Summary
In the article we present pathological intracranial substances and lesions, which produce high signal intensity on T1-weighted MR images. Six groups of substances are discussed: 1. Gadolinium – based contrast agents, 2.hemoglobin degradation products (intra- and extra-cellular methemoglobin), 3. lipid-containing lesions (lipoma, dermoid cyst, implanted fatty materials, laminar cortical necrosis), 4. substances with high concentration of proteins (colloid cyst, craniopharyngioma, Rathke’s cleft cyst, ectopic posterior pituitary gland), 5. melanin (metastatic melanoma), 6. lesions containing mineral substances such as: calcium (calcifications, Fahr’s disease), copper (Wilson’s disease) and manganese (hepatic encephalopathy, manganese intoxication in intravenous drug abusers). Appropriate interpretation of signal intensity as well as analysis of location of lesions and clinical symptoms enables planning of further diagnostics and, in many cases, establishing the final diagnosis based on MR examination.
Keywords: hyperintense, MRI, T1-weighted pictures
Background
Magnetic resonance (MR) is the method of choice for the diagnosis of intracranial pathologies due to the possibility of imaging tissues with high resolution. T1-weighted pictures acquired with short repetition time (TR ≤500 ms) and short echo time (TE ≤30 ms) are basic MR sequences. On T1-weighted images substances with longitudinal relaxation time, e.g. water demonstrate low signal (are hypointense), while substances with short longitudinal relaxation time (e.g. fat) display high signal (are hyperintense) [1]. Typically white matter is brighter than grey matter on T1-weighted images, while cerebrospinal fluid is dark.
Most substances and intracranial pathologies are hypointense on T1-weighted images. In this article we present a review of substances and pathological lesions exhibiting high signal on T1-weighted images. In many cases knowledge of MR signal characteristics of various substances together with analysis of anatomical location of those lesions may significantly narrow down the differential diagnosis of assessed pathologies. In this publication we will discuss substances that are hyperintense on T1-weighted images including: 1. gadolinium contrast agents, 2. hemoglobin degradation products, 3. fats (lipids, cholesterol), 4. high-protein substances, 5. melanin and 6. minerals (e.g. calcium, manganese, iron, copper).
Contrast Agents
Contrast agents are paramagnetic substances containing unpaired electron magnetic moments that shorten the T1 and T2 relaxation time of surrounding protons. Gadolinium containing paramagnetics are most commonly used in clinical practice (Gd3+ ions contain seven unpaired electrons) [1]. Contrast enhancement is obtained in two main mechanisms: due to increased vascularization within pathological lesion and/or due to diffusion of contrast through the damaged blood-brain barrier. MR examination with contrast is performed for precise imaging of tumors, inflammatory lesions, ischemic changes or demyelination. A standard dose is 1 mmol/kg of body mass. Improved detection may be achieved by increasing contrast concentration 2- or 3-fold or lengthening the time between contrast administration and data acquisition (Figure 1), which is particularly useful for imaging of metastatic and demyelination lesions [2]. It should be emphasized that currently using increased doses of contrast should be avoided in order to prevent development of nephrogenic systemic fibrosis [3].
Figure 1.
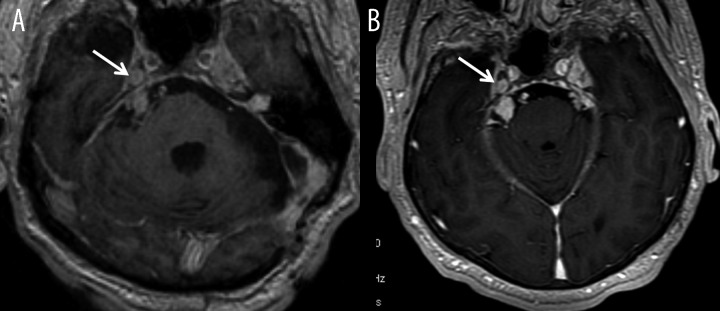
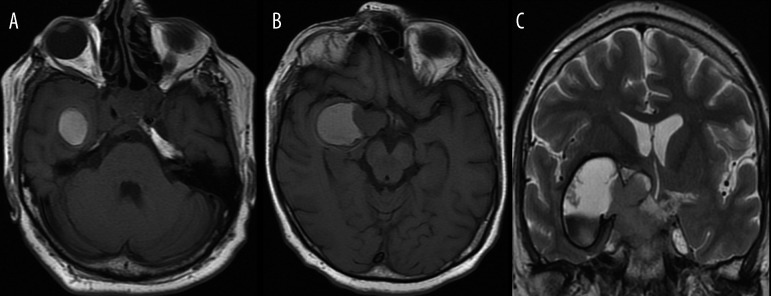
Patient with type 2 neurofibromatosis, (A, B) – axial T1-weighted images after contrast administration. Image (A) was acquired few minutes after intravenous contrast injection while image (B) 10 minutes after injection of double dose of contrast agent. Image (B) shows significant improvement in detection of nerve V neuromas within the right cerebellopontine angle and Meckel’s cave (arrows).
