortex. They further suggest that the integrated functioning of the amygdala, caudate and orbitofrontal cortex may be disrupted in individuals with this disorder.
INTRODUCTION
Youths with conduct disorder and oppositional defiant disorder show increased rates of aggressive and antisocial behaviors (1). A subset of these youths also displays callousness and psychopathic traits, including lack of guilt, empathy, and remorse (2, 3). This subset of youths is at highest risk for recurrent antisocial acts and future criminal behaviors (3–6). While psychopathic traits are detectable early in childhood and persist into adulthood (7), little is known about the pathophysiology (8–10).
The presence of psychopathic traits has long been linked to problems in emotional learning (11, 12). Specifically, it has been argued that these traits reflect impairment in stimulus-reinforcement learning and decision-making (13, 14). An early task indexing this impairment was the passive avoidance task (11, 15, 16). The passive avoidance task requires participants to learn to respond to (i.e., approach) stimuli that engender reward and refrain from responding to (i.e., passively avoid) stimuli that engender punishment. Adults and youths with psychopathic traits show a deficient capability to learn to avoid the stimuli that predict punishment regardless of whether the punishment is shock, loss of money or loss of points (11, 15, 16).
The passive avoidance learning task is particularly informative as both electro-physiologic and lesion studies map its neural basis, implicating the amygdala, orbitofrontal cortex, and striatum (17–21). In addition, a recent functional MRI study of the task has confirmed the importance of these regions in healthy human adults (22). Computationally, it is argued that the amygdala allows the formation of stimulus-reinforcement associations, determining which of the stimuli are “good” and which “bad”, and that this information is fed forward as reinforcement expectations to orbitofrontal cortex (19–21), potentially via ventral striatum (23). Orbitofrontal cortex is critical for the representation of this reinforcement-expectation information, thereby facilitating successful decision-making (24, 25). Orbitofrontal cortex and caudate are also critical for error prediction, which facilitates learning of reward or punishment contingencies (26–28). The detection of prediction errors prompts enhanced reinforcement based learning (29). These prediction errors are maximal after the individual’s first exposure to the cue and reinforcement but rapidly lessen (unless the reinforcement contingency changes) as the individual develops an expectation of the reinforcement associated with the cue (29).
Originally, the deficits shown by individuals with psychopathic traits on the passive avoidance task were attributed to reduced processing of punishment information (11). However, work has shown that individuals with psychopathic traits appropriately use punishment information to modify responses immediately following an error. Instead, it is argued that the deficits arise from impairment in the formation and use of stimulus-reinforcement associations in decision-making (30). The individual with psychopathic traits is less able to use reinforcement information to change their representation of the outcome associated with the response and consequently is less able to use reinforcement expectancies to guide behavior (13). A deficit in stimulus-reinforcement processing is thought to result from dysfunction in the integrated functioning of the amygdala and orbitofrontal cortex (13). In line with this model, recent fMRI work has indicated reduced amygdala responding and reduced amygdala- orbitofrontal cortex functional connectivity in response to fearful expressions in youths with psychopathic traits (9, 31). Moreover, anomalous neural responses in orbitofrontal cortex to reversal errors have also been identified in this population (10).
The goal of the current study is to assess the nature of the computational irregularities within the amygdala, orbitofrontal cortex, and striatum of youths with conduct disorder or oppositional defiant disorder plus psychopathic traits. Specifically, the study tests three hypotheses: first, we hypothesized that early reinforcement processing would be abnormal in conduct disorder or oppositional defiant disorder plus psychopathic traits. For initial exposures to reinforced outcomes, prediction errors are high; the participant has no reinforcement expectancy associated with the stimulus and thus all reinforcement received is unexpected (29). Healthy individuals show marked increases in activity, particularly within orbitofrontal cortex and striatum, to unexpected reinforcements (26–28). We predict, however, that this initial heightened responding to early trials will not be seen in conduct disorder or oppositional defiant disorder plus psychopathic traits, at least within orbitofrontal cortex. Our second hypothesis is that reward and punishment-outcome signaling would be abnormal in conduct disorder or oppositional defiant disorder plus psychopathic traits. Several studies document heightened responses to rewarded relative to punished outcomes within the amygdala, orbitofrontal cortex and striatum during decision-making (23, 24, 32). We predict that this heightened responding to rewarded outcomes will be seen only in healthy youth and not in conduct disorder or oppositional defiant disorder plus psychopathic traits.
METHODS
Participants
30 youths participated in this study: 15 youths with psychopathic traits and conduct disorder or oppositional defiant disorder, and 15 healthy comparison youths (Table 1). Youths were recruited from the community through newspaper ads, fliers, and referrals from area mental health practitioners. A statement of informed assent and consent was obtained from participating children and parents. This study was approved by the NIMH IRB.
Table 1.
