Abstract
Steroid induced skin atrophy is the most frequent and perhaps most important cutaneous side effect of topical glucocorticoid therapy. To date, it has not been described in vulvar skin. We describe a patient with significant vulvar skin atrophy following prolonged steroid application to treat vulvar dermatitis. The extensive atrophy in the perineum resulted in secondary ‘webbing’ and partial obstruction of genital hiatus and superimposed dyspareunia. Prolonged topical steroids may result in atrophic changes in vulvar skin. Therefore, further research in clinical correlates of steroid-induced atrophy in the vulvar region is warranted.
Introduction
Since the 1960s, skin atrophy has been noted as the most common cutaneous side effect of topical steroid therapy [1, 2]. Potency, duration and frequency of application are risk factors known to contribute to the atrophogenic potential of topical steroids [1]. Skin atrophy is clinically diagnosed and characterized by 1) an increase in transparency of skin, 2) “cigarette paper- like” consistency, and 3) fragility and tearing [1]. It had been noted that moist humid regions of the body (i.e. axilla and groin) have an increased vulnerability to the atrophogenic potential of topical steroids because moisture and occlusion lead to increased absorption [2]. Surprisingly, despite similar anatomical vulnerability, steroid induced atrophy has not been described in vulvar regions [3]. We report a case of vulvar skin atrophy in a woman with a 5-year history of intermittent topical steroid use.
Case Report
A 55 year old woman presented to our clinic with a three-year history of post coital bleeding and worsening dyspareunia. In addition, she reported suffering from intermittent vulvar pain in her forties which had become constant following menopause at age 50–52. Her vulvar pain had been treated with various forms of estrogen therapy and when she failed to respond, topical steroids were prescribed. During the initial four months of steroid therapy, her vulvar pain improved. However, she then began experiencing new onset vulvar itching and worsening dyspareunia. In an attempt to alleviate her worsening symptoms, the frequency and potency of the topical steroids were increased. Over the two years prior to her first evaluation at our clinic, she experienced 4–6 episodes per year of increasing sensitivity, with each episode lasting 2–3 weeks and decreasing after the application of ‘left over steroids.’ Biopsies were performed and were reported to be negative for any known dermatological condition that could be associated with underlying atrophy. Of note, post-coital bleeding was noted with the initiation of steroid therapy.
At her initial presentation, the patient was unable to wear slacks or fitted undergarments. She reported that her skin splitting initially provoked by intercourse now occurred readily with toileting or the slightest contact. Upon physical examination she had a profound loss of subcutaneous fat as demonstrated by the drooping of labia minora and its secondary elongation (Fig. 1a). This finding was more pronounced than would be expected in the post-menopausal woman. The perineal skin was extremely thin (Figs. 1b, 2). Separation of labia majora during examination resulted in near complete obstruction of the genital opening by a web of perineal skin (Fig. 2). Pressure application with a cotton swab caused the sensation of “splitting” that she experienced with intercourse. Using a mirror, the patient was educated on anatomical and functional correlates of her symptoms.
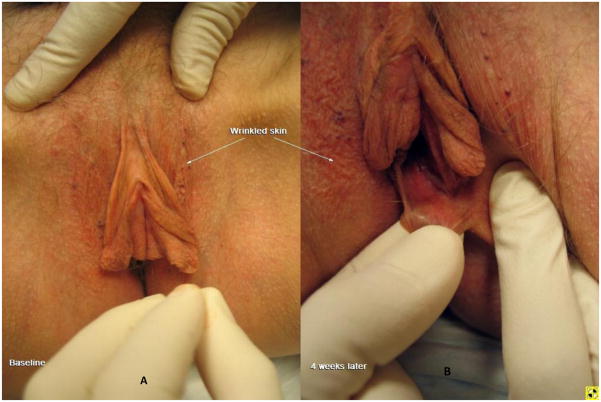
Figure 1.
A. Extensive loss of subcutaneous fat as demonstrated by flattening of labia majora and drooping of the labia minora akin to an “elephant’s ear” (baseline visit). B. After 4 weeks of therapy with 0.05% estradiol; despite persistence of stretchy elongated skin, the labia minora are retracted upward and are fuller in appearance presumably due to estrogen’s effect on bulbous tissue and blood flow.
Figure 2.