Hemoglobin Degradation Products
On T1-weighted images blood exhibits high signal only in the methemoglobin phase, which possesses the strongest paramagnetic properties, since it contains a Fe3+ ion with five unpaired electrons on electron shells. Methemoglobin appears around the 3rd day of the early subacute phase of bleeding and initially, when the integrity of erythrocytes remains intact, leading to shortening of T1- and T2-weighted relaxation times. It is therefore hyperintense on T1-weighted images and hypointense in T2-weighted images (Figure 2). About the 7th day, in the late subacute phase of hemorrhage, erythrocytes undergo decay and extracellular methemoglobin appears, which shortens the T1 and elongates the T2 relaxation time – thus, it is hyperintense on both T1- as well as T2-weighted images (Figure 2). After about 2 weeks methemoglobin becomes oxidized to hemosiderin, causing gradual signal hypointensity on T1- and T2-weighted images [4]. The described processes of hemoglobin degradation may be observed both in intracerebral and extracerebral (extra- and subdural) bleeding (Figure 3), intraventricular hemorrhages as well as bleeding into tumors or vascular malformations. In case of intracerebral hemorrhage, the process of methemoglobin transformation does not take place simultaneously within the entire hemorrhagic focus, but progresses gradually from periphery into the center of a lesion. In intratumoral hemorrhage hemoglobin degradation time may be longer than in intracerebral hemorrhage and the products of its degradation may appear later. Primary tumors most frequently presenting with bleeding include glioblastoma multiforme, anaplastic glioma, pituitary macroadenoma of the prolactinoma type and secondary tumors include metastases from bronchial cancer, clear cell carcinoma, thyroid cancer and choriocarcinoma [5,6].
Figure 2.
Solid/cystic pituitary macroadenoma of prolactinoma type with hemorrhage during therapy with bromocriptine.
(A, B) – axial unenhanced T1-weighted images show high signal corresponding to methemoglobin.
(C) – coronal T2-weighted image allows for differentiation of methemoglobin types. The lower part of the tumor contains hypointense intracellular methemoglobin and the upper part of a lesion contains hyperintense extracellular methemoglobin.
Figure 3.
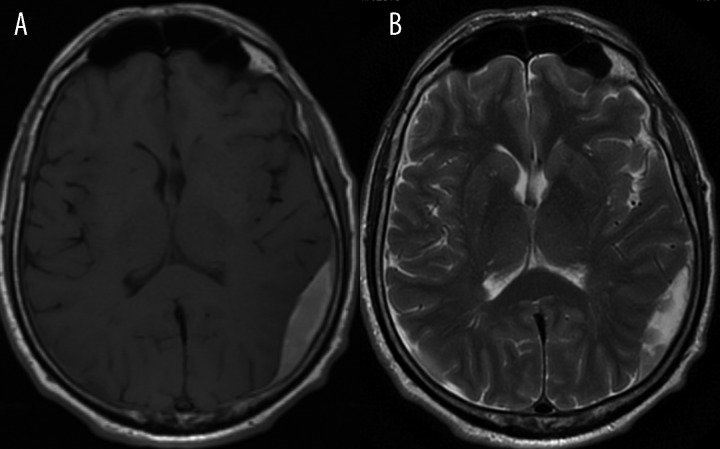
Left parietal epidural hematoma, (A) – T1-weighted image, (B) – T2-weighted image. Hematoma shows high signal on both images, which is consistent with extracellular methemoglobin.
One should remember that processes of hemoglobin degradation and metabolism also take place within thrombi located in cerebral vessels. Intravascular thrombi containing methemoglobin are hyperintense in T1-weighted images as in hematomas (Figure 4) [7]. Therefore, T1-weighted images may be used to localize thrombi within venous vessels, particularly of small caliber, which are difficult to image with other methods such as angio-MR or angio-CT (Figure 4D).