Demographic and clinical characteristics of the children with conduct disorder or oppositional defiant disorder plus psychopathic traits and healthy comparison subjects
| Psychopathic traits + Conduct Disorder or Oppositional Defiant Disorder (n = 15) | Comparison Subjects (n = 15) | |||
|---|---|---|---|---|
| Age (S.D.) | 14.1 | (1.8) | 13.2 | (1.1) |
| IQ (S.D.) | 100.3 | (10.5) | 108.2 | (14.6) |
| Male sex, No. [%] | 9 | [60] | 9 | [60] |
| DSM-IV diagnoses (current), No. [%] | ||||
| Conduct disorder | 9 | [71] | 0 | (0) |
| Oppositional-defiant disorder | 6 | [43] | 0 | (0) |
| Attention Deficit Hyperactivity Disorder | 10 | [57] | 0 | (0) |
| Pediatric psychopathy rating scale scores (S.D.) | ||||
| Antisocial process screening device | 28.9 | (4.0) | 6.9 | (4.1) |
| Psychopathy Checklist: Youth Version | 24.7 | (3.1) | - | |
All youths and parents completed Kiddie Schedule for Affective Disorders and Schizophrenia (K-SAD) (33) assessments with an experienced clinician trained and supervised by expert child psychiatrists, with good inter-rater reliability (kappa >0.75 for all diagnoses) and youths the Wechsler Abbrieviated Scale of Intelligence (two-subtest form). Exclusion criteria were pervasive developmental disorder, Tourette’s syndrome, lifetime history of psychosis, depression, bipolar disorder, generalized, social or separation anxiety disorder, PTSD, neurologic disorder, history of head trauma, and IQ<80. In addition, parents completed the Antisocial Process Screening Device, a measure of psychopathic traits. Youths meeting K-SAD criteria for CD or ODD who had APSD scores of 20 or greater returned to complete the Psychopathy Checklist-Youths Version assessment. Youths scoring ≥20 on the Psychopathy Checklist-Youths Version were included in the psychopathic traits group, those scoring <20 were excluded from the study. Comparison subjects did not meet criteria for any K-SAD diagnosis and scored <20 on the Antisocial Process Screening Device.
Clinical Measures
Antisocial Process Screening Device (ASPD; 34)
A 20 item parent completed rating of callous-unemotional traits and conduct and impulsivity problems for the detection of antisocial processes in youth. A three factor structure has been characterized comprised of the following dimensions: Callous/Unemotional, Narcissism, and Impulsivity (34). There is no established cutoff score on the Antisocial Process Screening Device for classification of high psychopathic traits (35–37). For research purposes studies of adolescents have used cutoff of scores of 20(10, 31, 38), median splits (>11 for males, 9 for females)(39), or percentile rankings (top 1/3, score>18)(36). Following our previous work (10, 31) a cutoff score of ≥20 was used in this study. All healthy comparison subjects scored less than 15 on this measure.
Psychopathy Checklist:Youths Version (PCL-YV; 5)
A 20 item rating scale for assessment of interpersonal, affective and behavioral features related to psychopathic traits in adolescents based on semi-structured interview and collateral information. A cutoff score of ≥20 was used for defining the high psychopathic traits group(10, 31), as there are no standard cut point scores for classifying youth on this measure to date (40).
The Passive Avoidance fMRI Task
A modified version of the previously reported fMRI passive avoidance task was used (see figure 1) (22, 41). Task parameters were equivalent to those used in Finger et al. 2008 with the exception that 8 stimuli rather than 12 were presented in each fMRI run. Each of the 4 runs involved a new set of 8 stimuli for the participant to learn about. Trials began with the presentation for 1100ms of one of these 8 stimuli (Snodgrass line drawings (42) on a black background. Button presses to four of the stimuli engendered reward (“You have WON 100 points”). Button presses to the other four stimuli engendered punishment (“You have LOST 100 points”). If no response was made, a fixation cross was presented in lieu of reinforcement. The reinforcement phase (reward, punishment or fixation cross) lasted for 1000 ms. Following this, there was a 200ms fixation cross before the commencement of the next trial. The participant’s goal was to win as many points as possible; they had to learn to respond to the stimuli engendering reward and withhold from responding to the stimuli engendering punishment.
Figure 1.
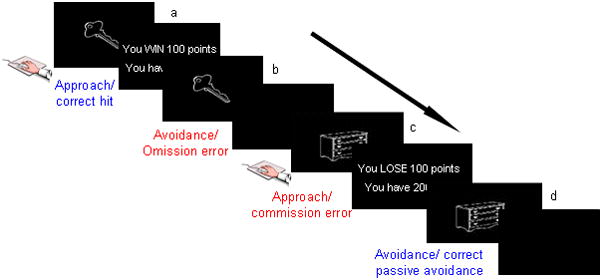
Passive Avoidance Learning Task: Participants received the following instructions: “In this task, you are going to be presented with a series of images. Some of these images are good and will gain you points if you press the button when they are showing. Some are bad and will lose you points if you press the button when they are showing. If you do nothing you will neither gain nor lose points. Your goal is to win as many points as you can.” Depiction of the four event types: a) correct hit- participant is presented with a stimulus (key) associated with reward. Upon responding to this stimulus with a mouse click, the participant is presented with positive feedback. b) omission error- upon presentation of a stimulus associated with reward, the participant does not make any response. A fixation cross is presented during the feedback interval. c) commission error-a stimulus (dresser) associated with punishment is presented, and the participant responds with a mouse click, result in the display of negative feedback. d) correct passive avoidance- the participant refrains from responding to a stimulus associated with punishment and a fixation cross is presented during the feedback interval.
Stimuli were presented in random order once per block for 8 acquisition blocks. After the 8 acquisition blocks, the reinforcement value associated with half of the stimuli changed (this extinction component of the study is considered in a separate paper). Within each block of 8 stimuli, there were an additional 4 fixation point trials (2300 ms of fixation point) to serve as a baseline. Each of the fMRI runs contained a different set of 8 stimuli.
Each participant completed a brief practice session and then 4 runs of the task in a 3.0 T GE scanner. Four versions of the task were developed to counterbalance the reinforcements associated with each of the stimuli. The task version and run order were randomized across participants.
