Abstract
Background
The role of concomitant aspirin therapy in patients with atrial fibrillation (AF) receiving oral anticoagulation (OAC) is unclear. We assessed concomitant aspirin use and its association with clinical outcomes among AF patients treated with OAC.
Methods and Results
The Outcomes Registry for Better Informed Treatment (ORBIT) of Atrial Fibrillation registry enrolled 10,126 AF patients from 176 US practices from June, 2010 through August, 2011. The study population was limited to those on OAC (n=7,347).
Hierarchical multivariable logistic regression models were used to assess factors associated with concomitant aspirin therapy. Primary outcomes were 6-month bleeding, hospitalization, ischemic events, and mortality. Overall, 35% (n=2543) of AF patients on OAC also received aspirin (OAC+ASA). Patients receiving OAC+ASA were more likely male (66% vs. 53%, p<0.0001) and had more comorbid illness than those on OAC alone. Over one-third (39%) of OAC+ASA did not have a history of atherosclerotic disease, yet 17% had elevated ATRIA bleeding risk scores (≥5). Major bleeding (adjusted HR 1.53, 95% CI 1.20–1.96) and bleeding hospitalizations (adjusted HR 1.52, 95% CI 1.17–1.97) were significantly higher in those on OAC+ASA versus OAC alone. Rates of ischemic events were low.
Conclusions
Patients with AF receiving OAC are often treated with concomitant aspirin, even when they do not have cardiovascular disease. Use of OAC+ASA was associated with significantly increased risk for bleeding, emphasizing the need to carefully determine if and when the benefits of concomitant aspirin outweigh the risks in AF patients already on OAC.
Keywords: atrial fibrillation, anticoagulants, aspirin, hemorrhage, outcomes research
Atrial fibrillation (AF) represents the most common arrhythmia in the US, and it substantially increases the risk of stroke.1, 2 Oral anticoagulant (OAC) therapy is the mainstay of treatment for AF patients at risk for stroke. Many patients with AF also have coexistent atherosclerotic cardiovascular disease,3 and may be also put on antiplatelet therapy in addition to OAC medications. However, the incremental benefit of antiplatelet therapy added to anticoagulation in patients with AF is unclear. While European guidelines support the use of more aggressive concomitant antiplatelet therapy over short periods of time in patients at acceptably low risk for bleeding, US guidelines are more reserved.4, 5 To date, there have been limited data available to define current patterns of use of concomitant antiplatelet therapy along with OAC in AF patients in the US. Furthermore, the risks of such combinations in community practice remain poorly defined.
To address these important questions, we used data from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) to investigate contemporary implementation of aspirin therapy in patients with AF receiving OAC, and associated clinical outcomes. Our objectives were to:(1) describe overall use of concomitant antiplatelet and OAC therapy in AF patients; (2) identify clinical factors associated with concomitant therapy; (3) note use of dual therapy among those without any known cardiovascular disease; and (4) determine if the addition of antiplatelet therapy is associated with risk for subsequent bleeding events.
Methods
The ORBIT-AF registry is an observational study of outpatients with AF who are managed by primary care providers, cardiologists, and electrophysiologists. The design and rationale of the ORBIT-AF registry have been described previously.6 Briefly, patients with electrocardiographically-proven AF aged 18 years and older, who are able to provide informed consent and follow-up at least every 6 months, were eligible for enrollment. Those with reversible causes of AF (e.g., thyroid disease, post-operative AF) or patients with a lifeexpectancy of less than 6 months are excluded.
This population was then stratified by the use of aspirin: (1) OAC alone versus (2) dual therapy with OAC and aspirin (OAC+ASA). Baseline characteristics, AF status, stroke and bleeding risk profiles, and vascular history were compared between these groups. Risk scores for stroke and bleeding risk were calculated, including the CHADS2, CHA2DS2-VASc, and ATRIA scores.7–9 To isolate the adjusted impact of concomitant aspirin use separate from newer antiplatelet agents (clopidogrel, prasugrel), patients receiving other antiplatelet drugs were excluded.
In the entire study population, baseline characteristics associated with the use of OAC+ASA versus OAC alone were measured in multivariable modeling. In patients with 6-month follow-up (6540/7347, 89%), consistency of therapy was measured. Lastly, 6-month clinical outcomes across all treatment groups were assessed, and included death, first hospitalization by cause, bleeding events (major bleeding classified by International Society on Thrombosis and Haemostasis criteria [ISTH],10 and minor bleeding), and ischemic events (myocardial infarction [MI], revascularization, stroke or non-CNS embolism, and TIA). Additional, absolute rates of ischemic and bleeding events are also presented in high-risk subgroups.
Statistical Methods
Patient characteristics and outcomes are stratified by type of therapy. Continuous variables are presented as medians (interquartile range) and differences between the groups were assessed using the Kruskal-Wallis test. Categorical variables are presented as counts (proportions) and differences were assessed using the Chi-Square test.