Figure 4.
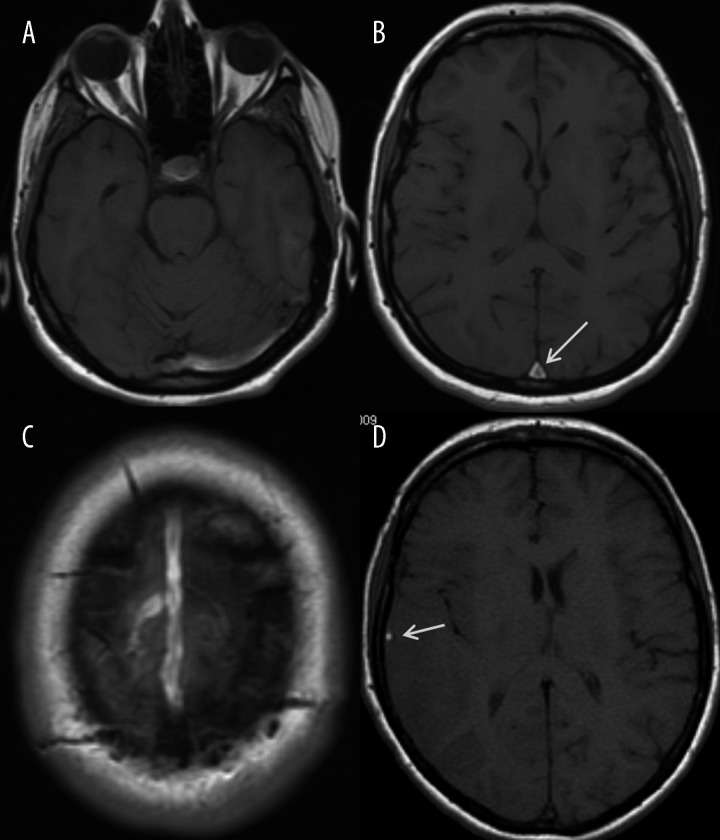
Cerebral venous thrombosis, axial T1-weighted images. (A) – left sigmoid sinus thrombosis, (B) – superior sagittal sinus thrombosis in the inferior-posterior portion (arrow), (C) – superior sagittal sinus thrombosis at the convexity with a thrombosed draining cortical vein, (D) – thrombosis of the right vein of Labbe (arrow).
Fatty Substances
High signal intensity of fat-containing substances ensues from their triglyceride content, which shortens the T1 relaxation time. Additional fat saturation sequence (T1 FatSat) makes it possible to confirm the presence of fat in the lesion - signal from fat tissue becomes attenuated [8].
Pathological lesions hyperintense on T1-weighted images due to increased fat content include: lipomas, dermoid cyst and other lipid-containing tumors (mainly teratomas and fat-rich subtypes of meningiomas or ependymomas), iatrogenically inserted filling material (most frequently used during transsphenoidal surgery of the pituitary gland) and changes in the course of cortical laminar necrosis.
Intracranial lipomas are congenital lesions. They are formed as a result of improper differentiation of primary meninx as it develops into the subarachnoid space. They are rare, comprising less than 0.1% of intracranial tumors, and asymptomatic [5,6]. Lipomas are most frequently located in the midline of the brain or nearby (80–95%) (Figure 5), particularly in the interhemispheric fissure or near corpus callosum (25–50%) [9]. They often coexist with total or partial agenesis of corpus callosum. Lipomas are well demarcated lesions, hyperintense on T1-weighted images and somewhat less hyperintense on T2-weighted images that do not enhance following administration of contrast medium (Figure 6A). In CT they exhibit low density (ranging between −150 and 0 HU) typical for fat [5,6,8].
Figure 5.