MRI Parameters
Participants were scanned during task performance using a 3.0 Tesla GE Signa scanner. A total of 189 functional images per run were taken with a gradient echo planar imaging (EPI) sequence (repetition time=2300ms, echo time=23 ms, 64×64 matrix, flip angle 90°, FOV 24cm). Whole brain coverage was obtained with 34 axial slices (thickness 3.3mm). A high resolution anatomical scan (three-dimensional Fast Spoiled Gradient Echo sequence; repetition time=6 ms, echo time=2.5ms; field of view=24cm; flip angle=12°; 124 axial slices; thickness=1.0 mm; 224×224 matrix) in register with the EPI dataset was obtained covering the whole brain.
Imaging Data Pre-processing
Imaging data were pre-processed and analyzed in AFNI (43). At the individual level, functional images from the first 5 volumes of each run collected before equilibrium magnetization was reached were discarded. Functional images from the 4 time series were motion corrected and spatially smoothed with a 6 mm full-width half-maximum Gaussian filter. The time series were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percent signal change from the mean.
Following this, regressors characterizing the trial and response types were generated: correct hits (responses to a stimulus associated with reward), commission errors (responses to stimuli associated with punishment), correct avoidances (no response made to stimuli associated with punishment), and omission errors (no response made to a stimulus associated with reward). To examine differential responsiveness to initial exposure as opposed to later learning, we evaluated neural responses during first exposures to the stimulus-reinforcement associations in block 1 in comparison to subsequent exposures in blocks 2–8.
All regressors were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function to account for the slow hemodynamic response. Linear regression modelling was performed using the regressors described above plus regressors to model a first order baseline drift function. This produced a beta coefficient and associated t-statistic for each voxel and regressor. Following findings that that normalization of brain volumes from age 7–8 years onward does not introduce major age related distortions in localization or time course of the BOLD signal in event related fMRI (44, 45), participants’ anatomical scans were individually registered to the Talaraich and Tourneoux Atlas (46). The individuals’ functional EPI data were then registered to their Talaraiched anatomical scan within AFNI.
fMRI Data Analysis
The group analysis of the BOLD data was then performed on regression coefficients from individual subject analyses using a 2(diagnosis: psychopathic traits vs. comparison subjects) × 2 (response type: correct hits vs. commission errors) × (learning phase: early [block 1] vs. late [Block 2–8]) ANOVA on BOLD response data. The initial probability threshold was set at p<0.005 (corrected at p<0.05 for multiple comparisons). Extent threshold correction for multiple comparisons was done using the AlphaSim program in AFNI. Average percent signal change was measured within each significant cluster of 547 mm3 or greater. Post-hoc analysis of significant interactions was assessed with planned ANOVAs within SPSS.
RESULTS
Behavioural Results
There were no significant group differences in age or IQ (p= n.s.; table 1). Group differences in omission and commission errors were analyzed through a 2(diagnosis: conduct disorder or oppositional defiant disorder plus psychopathic traits vs. comparison subjects) × 2(learning phase: early[block 1] vs. late[Block 2–8]) × 2(Error type: commission vs. omission) ANOVA. This revealed the expected diagnosis-by-learning phase-by-error type interaction (F(1,28)=4.6, p<0.05); conduct disorder or oppositional defiant disorder plus psychopathic traits youths made significantly more commission errors during the late learning phase (F(1,28)=5.4, p<0.05)(Mean commission errors per block early phase: psychopathic traits 3.31 (SD 0.39), comparison subjects 3.65 (SD 0.62); late phase: psychopathic traits 1.15 (SD 0.79), comparison subjects 0.05 (SD 0.23)). There was also a significant diagnosis-by-learning phase interaction (F(1,28)=10.5, p<0.005); conduct disorder or oppositional defiant disorder plus psychopathic made significantly more errors during the late learning phase (F(1,28)=7.4, p<0.01). There was no main effect of diagnosis.
fMRI Results
The goal of the current study is to assess the nature of the computational irregularities within the amygdala, orbitofrontal cortex and striatum in conduct disorder or oppositional defiant disorder plus psychopathic traits during passive avoidance learning. We examined this issue through a 2(Diagnosis: conduct disorder or oppositional defiant disorder plus psychopathic traits vs. comparison subjects) × 2(learning phase: early[block 1] vs. late[Block 2–8]) × 2(response type: commission error vs. correct hit) ANOVA conducted on the BOLD response data (Table 2). The key interactions with respect to our predictions (diagnosis-by-phase, diagnosis-by-response type) and the main effect of diagnosis are described, providing a test of our a priori hypotheses (no significant activation was seen for the 3-way diagnosis-by-phase-by-response type interaction).
Table 2.