Hierarchical multivariable logistic regression was used, and odds ratio (OR) and corresponding 95% confidence interval (CI) were presented to assess factors associated with OAC+ASA (versus OAC alone), while accounting for variability in the use of dual therapy between sites. Backward selection was used to select from among the baseline characteristics, with an alpha for exclusion of 0.05, using hierarchical logistic regression.
A shared frailty model was used to assess the association between of OAC+ASA (versus OAC alone) and 6-month outcomes, and hazard ratio (HR) and corresponding 95% CIs were presented. A shared frailty model was used to account for correlation between subjects from the same site. For nuisance bleeding, hierarchical logistic regression was used, and OR and corresponding 95% CI were presented since the dates of events were not available. To minimize confounding, an adjusted regression model was developed by inverse weighting according to the propensity score for getting OAC+ASA. Propensity scores were calculated using a hierarchical logistic regression model adjusted for selected baseline characteristics. After backward selection with an alpha for exclusion of 0.10, the following variables were included in the propensity model: age, weight, creatinine clearance, diastolic blood pressure, sex (female), hyperlipidemia, liver disease, hypertension, diabetes, smoking, thyroid disease, prior gastrointestinal bleed, history of stroke or transient ischemic attack, history of coronary artery disease, peripheral arterial disease, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), drug-eluting stent (DES), prior antiarrhythmic drug (AAD), prior interventional therapy for AF, renal disease, valvular disease, prior valve replacement or repair, level of education, electrocardiographic evidence of LVH, provider specialty, type of AF, and region.
All continuous variables were tested for linearity, and non-linear relationships were accounted for using linear splines. Analyses of the summary, de-identified data were performed by the Duke Clinical Research Institute using SAS software (versions 9.3, SAS Institute, Cary, North Carolina, USA). All p-values were two-sided. The ORBIT-AF Registry is approved by the Duke institutional review board (IRB), and all participating sites obtained IRB approval pursuant to local requirements. All subjects participating in ORBIT-AF provided written, informed consent. The authors had open access to the primary data and take full responsibility for the validity herein, as well as the formulation and drafting of the manuscript.
Results
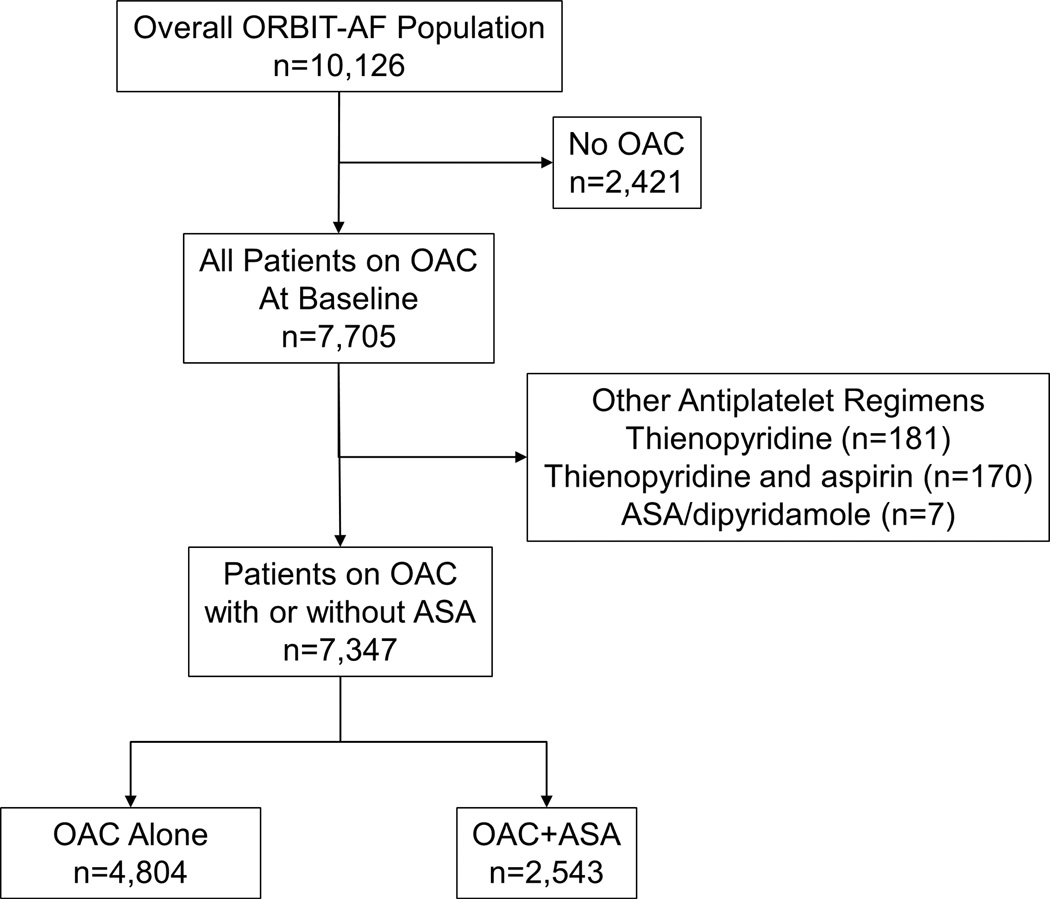
The overall ORBIT-AF population included 10,126 patients enrolled at 176 sites from June 29th, 2010 through August 29th, 2011. For the purpose of this analysis, patients who were not taking an oral anticoagulant were excluded. Additionally, patients on antiplatelet therapies other than aspirin were excluded. This yielded a study population of 7,347 patients from 174 sites (Figure 1). Over one third (35%, n=2543) of our study population received concomitant aspirin therapy. The dosing of aspirin included 81 mg (n=2251/2543, 88.5 %), 162 mg (n=12/2543, 0.5%), 325 mg (n=272/2543, 10.7%), and other doses (n=8/2543). Overall, dabigatran use was 6.9% in the OAC alone group (n=332/4804) and 6.0% in the OAC+ASA group (n=153/2543).