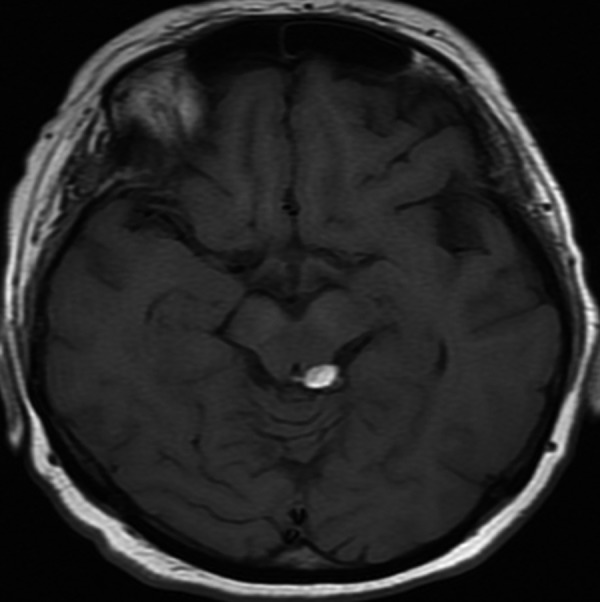
Intracranial lipoma. Axial T1-weighted MR-image shows small hyperintense lipoma located near the midline in the quadrigeminal cistern on the left side.
Figure 6.
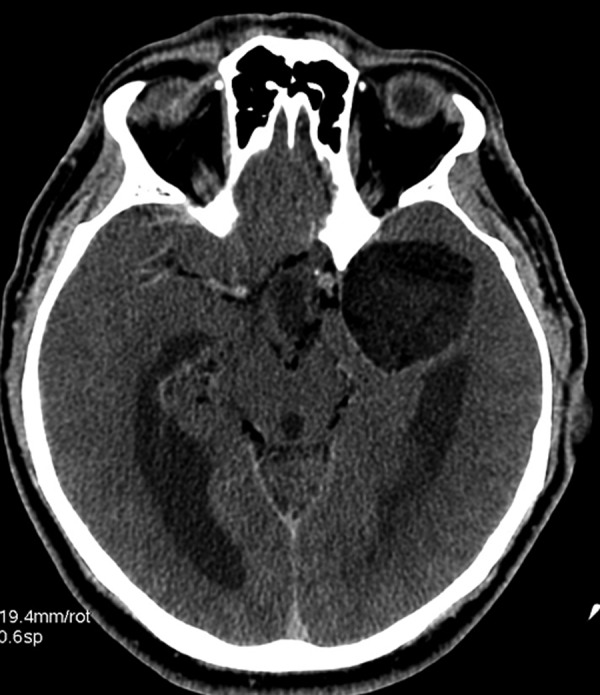
Ruptured dermoid cyst, axial CT image. Low density parasellar dermoid cyst in the middle cranial fossa on the left side. Numerous hypodense foci of fat within interpeduncular cisterns and along the right middle cerebral artery indicate rupture of a cyst into the subarachnoid space.
Dermoid cyst is a rare congenital lesion (comprising less than 1% of intracranial lesions), which develops as a result of incorporating ectodermal cells into neural tube during embryogenesis. Dermoid cyst is lined with stratified epithelium and filled with fatty substances, cholesterol, exfoliated epithelial cells and keratin, which affects its high signal on T1-weighted images [5,6,8]. Moreover, it may contain fully developed skin appendages such as: hair follicles, sebaceous and sweat glands, giving it a heterogeneous signal on T2-weighted images. Dermoid cyst is located adjacent to the midline, most frequently in the posterior cranial cavity (4th ventricle, cisterna magna) and in the supra/perisellar region. Dermoid cyst does not exhibit diffusion restriction in DWI and does not undergo enhancement following contrast administration. Dermoid cysts may rupture spontaneously, after trauma or during surgery and empty their contents into subarachnoid space or ventricular system, causing chemical meningitis [10]. In such cases T1-weighted images show numerous, minute hyperintense foci (fat drops) and fat-fluid levels in the subarachnoid space and ventricular system. Similar to lipomas dermoid cysts exhibit low density (−150 to 0 HU) in CT [5,6,8] (Figure 6).
Fatty substances may be also introduced into the intracranial space iatrogenically, e.g. as a filling material into the cavernous sinus after transsphenoidal resection of pituitary tumors (Figure 7). Such materials are used to fill the empty space after excised tissue at the bottom of sella turcica and prevent cerebrospinal fluid leakage. It should be emphasized that fragments of implanted fat tissue may be identified as long as 10 years after the procedure [11].
Figure 7.