Regions Demonstrating Differential BOLD Responses during acquisition of Passive Avoidance Learning
| Region | L/R | BA | x | y | z | F | volume | |
|---|---|---|---|---|---|---|---|---|
| Diagnosis-by-Phase interaction | ||||||||
| Orbital frontal cortex | R | 11 | 23 | 36 | −13 | 14.4 | 675 | |
| Middle frontal gyrus | L | 10 | −32 | 41 | 25 | 24.0 | 3834 | |
| Superior frontal gyrus | L | 8 | −17 | 46 | 44 | 15.5 | 4863 | |
| L | 6 | −29 | −4 | 45 | 15.2 | 1269 | ||
| Inferior frontal gyrus | R | 46 | 50 | 38 | 11 | 17.7 | 3942 | |
| L | 44 | −59 | 13 | 20 | 14.4 | 1836 | ||
| Inferior parietal lobule | L | 7 | −41 | −69 | 45 | 16.8 | 3159 | |
| Middle temporal gyrus | R | 39 | 47 | −58 | 10 | 18.9 | 2808 | |
| R | 21 | 44 | 3 | −33 | 21.0 | 2106 | ||
| Middle temporal/Angular gyrus | L | 39 | −50 | −54 | 3 | 19.4 | 8289 | |
| Caudate | L | −2 | 16 | 13 | 13.3 | 1458 | ||
| Cerebellum | L | −4 | −50 | −25 | 27.6 | 7371 | ||
| Diagnosis-by-Response type interaction | ||||||||
| Orbital frontal cortex | R | 10 | 5 | 64 | −8 | 12.3 | 162 | |
| Middle frontal gyrus | L | 8 | −44 | 12 | 49 | 13.6 | 486 | |
| Parahippocampal gyrus | R | 37 | 35 | −41 | −10 | 11.9 | 216 | |
| Main effect of Diagnosis | ||||||||
| Superior frontal gyrus | R | 6 | 11 | 26 | 66 | 17.4 | 2538 | |
| L | 6 | −8 | 1 | 68 | 17.3 | 6183 | ||
| R | 6 | 47 | 2 | 49 | 18.5 | 1701 | ||
| R | 6 | 37 | 14 | 66 | 15.8 | 1269 | ||
| Medial frontal gyrus | R | 8 | 11 | 27 | 43 | 13.2 | 1134 | |
| Middle frontal gyrus | L | 10 | −35 | 47 | 28 | 12.3 | 1350 | |
| Inferior frontal gyrus/insula | R | 44 | 44 | 13 | 13 | 12.6 | 945 | |
| Insula | L | 13 | −41 | 14 | 0 | 14.6 | 2295 | |
| Superior parietal lobule | L | 7 | −35 | −72 | 45 | 18.7 | 5508 | |
| Superior temporal gyrus | L | 22 | −53 | −33 | 4 | 20.5 | 7992 | |
| Middle temporal gyrus | R | 39 | 44 | −64 | 19 | 16.5 | 4482 | |
| Lingual gyrus | L | 18 | −20 | −88 | −13 | 14.1 | 1890 | |
| Fusiform gyrus | R | 19 | 32 | −81 | −20 | 23.8 | 13041 | |
| Amygdala | R | 20 | −10 | −26 | 10.7 | 216 | ||
| Caudate | L | −8 | 7 | 16 | 16.8 | 12204 | ||
| Thalamus/pulvinar | R | 5 | −30 | 8 | 13.9 | 1026 | ||
MNI coordinates of peak activation. Regions and BA areas according to Talairach Daemon Atlas. All regions initially thresholded at p<0.005. Regions >547 mm3 survive whole brain correction for multiple comparisons (p < 0.05).
Our first interaction of interest, diagnosis-by-phase, examined whether conduct disorder or oppositional defiant disorder plus psychopathic traits youths showed atypical BOLD responses during early trial learning. In line with predictions, significant diagnosis–by-phase interactions were observed within orbitofrontal cortex and the caudate (though not within the amygdala). In addition, significant diagnosis-by-phase interactions were seen in a network of attentional regions including inferior frontal cortex, middle frontal gyrus and the inferior parietal lobule (Table 2). In all the regions identified by this interaction, comparison subjects showed increased activity during early, but not late, trials relative to conduct disorder or oppositional defiant disorder plus psychopathic traits (orbitofrontal cortex (BA11): early t=3.2, p<0.005, late p= n.s.; caudate: early t=4.2, p<0.001, late p=n.s. (see figure 3).
Figure 3.
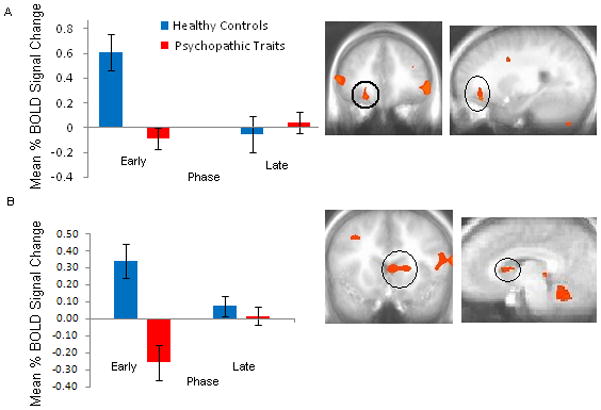
Diagnosis x Phase Interaction: In (a) right orbitofrontal cortex and (b) left caudate, conduct disorder or oppositional defiant disorder plus psychopathic traits youths demonstrated reduced mean BOLD activation during the early learning phase in comparison to healthy youths. Error bars represent standard errors.
Our second interaction of interest, diagnosis-by-response type, examined whether conduct disorder or oppositional defiant disorder plus psychopathic traits showed atypical BOLD responses to rewarded correct hits relative to punished commission errors. Significant diagnosis-by-response type interactions were observed within an anterior region of orbitofrontal cortex, middle frontal gyrus and the parahippocampal gyrus. Within orbitofrontal cortex, conduct disorder or oppositional defiant disorder plus psychopathic traits youths showed reduced activation during rewarded correct hits compared to comparison subjects (F(1,29)=4.6, p<0.05), while responses during punished commission errors were comparable (F(1,29) <1, p=0.8). In contrast, right middle frontal and parahippocampal gyrus, comparison subjects showed increased activation during commission errors relative to conduct disorder or oppositional defiant disorder plus psychopathic traits (F(1,29)=14.2 & 5.4, p < 0.001 & 0.05 respectively), while activation during correct hits was comparable across both groups (F(1,29)<1).