Figure 1.
Flow diagram of exclusion criteria in the current study, yielding the final analysis population of 7,347 patients. OAC: oral anticoagulation; ASA: aspirin.
Factors Associated with Concomitant Aspirin
Baseline characteristics and medical history are shown in Table 1. Patients receiving OAC+ASA therapy were slightly younger, less likely to be female, but had more medical co-morbidities, including hypertension, hyperlipidemia, diabetes, pulmonary disease, and heart failure than patients receiving OAC alone. Rates of prior GI bleeding were not significantly different (8.2% for OAC alone vs. 7.1% for OAC+ASA, p=0.1). Patients receiving OAC+ASA were more likely to have new onset or paroxysmal AF (4.6% vs. 3.8% for new onset, 47% vs. 45% for paroxysmal) versus longstanding persistent AF (30% vs. 33%, p=0.003 for overall comparison) (Table 2). Stroke risk scores were higher in patients receiving concomitant aspirin (CHADS2 ≥2 for 72% for OAC alone vs. 79% for OAC+ASA, p<0.0001), while ATRIA bleeding risk scores were no different (17% with ATRIA score ≥5 in each group, p=0.7).
Table 1.
Baseline Characteristics Stratified by Additional Antiplatelet Therapy
| Overall (n=7347) |
OAC Alone (n=4804) |
OAC+ASA (n=2543) |
p-Value | |
|---|---|---|---|---|
| Age | 75 (68–82) | 76 (68–82) | 75 (67–81) | 0.0003 |
| Female | 43 | 47 | 34 | <0.0001 |
| Race | <0.0001 | |||
| White | 89 | 88 | 92 | |
| Black or African American | 4.8 | 5.0 | 4.3 | |
| Hispanic | 4.5 | 5.8 | 2.1 | |
| Past Medical History | ||||
| Smoking | 48 | 45 | 54 | <0.0001 |
| Hypertension | 85 | 83 | 87 | <0.0001 |
| Hyperlipidemia | 72 | 69 | 79 | <0.0001 |
| Diabetes | 30 | 28 | 35 | <0.0001 |
| Obstructive Sleep Apnea | 18.5 | 17 | 21 | <0.0001 |
| Heart Failure | 34 | 31 | 39 | <0.0001 |
| Gastrointestinal Bleeding | 7.8 | 8.2 | 7.1 | 0.11 |
| Anemia | 17 | 16 | 19 | 0.002 |
| Chronic Obstructive Pulmonary Disease | 16 | 15 | 18 | 0.0001 |
| Hemoglobin (g/dl) | 13.5 (12.3–14.6) | 13.6 (12.3–14.6) | 13.5 (12.3–14.7) | 0.05 |
| Calculated creatinine clearance (mL/min/1.73m2)* | 70 (51–97) | 69 (50–96) | 72 (52–98) | 0.004 |
| LVEF (%), mean (SD) | 54.3 (12) | 55.2 (12) | 52.6 (13) | <0.0001 |
Values are presented as % or median (interquartile range), except where noted.
Excluding patients on dialysis.
OAC: oral anticoagulation; ASA: aspirin; BMI: body mass index; LVEF: left-ventricular ejection fraction.
Table 2.
Atrial Fibrillation History and Risk Scores
| Overall (n=7347) |
OAC Alone (n=4804) |
OAC+ASA (n=2543) |
p-Value | |
|---|---|---|---|---|
| AF Type | 0.003 | |||
| New onset | 4.0 | 3.8 | 4.6 | |
| Paroxysmal | 46 | 45 | 47 | |
| Persistent | 18 | 18 | 18 | |
| Longstanding Persistent | 32 | 33 | 30 | |
| Duration of OAC, median months (IQR) | 46 (19–90) | 50 (21–95) | 41 (15–79) | <0.0001 |
| OAC Started Within 3 Months | 4.3 | 3.7 | 5.5 | 0.001 |
| Prior Treatment with Antiarrhythmic Drug | 45 | 43 | 48 | <0.0001 |
| Prior Catheter Ablation of AF | 5.3 | 4.8 | 6.3 | 0.001 |
| CHADS2 Score | <0.0001 | |||
| 0 | 4.6 | 5.2 | 3.6 | |
| 1 | 21 | 23 | 17 | |
| ≥2 | 75 | 72 | 79 | |
| CHA2DS2-VASa Score | 0.002 | |||
| 0 | 1.3 | 1.5 | 0.8 | |
| 1 | 5.4 | 5.7 | 4.7 | |
| ≥2 | 85 | 84 | 88 | |
| ATRIA Score | 0.74 | |||
| Low (0–3) | 75 | 75 | 74 | |
| Intermediate (4) | 8.6 | 8.4 | 8.8 | |
| High (5+) | 17 | 17 | 17 | |
| Site Provider Specialty | <0.0001 | |||
| Cardiology | 66 | 65 | 68 | |
| Electrophysiology | 15 | 14 | 18 | |
| Primary Care Provider | 19 | 22 | 15 |
Values are presented as percentages, except where noted.