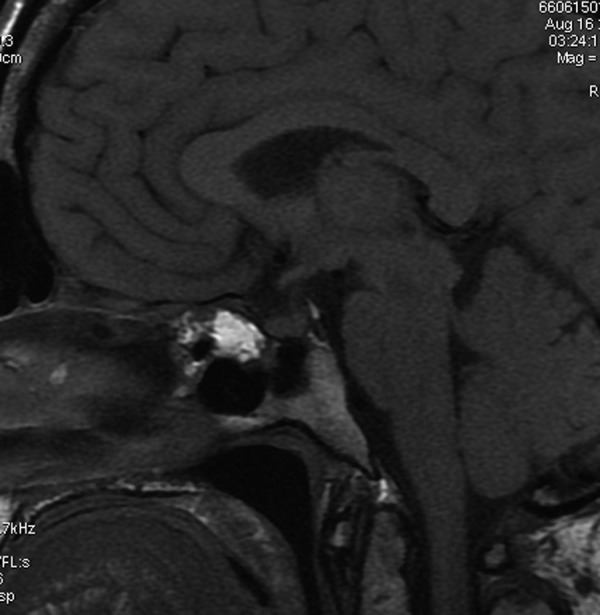
Lipid-containing filling material in the sphenoid sinus. Sagittal T1-weighted image shows iatrogenic hyperintense lipid-containing filling material in the sphenoid sinus in a patient after transsphenoidal resection of a pituitary tumor.
Another lesion hyperintense on T1-weighted images is cortical laminar necrosis. Predisposing factors include general hypoxia and hypoglycemia, which may occur in an ischemic stroke, anoxia, status epilepticus or in the course of treatment with immunosupressants or cytostatics [12,13]. In cortical laminar necrosis cortical hyperintensity appears on T1-weighted images around the second week following the hypoxic-ischemic event and persists for 1–2 months, in some cases as long as a few years [5,6,8]. Neural cell necrosis leads to protein denaturation, glial reaction and accumulation of fat-loaded macrophages, leading to shortening of T1 time. The third cortical layer is most sensitive to ischemia [8]. Hyperintense, linear bands within the cortex are best visible in the sulci and lateral parts of gyri (Figure 8). It should be emphasized that in some cases picture of hyperintense cortical bands may appear within 2–3 days from the hypoxic-ischemic event. High cortical signal is related to appearance of petechiae and methemoglobin (hemorrhagic cortical necrosis), while the processes associated with cortical laminar necrosis take place later, around the second week (Figure 9).
Figure 8.
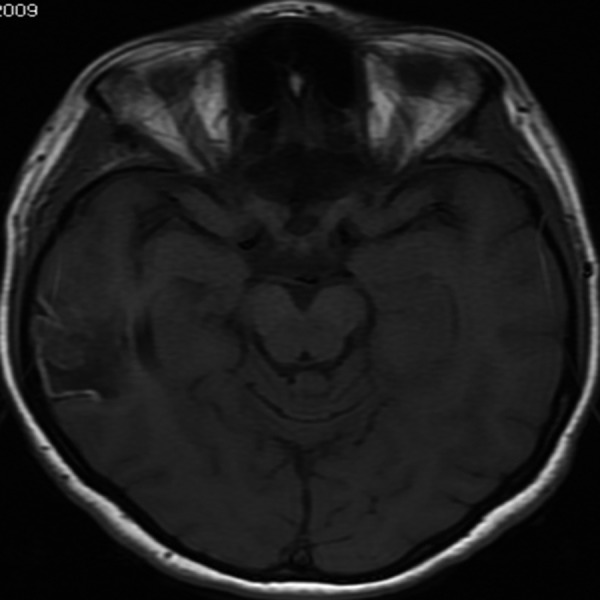
Cortical laminar necrosis. Axial T1-weighted image, transverse section, demonstrates segmental necrosis of cerebral cortex visible as linear bands of high signal intensity in the right temporal cortex at the periphery of a chronic ischemic lesion.
Figure 9.
Hemorrhagic necrosis of the cortex and basal ganglia, axial T1-weighted images. Hyperintense basal ganglia (A) and cortex along both central sulci (B) consistent with necrosis with petechial hemorrhage in a patient 3 days after cardiopulmonary resuscitation following cardiac arrest.
Protein-rich Substances
Intracranial lesions characterized by high signal on T1-weighted images due to their protein-rich content include: colloid cyst, some craniopharyngiomas, Rathke’s cleft cyst or ectopic posterior pituitary lobe.