In addition, there was a significant main effect of diagnosis within the amygdala, caudate, insula and a network of regions implicated in attention processes including dorsolateral prefrontal cortex and parietal cortex (Table 2). In all of these regions, conduct disorder or oppositional defiant disorder plus psychopathic traits showed significantly less activation relative to comparison subjects.
To account for possible effects of medication use on the BOLD responses, the above analysis were repeated excluding the 7 youths in the conduct disorder or oppositional defiant disorder plus psychopathic traits group who were taking medication and their 7 age and IQ matched comparison subjects. The above effects of interest were replicated. Thus, the diagnosis-by-phase interaction was replicated in middle orbitofrontal cortex; conduct disorder or oppositional defiant disorder plus psychopathic traits showed reduced early activation relative to comparison subjects. Similarly, the diagnosis-by-response type interaction was observed in orbitofrontal cortex. Moreover, the main effects of diagnosis in the amygdala, caudate, and dorsolateral prefrontal cortex again revealed decreased activation in conduct disorder or oppositional defiant disorder plus psychopathic traits compared to comparison subjects.
DISCUSSION
The goal of the current study was to assess the nature of the irregularities within the amygdala, orbitofrontal cortex and striatum in conduct disorder or oppositional defiant disorder plus psychopathic traits during passive avoidance learning. Specifically, this study examined group differences in neural responses to two features critical to emotional learning: the level of experience with the stimulus (early vs. late trials) and the type of reinforcement (rewarded correct responses vs. punished incorrect responses). The results revealed that the youths with conduct disorder or oppositional defiant disorder plus psychopathic traits, relative to healthy youths, showed significantly less activity within orbitofrontal cortex and caudate in response to early exposures to reinforced outcomes. Moreover, conduct disorder or oppositional defiant disorder plus psychopathic traits showed significantly less activity within orbitofrontal cortex relative to comparison youths to rewarded correct responses. These task parameters did not reveal group differences within the amygdala though conduct disorder or oppositional defiant disorder plus psychopathic traits was associated with generally reduced activity within this region across the task.
PA impairments have been repeatedly reported in individuals with psychopathic traits (11, 15, 16, 47). Our model of passive avoidance learning and its dysfunction in psychopathic traits is highly influenced by the work of Schoenbaum/Gallagher and colleagues (19, 48, 49). In essence, we assume that in healthy individuals prediction errors to unexpected reinforcements (mediated by orbitofrontal cortex and caudate) serve to initiate rapid stimulus-reinforcement learning in the amygdala. As learning progresses, more accurate reinforcement expectancies are fed from the amygdala to orbitofrontal cortex where their representation allows successful decision making to occur; initiating approaches to objects associated with reward and avoidances of those associated with punishment. In individuals with high psychopathic traits, deficits in prediction error signaling lead to slower and weaker stimulus-reinforcement learning which in turn leads to weaker and less accurate reinforcement expectancies fed forward to orbitofrontal cortex. The individual becomes progressively less accurate in their decisions relative to comparison individuals because they are less able to represent the reward expectancy value associated the presented stimulus and respond accordingly (50).
Previous work has indicated orbitofrontal cortex dysfunction in individuals with psychopathic traits (51, 52). The current paper extends this by providing information on the nature of the functional impairment. In line with the model, conduct disorder or oppositional defiant disorder plus psychopathic traits youths, relative to comparison youths, showed significantly reduced orbitofrontal cortex responses during early trial learning and to rewarded responses. The first of these is compatible with the suggestion of disrupted prediction error signaling in orbitofrontal cortex in conduct disorder or oppositional defiant disorder plus psychopathic traits (cf. 28). Prediction errors are maximal after the individual’s first exposure of the relationship between the cue and the reinforcement but rapidly lessen as the individual develops an expectation of the reinforcement associated with the cue (29). In this regard it is notable that we have previously documented decreased orbitofrontal cortex responses, relative to comparison populations, to unexpected failures to receive rewards in conduct disorder or oppositional defiant disorder plus psychopathic traits (10). The finding of reduced orbitofrontal cortex responses to rewarded responses in conduct disorder or oppositional defiant disorder plus psychopathic traits is consistent with the suggestion that this population is less able to represent reward expectancy values. As such, these data are consistent with a previous study examining youths with CD, undifferentiated by psychopathic traits (53). This study also reported reduced responsiveness to reward outcome information within orbitofrontal cortex in youths with CD.
Neurobiological accounts of psychopathic traits (13, 54, 55) have neglected consideration of caudate (and for that matter, ventral tegmental area and ventral striatum), despite the critical role of these regions in prediction error signaling and/or reinforcement based learning (26, 27, 56). Part of this likely reflects the choice of paradigms used in the majority of previous fMRI work with youths or adults with psychopathic traits; i.e., expression processing (9, 31, 57), emotional memory (58) and moral reasoning (59). However, the current study revealed reduced caudate (though not ventral tegmental area or ventral striatal) responsiveness to early reinforced outcomes relative to later trials in the youths with psychopathic traits. This is compatible with the suggestion of disrupted prediction error signaling in caudate in conduct disorder or oppositional defiant disorder plus psychopathic traits. Indeed, it is notable that the only previous study examining operant responding in individuals with psychopathic traits also reported dysfunctional caudate but not ventral striatum responding in conduct disorder or oppositional defiant disorder plus psychopathic traits to reversal errors (10). This pattern is echoed by recent structural MRI work also indicating selective structural abnormalities within dorsal striatum in adult psychopathic traits populations (60). A recent study on healthy adults focused on the nucleus accumbens and found a correlation between individuals scoring higher on the impulsive aggressivity components of the psychopathic traits inventory and increased pharmacologically triggered dopamine release and increased activity during reward (61). Though intriguing results, these data were somewhat inconsistent with the only previous study reporting ventral striatal abnormalities in adults meeting criteria for psychopathy (62). This latter study reported reduced ventral striatal activity to emotional stimuli in the adults with psychopathy - albeit in response to a very different task. The current study also did not identify any regions demonstrating increased activity during rewarding trials in youths with psychopathic traits. These differences may reflect the hypothesized distinction between individuals showing heightened aggressive impulsivity in the absence of the core callous traits of psychopathy (who are believed to reflect heightened limbic responsiveness; 14) and those who do show these core callous traits (who show reduced limbic responsiveness).