OAC: oral anticoagulation; ASA: aspirin; AF: atrial fibrillation; IQR: interquartile range.
Vascular disease, including coronary, cerebral, or peripheral arterial disease, was common in the study cohort (Table 3). Overall 39% of patients receiving OAC+ASA had no history of atherosclerotic disease. Conversely, 37% of those on OAC alone had a history of atherosclerotic disease.
Table 3.
Vascular History
| OAC Alone (n=4804) | OAC+ASA (n=2543) | |
|---|---|---|
| Any Vascular History (CAD, PAD, or CVD) | 37 | 61 |
| CAD | 20 | 47 |
| Prior MI | 9.7 | 23 |
| Prior CABG | 9.1 | 24 |
| Prior PCI | 9.2 | 24 |
| Prior DES | 2.4 | 8.9 |
| Prior Cerebrovascular Events (stroke or TIA) | 15 | 20 |
| Stroke – non-hemorrhagic | 7.8 | 10 |
| Stroke – hemorrhagic | 0.5 | 0.7 |
| TIA | 7.7 | 11 |
Values are presented as percentages. All comparison p-values <0.0001, except MI in previous year (p=0.003), DES in previous year (p=0.004), non-hemorrhagic stroke (p=0.0001), and hemorrhagic stroke (p=0.3).
OAC: oral anticoagulation; ASA: aspirin; CAD: coronary artery disease; PAD: peripheral artery disease; CVD: cerebrovascular disease; MI: myocardial infarction; CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; DES: drug-eluting stent; TIA: transient ischemic attack.
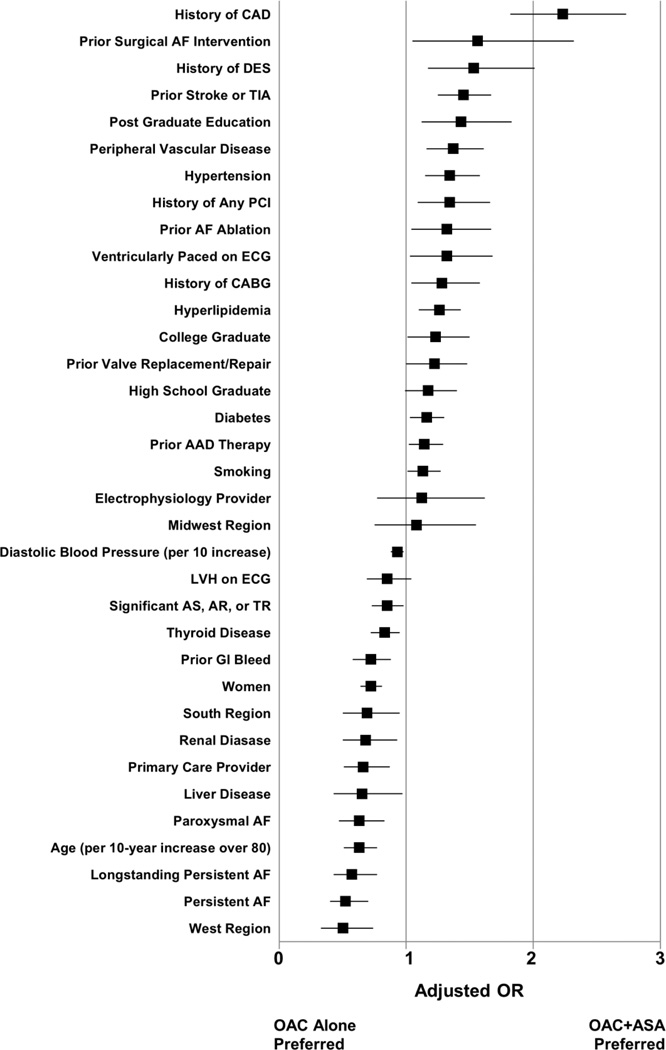
Multivariable factors associated with the use of additional aspirin (versus OAC alone) are provided in Figure 2. The strongest positive effect estimates were observed with a history of CAD (adjusted OR 2.23, 95% CI 1.82–2.73), prior MAZE (adjusted OR 1.56, 95% CI 1.05–2.32), any prior DES (adjusted OR 1.53, 95% CI 1.17–2.01), and prior stroke or TIA (adjusted OR 1.45, 95% CI 1.25–1.67).