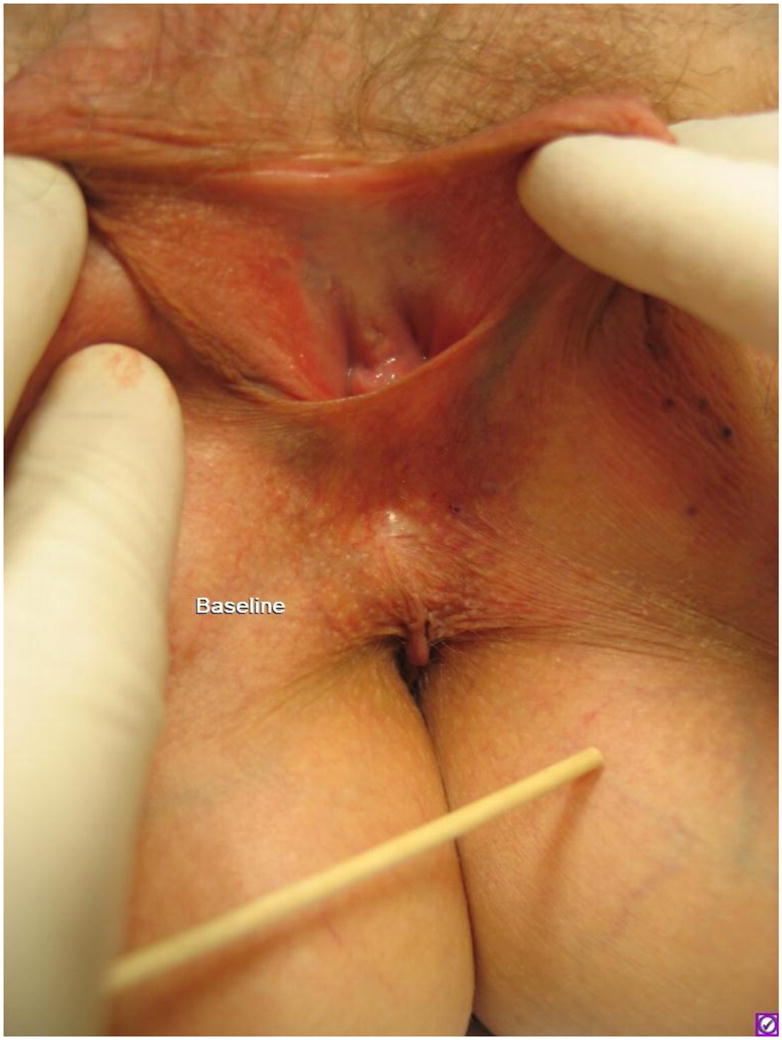
A thin redundant web of perineal skin which obstructs the genital hiatus (baseline visit). Note: puckering of the skin (pointed toward by the wooden end of swab) and background telangiectasia in perineum.
In addition to stopping steroids, she was instructed to apply Crisco® shortening to the labial surface including the inter-labial fold: the anatomical point of maximal friction. This was done to increase the lubrication of the skin surface and to prevent further thinning and irritation caused by friction. At her follow up appointment, the patient reported a significant reduction of her symptoms with this regimen. To address the remaining symptoms, we started her on nightly high dose topical estrogen therapy. In our experience, most patients with long lasting symptoms and moderate to severe atrophic changes do not respond to commercially available formulations of estrogen. Thus, we use topical estradiol (0.05%) compounded in hydrophilic petrolatum for a brief period of 6–8 weeks; application consists of 1–3 cc of cream strategically applied to inner labial folds (medial aspect of the labia minora) and inter-labial sulcus (the junction between labia minora and majora).
After 8 weeks of therapy, the patient experienced profound improvement. Upon examination, while the keratinized skin of the perineum remained thin, the erectile tissue of the labia minora and bulbocavernosus muscles and mucosa had nearly normalized in appearance (Fig. 3). Using a mirror, the patient was educated on positioning and ways of avoiding traction on the web of skin during intercourse (Fig. 3). After the initial eight weeks of therapy, the high dose topical estradiol was discontinued and she was successfully maintained on a vaginal estrogen ring and Crisco® shortening. Twelve months later, she resumed intercourse (after a hiatus of 4–5 years). However, intercourse continued to be painful despite near complete resolution of her vulvo-vaginal symptoms. The extent to which her residual pain with intercourse can be attributed to the redundant web of skin, versus underlying vulvodynia, remains unclear.
Figure 3.