Dorsal anterior cingulate cortex (dACC) has been implicated in conflict monitoring (63) and the use of error signals and the integration of reinforcement history to select between competing responses (64, 65). The current study was not designed to distinguish between these models. However, it is important to note the significant main effect of response type (commission error vs. correct hit) identified in this region (Supplementary Table) was not modulated by a main effect of group or group-by-response type interaction. These data suggest that dACC responses to the conflict initiated by punishment/error feedback remains intact in youths with conduct disorder or oppositional defiant disorder plus psychopathic traits. Notably, this replicates prior results in conduct disorder or oppositional defiant disorder plus psychopathic traits during a reversal learning paradigm where both groups showed appropriate recruitment of dorsal anterior cingulate cortex in response to punished reversal errors (10) In contrast, functional (10) and now structural abnormalties (60) have been reported in these populations in the dorsal striatum (caudate), indicating abnormalities in reward processing may arise from deficits in the amygdala-insula-orbitofrontal cortex -caudate circuit.
The main effect of diagnosis revealed reduced activation in several brain regions in conduct disorder or oppositional defiant disorder plus psychopathic traits. This could indicate reduced engagement with the task. In contrast, regions showing a main effect of accuracy and phase (i.e., regions where both groups show appropriate responses as a function of task parameters) include dACC and inferior frontal cortex/anterior insula. Importantly, these data suggest that an engagement explanation appears unsatisfactory as there is no reason why less task engagement would lead to appropriate recruitment of systems necessary for response change but selectively reduced recruitment of regions implicated in stimulus-reinforcement based decision making.
One caveat of the present study is that though there is a high comorbidity between conduct disorder or oppositional defiant disorder and ADHD, we did not include a second ADHD comparison group in the current study because neither of our previous studies indicated amygdala/orbitofrontal cortex pathophysiology in the youths with ADHD, though this was seen in conduct disorder or oppositional defiant disorder plus psychopathic traits (10, 31). Rubia and colleagues have shown dysfunction in orbitofrontal cortex reward signaling in youths with conduct disorder who do not present with ADHD but not in youths with ADHD (53). Second, the youth with conduct disorder or oppositional defiant disorder plus psychopathic traits made significantly more commission errors during the late learning phase which could produce stronger parameter estimates for late commission errors for the youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. However, it should be noted that the groups did not differ in BOLD responses to late commission errors but rather to early trials and rewarded correct hits.
In short, the current results are highly compatible with developmental models of conduct disorder with psychopathic traits stressing that this disorder reflects a fundamental disruption in the neural systems necessary for appropriate stimulus-reinforcement based decision making (14). Problems in this basic form of emotional learning will make the child difficult to socialize; the child will be less likely to “learn from his/her mistakes”. Moreover, they will learn on the basis of emotional feedback more slowly – this is notable in the present study in both the behavioral data and also in the reduced sensitivity within both orbitofrontal cortex and the caudate to early reinforcement information. Appropriate representation of such information is critical for rapid, early emotional learning. In addition, the dysfunction in orbitofrontal cortex’s role reinforcement outcome signaling, documented in this study, will be associated with poor decision making. This will lead to the selection of non-optimal behavioral choices (actions that harm others and actions likely to result in the individual being frustrated and potentially reactively aggressive because they do not meet their goals).
These data have clear treatment implications. Specifically, they suggest that the development of pharmacologic or behavioral therapeutic approaches that augment the capacity to engage in stimulus-reinforcement based decision making may enable youths with high psychopathic traits to overcome their functional deficiencies. In this regard, it is notable the both serotonergic (your ATD study) and dopaminergic manipulations (My study with Hasler) influence the performance of the task described in this study as well as its neural substrates. Moreover, recent reports that prediction error signaling in the amygdala facilitates learning by increasing orienting and attention to the stimulus during the subsequent trial (66), an interesting behavioral approach might be to train youths to direct their attention to explicitly form predictions about the expected reward from a particular action, and explicitly attend to the difference between their verbalized prediction and the actual outcome for both advantageous and disadvantageous actions.
In summary, the current data support previous suggestions of amygdala and orbitofrontal cortex dysfunction in conduct disorder or oppositional defiant disorder plus psychopathic traits. More importantly they indicate that conduct disorder or oppositional defiant disorder plus psychopathic traits are marked by a compromised sensitivity to early reinforcement information in both orbitofrontal cortex and caudate and to reward outcome information within orbitofrontal cortex. In conjunction with established models of stimulus-reinforcement instrumental learning they suggest that the integrated functioning of the amygdala, caudate and orbitofrontal cortex may be disrupted in individuals with this disorder.