Figure 2.
Factors associated with OAC+ASA (versus OAC alone) in multivariable analysis. References groups are new onset AF (for AF type), some school (for education), cardiologist (for provider type), and Northeast (for region). OAC: oral anticoagulation; ASA: aspirin; OR: odds ratio; CAD: coronary artery disease; DES: drug-eluting stent; TIA: transient ischemic attack; PCI: percutaneous coronary intervention; AF: atrial fibrillation; ECG: electrocardiogram; CABG: coronary artery bypass grafting; AAD: antiarrhythmic drug; LVH: left-ventricular hypertrophy; GI: gastrointestinal; AS: aortic stenosis; AR: aortic regurgitation; TR: tricuspid regurgitation.
Follow-Up Therapy and Outcomes
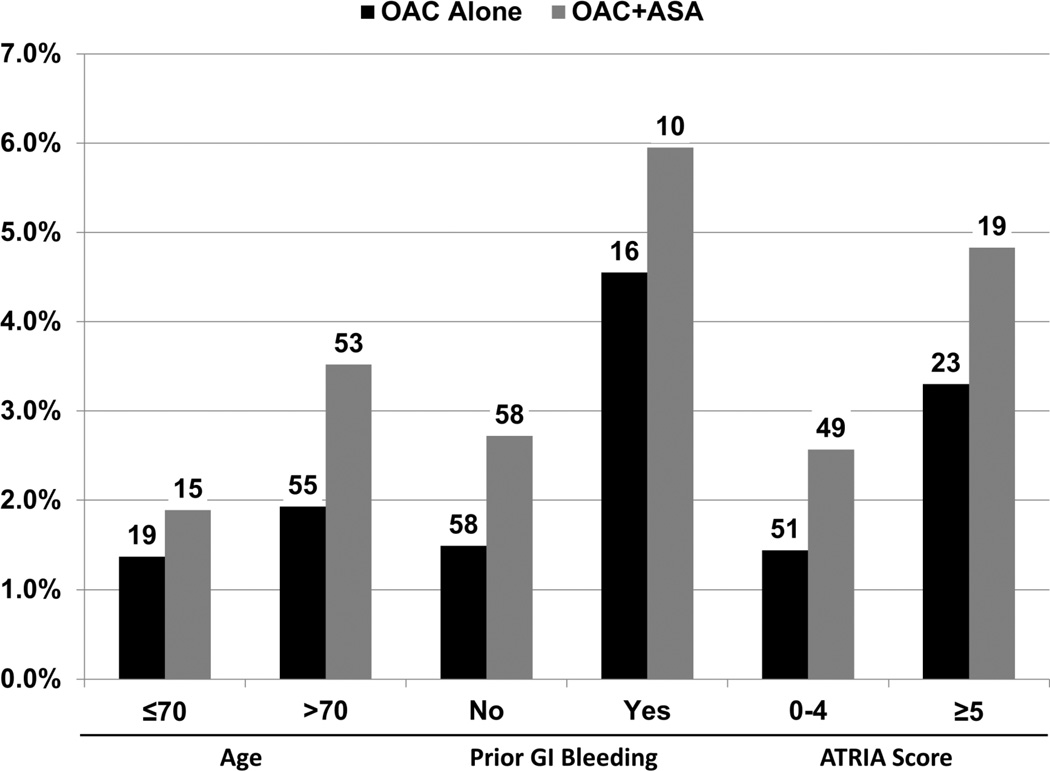
At 6-month follow-up, utilization rates of antithrombotic therapies were stable, with 89% of patients who started on OAC alone remaining on OAC alone and 81% of patients on OAC+ASA remaining on OAC+ASA. Characteristics of patients missing follow-up are shown in the supplement (Supplemental Table 1). Rates of major bleeding events at 6 months, among high-risk subgroups, are shown in Figure 3. Unadjusted and multivariable-adjusted rates of major adverse outcomes at 6-months are shown in Table 4. Major bleeding and bleeding hospitalization rates were significantly higher in the OAC+ASA group. There were 6 intracranial hemorrhage events in the OAC alone group, versus 10 in the OAC+ASA group (0.14% vs. 0.43%, p=0.02).After adjusting for baseline characteristics, major bleeding (HR 1.53, 95% CI 1.20–1.96) and bleeding hospitalization (HR 1.52, 95% CI 1.17–1.97) were more likely in patients receiving OAC+ASA (compared with OAC alone). Rates of ischemic events were low in both groups; MI occurred in 16 (0.38%) patients on OAC alone versus 11 on OAC+ASA (0.48%), coronary revascularization in 28 (0.66%) versus 31 (1.35%), stroke or non-CNS embolism in 18 (0.42%) versus 15 (0.65%), and TIA in 7 (0.17%) versus 3 (0.13%).
Figure 3.