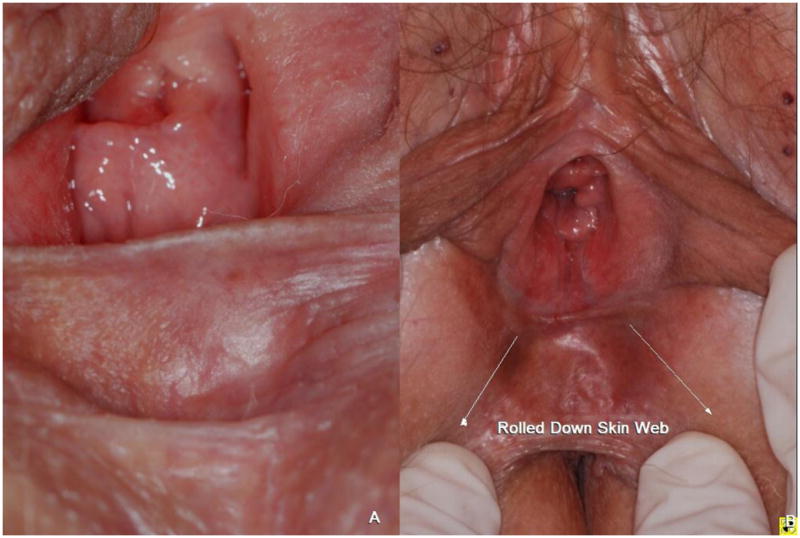
The resting (A) and rolled down (B) position of the perineal web of skin (2 years after therapy).The resting position of the perineal web continues to obstruct the genital hiatus as steroid related atrophy is often irreversible.
Discussion
Moisture inintertriginous areas (e.g. axilla, groin) allows greater absorption of steroids making them more vulnerable to steroid induced atrophy. The vulvo-vaginal region logically seems vulnerable, but steroid induced vulvar atrophy has not been documented [3, 4]. Given the extensive documentation of vulvar atrophy in this case, steroid related side effects in vulvar region seem likely. Poor sensory discrimination, overlapping symptomatology, and high anatomic variability of vulvar skin may serve to obscure the unwarranted side effects of steroids in gynecological practice [5]. Worsening symptoms after initial improvement, and/or a new onset of symptoms (e.g. insertional dyspareunia) may be early warning signs of steroid related side effects.
Apart from steroid induced skin atrophy, other conditions such as lichen sclerosus and senile skin atrophy may cause similar atrophic changes[1]. As previously mentioned, in our case, previous biopsies had been negative for any known dermatological condition that could be associated with underlying atrophy. Steroid induced atrophy is primarily a clinical diagnosis [1], and the histological correlates of our clinical observation would not have altered the patient’s care plan, thus we did not repeat the biopsy in this case.
Our case shows that vulvar atrophy is a potential risk of long term steroid use in the vulvar region. High potency steroids, such as clobetasol propionate, are commonly used in gynecology as first line therapies. Until further research is carried out, steroids should be used cautiously to decrease the likelihood of long term complications. In addition to starting with lower potency steroids for the shortest duration (e.g. 2 weeks) [3, 6], thorough patient instruction including the use of a mirror to demonstrate the amount and location of application may help to minimize the likelihood of unwanted side effects.
Footnotes
Disclosure(s): The authors do not have any potential conflicts of interest. This work, in part, was supported by an NIH mentored career development award (K23HD053631).
Contributor Information
Elisabeth Johnson, Email: edinkins@med.unc.edu.
Pamela Groben, Email: pgroben@med.unc.edu.
Alisa Eanes, Email: akeanes@med.unc.edu.
Priya Iyer, Email: iyerph@gmail.com.
Joseph Ugoeke, Email: jugoeke@med.unc.edu.
Denniz Zolnoun, Email: zolnound@med.unc.edu.
References
- 1.Schoepe S, Schacke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006;15(6):406–420. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 2.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Practice Bulletin No. 93: diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111(5):1243–1253. doi: 10.1097/AOG.0b013e31817578ba. [DOI] [PubMed] [Google Scholar]
- 4.Dalziel KL, Wojnarowska F. Long-term control of vulval lichen sclerosus after treatment with a potent topical steroid cream. J Reprod Med. 1993;38(1):25–27. [PubMed] [Google Scholar]
- 5.The vulva: anatomy, physiology, and pathology. New York: Informa Healthcare; 2006. [Google Scholar]
- 6.Lagos BR, Maibach HI. Frequency of application of topical corticosteroids: an overview. Br J Dermatol. 1998;139(5):763–766. doi: 10.1046/j.1365-2133.1998.02498.x. [DOI] [PubMed] [Google Scholar]