Supplementary Material
Figure 2.
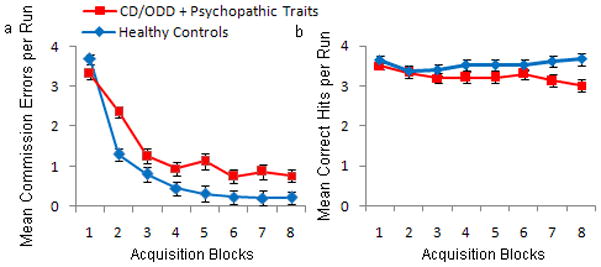
a) Mean commission errors (passive avoidance errors) and b) correct hits per block per run of the passive avoidance learning task. Youths with conduct disorder or oppositional defiant disorder plus psychopathic traits made significantly more errors in the late blocks (2–8) compared with healthy youths.
Figure 4.
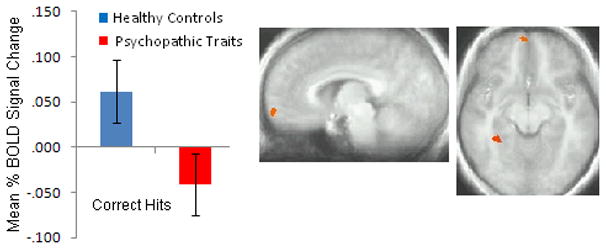
Diagnosis x Accuracy Interaction. In frontopolar orbitofrontal cortex (BA 10), in comparison to healthy youths, youths with conduct disorder or oppositional defiant disorder plus psychopathic traits showed reduced activation during rewarded responses relative to punished responses.
Table 3.
Patient Perspective: Youth with Conduct Disorder plus High Psychopathic Traits
| “Meghan” is a 14 y.o. girl with a history of disruptive behaviors since the age of 5. Despite being a bright and charming child, her parents report a long history of delinquent behaviors, including shoplifting expensive items, joy riding alone in her parents’ car, threatening her little sister with a knife, and promiscuous sexual behavior. Her parents describe her as completely self-centered, extremely manipulative, and a pathologic liar. She does not demonstrate empathy for family, and according to her parents, shows no remorse or guilt for her actions. Meghan describes herself as popular and smart and able to get A’s in school when she wants to. However, according to her parents she receives mainly C’s and D’s on her report card and has had no significant friendships. |
Acknowledgments
Grant support: This project was supported by the intramural research Program of the National Institutes of Mental Health
Footnotes
Prior presentations: none
Disclosures and Acknowledgements: The authors report no competing interests.
References
- 1.Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4:623–632. doi: 10.1586/14737175.4.4.623. [DOI] [PubMed] [Google Scholar]
- 2.Barry CT, Frick PJ, DeShazo TM, McCoy MG, Ellis M, Loney BR. The importance of callous-unemotional traits for extending the concept of psychopathy to children. J Abnorm Psychol. 2000 May;109(2):335–340. doi: 10.1037/0021-843X.109.2.335. [DOI] [PubMed] [Google Scholar]
- 3.Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report delinquency. Journal of Abnormal Child Psychology. 2003;31:457–470. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- 4.Dadds MR, Fraser J, Frost A, Hawes DJ. Disentangling the underlying dimensions of psychopathy and conduct problems in childhood: a community study. Journal of Consulting & Clinical Psychology. 2005;73(3):400–410. doi: 10.1037/0022-006X.73.3.400. [DOI] [PubMed] [Google Scholar]
- 5.Forth AE, Kosson DS, Hare RD. The Psychopathy Checklist: Youth Version. Toronto, Ontario, Canada: Multi-Health Systems; 2007. [Google Scholar]
- 6.Rowe R, Maughan B, Moran P, Ford T, Briskman J, Goodman R. The role of callous and unemotional traits in the diagnosis of conduct disorder. J Child Psychol Psychiatry. 2009 Dec 18; doi: 10.1111/j.1469-7610.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 7.Lynam DR, Caspi A, Moffitt TE, Loeber R, Stouthamer-Loeber M. Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. J Abnorm Psychol. 2007 Feb;116(1):155–165. doi: 10.1037/0021-843X.116.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh AA, Finger EC, Mitchell DG, et al. Reduced Amygdala Response to Fearful Expressions in Children and Adolescents With Callous-Unemotional Traits and Disruptive Behavior Disorders. Am J Psychiatry. 2008 Feb 15; doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 9.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 10.Finger EC, Marsh AA, Mitchell DG, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008 May;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lykken DT. A study of anxiety in the sociopathic personality. Journal of abnormal and social psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- 12.Hare RD. Psychopathy: Theory and Research. New York: Wiley; 1970. [Google Scholar]
- 13.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007 Sep;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Blair RJR. Dysfunctions of medial and lateral orbitofrontal cortex in psychopathy. Annals of the New York Academy of Sciences. 2007;1121:461–479. doi: 10.1196/annals.1401.017. [DOI] [PubMed] [Google Scholar]
- 15.Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986;95:252–256. [PubMed] [Google Scholar]
- 16.Newman JP, Widom CS, Nathan S. Passive avoidance in syndromes of disinhibition: Psychopathy and extraversion. Journal of Personality and Social Psychology. 1985;48:1316–1327. doi: 10.1037//0022-3514.48.5.1316. [DOI] [PubMed] [Google Scholar]
- 17.Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Neural topography and chronology of memory consolidation: A review of functional inactivation findings. Neurobiology of Learning and Memory. 1999;71:1–18. doi: 10.1006/nlme.1998.3865. [DOI] [PubMed] [Google Scholar]
- 18.Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response memory trace. Brain Research. 1997;768:242–248. doi: 10.1016/s0006-8993(97)00651-3. [DOI] [PubMed] [Google Scholar]
- 19.Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005 Sep 1;47(5):633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. Journal of Neuroscience. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosson DS, Budhani S, Nakic M, et al. The role of the amygdala and rostral anterior cingulate in encoding expected outcomes during learning. Neuroimage. 2006 Feb 15;29(4):1161–1172. doi: 10.1016/j.neuroimage.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 23.Roesch MR, Singh T, Brown PL, Mullins SE, Schoenbaum G. Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. Journal of Neuroscience. 2009;29:13365–13376. doi: 10.1523/JNEUROSCI.2572-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair KS, Marsh AA, Morton J, et al. Choosing the lesser of two evils, the better of two goods: Specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate cortex in object choice. Journal of Neuroscience. 2006;26(44):11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Shimojo S, O’Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol. 2006 Jul;4(8):e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006 Jan 5;49(1):157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putament and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology. 2006;95(2):948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- 28.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003 Apr 24;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 29.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II. Appleton: Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 30.Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. J Abnorm Psychol. 2006 Aug;115(3):552–558. doi: 10.1037/0021-843X.115.3.552. [DOI] [PubMed] [Google Scholar]