Unadjusted, 6-month major bleeding rates among high-risk subgroups are shown with absolute numbers of events per group noted above. OAC: oral anticoagulation; ASA: aspirin; GI: gastrointestinal.
Table 4.
Adjusted 6-Month Outcomes
| OAC Alone (n=4239) |
OAC+ASA (n=2301) |
Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Major Bleeding | 74 (1.8) | 68 (3.0) | 1.62 (1.16 – 2.26) | 0.004 | 1.53 (1.20 – 1.96) | 0.0006 |
| Nuisance Bleeding* | 428 (10) | 250 (11) | 1.33 (1.31 – 1.36) | <0.001 | 1.09 (0.96 – 1.25) | 0.18 |
| All-cause Hospitalization | 815 (19) | 523 (23) | 1.17 (1.04 – 1.31) | 0.008 | 1.08 (1.00 – 1.17) | 0.06 |
| Cardiovascular | 463 (10.9) | 306 (13.3) | 1.18 (1.01–1.37) | 0.03 | 1.08 (0.97–1.21) | 0.14 |
| Bleeding | 69 (1.6) | 60 (2.6) | 1.59 (1.12 – 2.26) | 0.009 | 1.52 (1.17 – 1.97) | 0.002 |
| Other | 366 (8.6) | 220 (9.6) | 1.09 (0.92 – 1.30) | 0.33 | 0.98 (0.87 – 1.11) | 0.72 |
| Death | 80 (1.9) | 59 (2.6) | 1.37 (0.98 – 1.93) | 0.07 | 1.26 (0.98 – 1.63) | 0.08 |
Raw rates presented as counts (%). Hazard ratios presented for OAC + ASA compared with OAC alone. Major bleeding defined by International Society on Thrombosis and Haemostasis criteria.
For nuisance bleeding odds ratios are presented, as dates of events are not included in the analysis.
OAC: oral anticoagulation; ASA: aspirin; HR: hazard ratio; CI: confidence interval.
For the subgroups of patients with prior MI or stroke/TIA, counts and rates of major adverse outcomes are shown in Table 5. Further statistical comparisons of these populations were not performed due to low power.
Table 5.
Outcomes in patients with prior atherosclerotic disease
| Patients with Prior MI | Patients with Prior Stroke/TIA | |||
|---|---|---|---|---|
| OAC Alone (n=418) |
OAC+ASA (n=529) |
OAC Alone (n=611) |
OAC+ASA (n=431) |
|
| Revascularization | 5 (1.20) | 5 (0.95) | 5 (0.82) | 5 (1.16) |
| MI | 3 (0.72) | 2 (0.38) | 2 (0.33) | 4 (0.93) |
| New-Onset/HF Diagnosis | 2 (0.48) | 3 (0.57) | 5 (0.82) | 6 (1.39) |
| Stroke or non-CNS embolism | 3 (0.72) | 7 (1.32) | 7 (1.15) | 1 (0.23) |
| TIA | 2 (0.48) | 1 (0.19) | 5 (0.82) | 1 (0.23) |
| Major Bleeding | 10 (2.39) | 18 (3.4) | 15 (2.45) | 14 (3.25) |
| Death | 16 (3.83) | 17 (3.21) | 14 (2.29) | 18 (4.18) |
Raw rates presented as counts (%).
MI: myocardial infarction; TIA: transient ischemic attack; OAC: oral anticoagulation; ASA: aspirin; CNS: central nervous system.
Discussion
Of 7,347 patients with AF taking OAC therapy, we found that 35% are also treated with aspirin. While those receiving concomitant aspirin therapy were more likely to have prior cardiovascular disease, more than one-third on concomitant aspirin did not have any history of atherosclerotic disease. Furthermore, patients on combined OAC and aspirin therapy had significantly higher adjusted rates of adverse clinical events, particularly bleeding events.
Recently, aspirin therapy for the primary prevention of cardiovascular events has been questioned as the risks appear to outweigh the benefits in several important populations.11 It is well-appreciated that antiplatelet therapy increases the risk of major bleeding in patients with or without oral anticoagulant therapy.11–15 Previous work has demonstrated significant use of dual-therapy with OAC and antiplatelet in patients without manifest cardiovascular disease in a single payer healthcare system, the Colorado Kaiser Permanente Healthcare System.16
Our analysis extends these findings to a broad, national, community-based patient population. Our study also points out the lack of clear logic or pattern to who is being treated with OAC+ASA versus OAC alone. For example, we observed no difference in ATRIA bleeding scores between those receiving OAC alone and those on OAC+ASA – approximately 25% of patients in both groups had elevated bleeding risk, with 17% in the highest risk category (≥5). Yet nearly 40% of patients in the OAC+ASA group lacked a convincing indication for aspirin (manifest atherosclerotic disease), while 37% of patients on OAC alone did have prior vascular disease. It is not clear that bleeding risk is being considered in the decision to use concomitant aspirin.