Colloid cyst develops from the inferior part of septum pellucidum and protrudes into the anterior part of third ventricle. About 99% of this type of lesions are located in the anteriosuperior part of the third ventricle, near the foramina of Monro, which may lead to their closing and development of obturative hydrocephalus (Figure 10). The most common clinical symptoms accompanying a colloid cyst include: headache, nausea, vomiting, diplopia [14]. Colloid cyst is a well demarcated, spherical or oval lesion that often demonstrates high signal on T1-weighted images and low signal on T2-weighted images (about 60% of cases), which is related to the presence of proteins and mucus within its lumen. In the remaining cases signal intensity may vary depending on the content of the cyst. Following contrast administration a slight marginal enhancement of the fibrous capsule may be observed.
Figure 10.
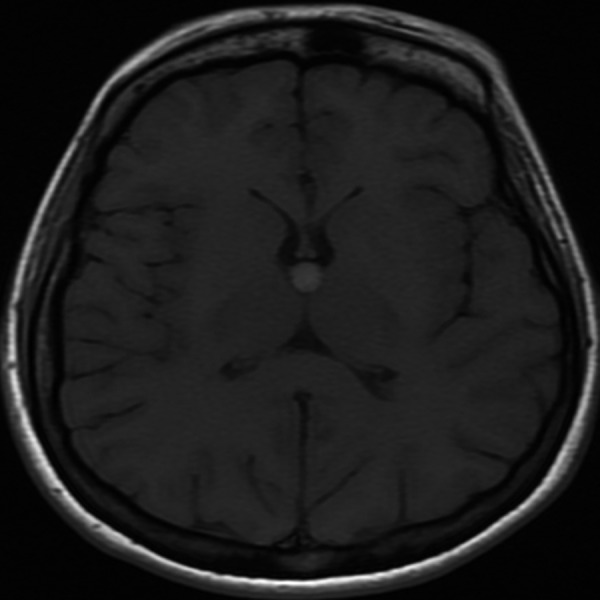
Colloid cyst. Axial T1-weighted image shows an ovoid hyperintense lesion in the typical location near foramina of Monro diagnostic of a colloid cyst.
Craniopharyngioma is a histologically benign (WHO grade 1), slow-growing tumor located in the perisellar region (about 75%), sellar-suprasellar (about 21%) or intrasellar (about 4%) region. It constitutes about 1–5% of primary brain tumors and is the most common suprasellar tumor in children. It develops from the remnants of epithelium in the anterior part of Rathke’s pouch. The most common symptoms associated with this tumor include headache, visual disturbances and hormonal disorders ensuing from hypothalamic dysfunction [5,6,8]. There are two types of craniopharyngiomas: adamantinomatous – more common in children, characterized by cystic or solid-cystic structure with protein-rich component, often containing calcifications, and papillary – more frequent in adults, more often solid, rarely containing calcifications [15]. Depending on their content, craniopharyngiomas may exhibit high, low or medium signal both on T1- and T2-weighted images. High signal intensity on T1-weighted images is typical for the adamantinomatous type and ensues from high protein content, presence of cholesterol crystals or hemorrhage (Figure 11). The solid part of the tumor and cystic capsule become heterogeneously enhanced following contrast administration [5,6,8,15].
Figure 11.
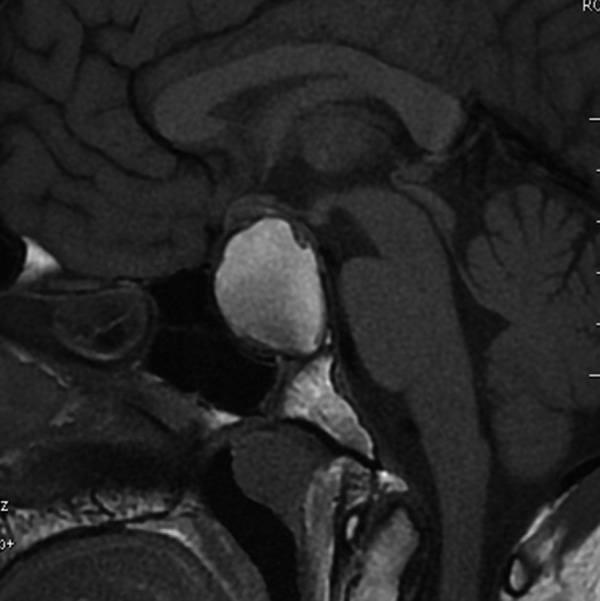
A 21-year-old patient with a solid/cystic craniopharyngioma, located in the sellar-suprasellar region. Sagittal T1-weighted image shows high signal intensity of the cystic portion of the tumor as well as a significant enlargement of sella turcica and compression of the optic chiasm.