- 31.Marsh AA, Finger EC, Mitchell DG, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008 Jun;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W, Preuschoff K, Camerer C, et al. Explicit neural signals reflecting reward uncertainty. Philos Trans R Soc Lond B Biol Sci. 2008;363:3801–3811. doi: 10.1098/rstb.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 Jul;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Frick PJ, Hare RD. The antisocial process screening device. Toronto: Multi-Health Systems; 2001. [Google Scholar]
- 35.Edens JF, Skeem JL, Cruise KR, Cauffman E. Assessment of “juvenile psychopathy” and its association with violence: a critical review. Behav Sci Law. 2001;19(1):53–80. doi: 10.1002/bsl.425. [DOI] [PubMed] [Google Scholar]
- 36.Murrie DC, Cornell DG. Psychopathy screening of incarcerated juveniles: a comparison of measures. Psychol Assess. 2002 Dec;14(4):390–396. [PubMed] [Google Scholar]
- 37.Frick PJ, Hare RD. Antisocial Process Screening Device. Toronto: Multi-Health Systems Inc; 2001. [Google Scholar]
- 38.Budhani S, Blair RJ. Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J Child Psychol Psychiatry. 2005 Sep;46(9):972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 39.Vitale JE, Newman JP, Bates JE, Goodnight J, Dodge KA, Pettit GS. Deficient behavioral inhibition and anomalous selective attention in a community sample of adolescents with psychopathic traits and low-anxiety traits. J Abnorm Child Psychol. 2005 Aug;33(4):461–470. doi: 10.1007/s10802-005-5727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forth A, Kosson DS, Hare RD. Hare Psychopathy Checklist-Youth Version. Toronto: Multi-Health Systems Inc; 2004. [Google Scholar]
- 41.Finger EC, Mitchell DGV, Jones M, Blair RJR. Dissociable roles of medial orbital frontal cortex in human operant extinction learning. Neuroimage. 2008;43:748–755. doi: 10.1016/j.neuroimage.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 43.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 44.Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 45.Burgund ED, Kang HC, Kelly JE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- 46.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- 47.Blair RJR, Mitchell DGV, Leonard A, Budhani S, Peschardt KS, Newman C. Passive avoidance learning in individuals with psychopathy: modulation by reward but not by punishment. Personality and Individual Differences. 2004;37:1179–1192. [Google Scholar]
- 48.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999 Mar 1;19(5):1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003 Aug 28;39(5):855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 50.Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007 Sep;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Birbaumer N, Veit R, Lotze M, et al. Deficient Fear Conditioning in Psychopathy: A Functional Magnetic Resonance Imaging Study. Arch Gen Psychiatry. 2005 Jul 1;62(7):799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 52.Rilling JK, Glenn AL, Jairam MR, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry. 2007 Jun 1;61(11):1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Rubia K, Smith AB, Halari R, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. American Journal of Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 54.Blair RJR. Neuro-cognitive models of aggression, the Antisocial Personality Disorders and Psychopathy. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Res. 2006 May 17; doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 57.Dolan MC, Fullam RS. Psychopathy and functional magnetic responance imaging Blood Oxygenation Level-Dependent respones to emotional faces in violence patients with schizophrenia. Biological Psychiatry. 2009;66:570–577. doi: 10.1016/j.biopsych.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Kiehl KA, Smith AM, Hare RD, et al. Limbic Abnormalities in Affective Processing By Criminal Psychopaths as Revealed by Functional Magnetic Resonance Imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- 59.Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2008;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- 60.Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in psychopathic individuals. Biol Psychiatry. Jan 1;67(1):52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckholtz JW, Treadway MT, Cowan RL, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. Apr;13(4):419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiehl KA, Smith AM, Hare RD, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001 Nov 1;50(9):677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- 63.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Science. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Holroyd CB, Coles MG. Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behavior. Cortex. 2008 May;44(5):548–559. doi: 10.1016/j.cortex.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007 Apr;11(4):168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Roesch MR, Calu DJ, Esber GR, Schoenbaum G. Neural correlates of variations in event processing during learning in basolateral amygdala. J Neurosci. Feb 17;30(7):2464–2471. doi: 10.1523/JNEUROSCI.5781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