Use of OAC+ASA appears to persist despite increasing concerns regarding the true bleeding risk of aspirin, and its lack of incremental benefit in preventing stroke in patients with AF already on OAC.11 One meta-analysis comparing OAC versus OAC+ASA identified an overall benefit only in patients receiving OAC for mechanical valves;17 the surfeit of bleeding events is a consistent finding across studies.17, 18 Patients in our analysis receiving OAC+ASA had significantly higher rates of major bleeding events and higher rates of hospitalization for bleeding. These results persisted after multivariable adjustment of measured baseline characteristics of the OAC+ASA populations, and mirror those seen in a prior international study. A claims-based study from Denmark found that aspirin increased the risk for bleeding events nearly two-fold relative to those on warfarin alone.15 When combining previously-published data on ischemic outcomes with the current analysis demonstrating increased bleeding, the available evidence suggests that concomitant antiplatelet therapy is associated with increased risk and minimal evidence of benefit.15, 19
The use of antiplatelet therapy alone for secondary prevention in patients with a history of cardiovascular disease has been demonstrated to improve outcomes within an acceptable risk profile,12 and remains a practice recommendation from the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.20 However, its use in patients with AF who are also treated with OAC is less clear and is not supported by randomized trials. Patients with AF and CAD have an increased risk of adverse events, including mortality, when compared with patients with CAD without AF.3 Yet those on OAC have a low risk of MI when treated with an OAC in recent randomized study populations (ranging from 0.5% to 1.1%).21, 22 Furthermore, several trials in patients without AF have demonstrated equal or superior efficacy of OAC versus aspirin for the secondary prevention of ischemic events in patients with MI.23–25 Lastly, preliminary data from the only randomized trial of dual therapy (OAC+clopidogrel) versus therapy triple (OAC plus aspirin and clopidogrel) in patients undergoing PCI demonstrated a significant reduction in bleeding events in the dual therapy group.26
Limitations
These data represent observations from a prospective, national registry; thus, they are subject to the limitations inherent in such methods, including site participation, patient selection, and reporting biases. Furthermore, the defined treatment groups (OAC alone and OAC+ASA) were not randomly assigned, and there are important differences in ischemic and bleeding risk levels between these two populations. Regression analyses cannot completely adjust for such differences, and therefore comparisons of outcomes in these groups are likely limited by residual bias. Additionally, low rates of prior and incident ischemic events (stroke or MI) limit the ability to detect meaningful differences in these endpoints. Lastly, every attempt was made to capture aspirin therapy, regardless of prescription or over-the-counter use. Aspirin use was ascertained on follow-up and includes both prescription and over-the-counter use; however, these data could potentially be susceptible to recall bias.
Conclusions
Patients with AF prescribed OAC therapy are often also treated with concomitant aspirin, even when they do not have vascular disease. Furthermore, patients with AF and known cardiovascular disease are often treated with OAC alone. The use of OCA+ASA was independently associated with significantly increased risk for bleeding compared with use of OCA alone. The optimal antithrombotic strategy for patients with AF remains unclear. These and other data raise the possibility that a ‘less is more’ strategy may be favorable among AF patients on OAC.27 However, we believe adequately-powered, prospective clinical studies of these regimens are warranted to definitely assess the benefit or harm of such strategies. In the interim, clinicians need to carefully weigh whether the potential benefits of adding aspirin is worth the risk among patients with AF on OAC. In lieu of clinical trials, automated risk assessment tools that calculate ischemic risk and bleeding risk might help guide concomitant antiplatelet therapy.
Supplementary Material
Acknowledgments
Funding Sources: The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. Dr. Steinberg was funded by NIH T-32 training grant #5 T32 HL 7101-37.
Dr. Steinberg reports modest educational support from Medtronic. Dr. Lopes reports modest Research Support and Consulting from Bristol-Myers Squibb. Dr. Ansell reports modest Consultant/Advisory Board from Bristol Myers Squibb, Pfizer, Janssen, Daiichi, Boehringer Ingelheim, and Alere. Dr. Hylek reports: modest Speakers Bureau support form Boehringer-Ingelheim and Bayer; Modest Consultant/Advisory Board to Johnson & Johnson, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, and Ortho-McNeil-Janssen. Dr. Mahaffey reports significant Research Grant support from Johnson and Johnson and significant Consultant/Advisory Board to Johnson and Johnson. Dr. Singer reports: significant Research Grant support from Johnson and Johnson; modest Consultant/Advisory Board support from Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson and Johnson, and Pfizer; and significant Consultant/Advisory Board support from Daiichi Sankyo. Dr. Ezekowitz reports significant Speakers Bureau support from Boehringer Ingelheim; modest Consultant/Advisory Board support from Pozen Inc., Eisai, and Astra Zeneca; And significant Consultant/Advisory Board support from Boehringer Ingelheim, ARYx Therapeutics, Pfizer, Sanofi, Bristol Myers Squibb, PORTOLA, Daiichi Sanko, Medtronic, MERCK, Gilead, and Janssen Scientific Affairs. Dr. Fonarow reports modest Consultant/Advisory Board support from Ortho McNeil. Dr. Kowey reports modest Consultant/Advisory Board support from Boehringer Ingelheim, Bristol Myers Squibb, Johnson & Johnson, Portola, Merck, Sanofi, and Daiichi Sankyo. Dr. Chang reports significant Employment with Johnson & Johnson. Dr. Piccini reports: significant Research Grant support from Johnson & Johnson / Janssen Pharmaceuticals; significant Other Research Support from Bayer HealthCare Pharmaceuticals Inc. (formerly Berlex Labs), Boston Scientific Corporation, Johnson & Johnson Pharmaceutical Research & Development; modest Consultant/Advisory Board support from Forest Laboratories, Inc. and Medtronic, Inc.; and significant Consultant/Advisory Board support from Johnson & Johnson / Janssen Pharmaceuticals. Dr. Peterson reports: significant Research Grant support from Eli Lilly & Company, Janssen Pharmaceuticals, Inc., and the American Heart Association; modest Consultant/Advisory Board support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceuticals, Inc., Pfizer, and Genentech Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration Information: ClinicalTrials.gov. Identifier: NCT01165710
Conflict of Interest Disclosures: Ms. Kim, Dr. Go, and Dr. Thomas report no disclosures.
References
- 1.Lloyd-Jones DM. Lifetime risk for development of atrial fibrillation: The framingham heart study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Lopes RD, Pieper KS, Horton JR, Al-Khatib SM, Newby LK, Mehta RH, Van de Werf F, Armstrong PW, Mahaffey KW, Harrington RA, Ohman EM, White HD, Wallentin L, Granger CB. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without st-segment elevation. Heart. 2008;94:867–873. doi: 10.1136/hrt.2007.134486. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the european society of cardiology (esc) Euro Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 5.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Tarkington LG, Yancy CW. 2011 accf/aha/hrs focused updates incorporated into the acc/aha/esc 2006 guidelines for the management of patients with atrial fibrillation: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 6.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: Rationale and design of orbit-af. Am Heart J. 2011;162:606–612. e601. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. Jama. 2001;285:2864-2864. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 9.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin-associated hemorrhage: The atria (anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 11.Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: An update of the evidence for the u.S. Preventive services task force. Ann Intern Med. 2009;150:405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]
- 12.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Berardis G, Lucisano G, D'Ettorre A, Pellegrini F, Lepore V, Tognoni G, Nicolucci A. Association of aspirin use with major bleeding in patients with and without diabetes. Jama. 2012;307:2286–2294. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
- 14.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: A summary of the evidence for the u.S. Preventive services task force. Ann Intern Med. 2002;136:161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 15.Hansen ML, Sorensen R, Clausen MT, Fog-Petersen ML, Raunso J, Gadsboll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrom SZ, Poulsen HE, Kober L, Torp-Pedersen C. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–1441. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SG, Witt DM, Eddy TR, Delate T. Warfarin and antiplatelet combination use among commercially insured patients enrolled in an anticoagulation management service. Chest. 2007;131:1500–1507. doi: 10.1378/chest.06-2374. [DOI] [PubMed] [Google Scholar]
- 17.Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: A meta-analysis of randomized trials. Arch Intern Med. 2007;167:117–124. doi: 10.1001/archinte.167.2.117. [DOI] [PubMed] [Google Scholar]
- 18.Flaker GC, Gruber M, Connolly SJ, Goldman S, Chaparro S, Vahanian A, Halinen MO, Horrow J, Halperin JL. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: An exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (sportif) trials. Am Heart J. 2006;152:967–973. doi: 10.1016/j.ahj.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Johnson SG, Rogers K, Delate T, Witt DM. Outcomes associated with combined antiplatelet and anticoagulant therapy. Chest. 2008;133:948–954. doi: 10.1378/chest.07-2627. [DOI] [PubMed] [Google Scholar]
- 20.Fraker TD, Jr, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Gardin JM, O'Rourke RA, Williams SV, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 chronic angina focused update of the acc/aha 2002 guidelines for the management of patients with chronic stable angina: A report of the american college of cardiology/american heart association task force on practice guidelines writing group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. Circulation. 2007;116:2762–2772. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 23.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–974. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 24.van Es RF, Jonker JJ, Verheugt FW, Deckers JW, Grobbee DE. Aspirin and coumadin after acute coronary syndromes (the aspect-2 study): A randomised controlled trial. Lancet. 2002;360:109–113. doi: 10.1016/S0140-6736(02)09409-6. [DOI] [PubMed] [Google Scholar]
- 25.Andreotti F, Testa L, Biondi-Zoccai GG, Crea F. Aspirin plus warfarin compared to aspirin alone after acute coronary syndromes: An updated and comprehensive meta-analysis of 25,307 patients. Eur Heart J. 2006;27:519–526. doi: 10.1093/eurheartj/ehi485. [DOI] [PubMed] [Google Scholar]
- 26.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van 't Hof AW, Ten Berg JM. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013 doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 27.Douketis JD. Combination warfarin-asa therapy: Which patients should receive it, which patients should not, why? Thrombosis Research. 2011;127:513–517. doi: 10.1016/j.thromres.2011.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.