Rathke’s cleft cyst is a benign cystic lesion, which develops from the remnants of the anterior part of Rathke’s pouch. Due to a small size it often does not give any clinical symptoms. Very rarely it is the cause of headaches, visual disturbances or diabetes insipidus. It is most often intrasellar (about 50%), rarely suprasellar (about 25%) or in both of those locations (about 25%). Intrasellar cyst is located in the middle part of pituitary gland (between anterior and posterior lobe) [5,6,8]. It may contain various amounts of proteins, mucopolysaccharides, cholesterol and fragments of exfoliated cells. MR image of Rathke’s cleft cyst depends largely on its content [16]. Serous cysts are hypointense on T1-weighted and hyperintense on T2-weighted images, while mucous cysts are hyperintense on T1-weighted images and typically exhibit very low signal on T2-weighted images (Figure 12). Rathke’s cleft cyst usually does not enhance with contrast of contrast [5,6,8,16].
Figure 12.
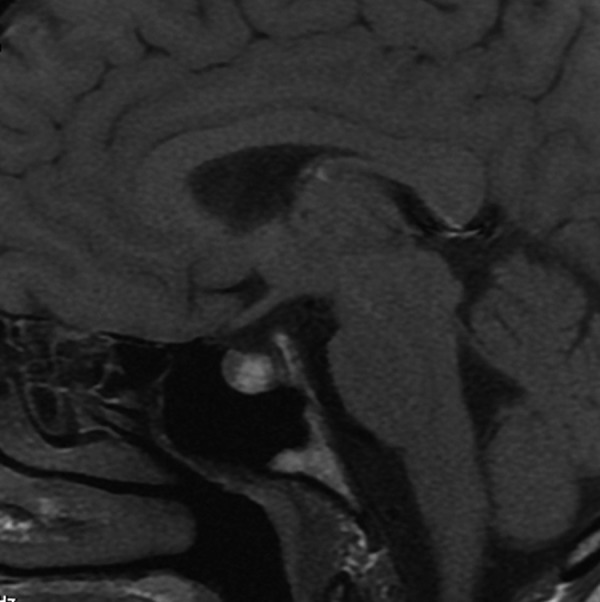
Rathke’s cleft cyst. Sagittal T1-weighted image demonstrates a hyperintense intrasellar cyst located between anterior and posterior pituitary lobes.
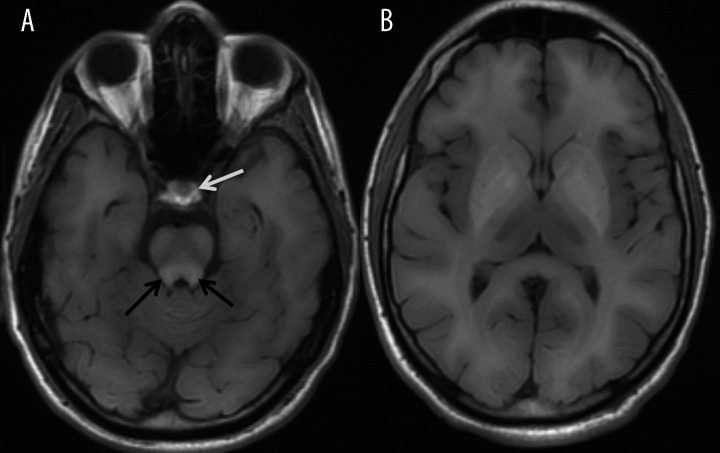
Ectopic posterior pituitary lobe is a rare congenital malformation associated with hypoplasia or aplasia of infundibulum. It may be also acquired as a result of traumatic or surgical injury to pituitary infundibulum. Ectopic posterior pituitary is most often located within the hypothalamus, on the floor of 3rd ventricle (Figure 13). Physiologically, posterior lobe of the pituitary contains a composition of vasopressin, neurophysin II and copeptin [8], which possess paramagnetic properties and induce high signal within the posterior lobe on T1-weighted images in 52–100% of studied population [17], although it should be emphasized that it is not visible in all healthy people. Posterior lobe hyperintensity on T1-weighted non-contrast MR images enables identification of ectopic pituitary.
Figure 13.
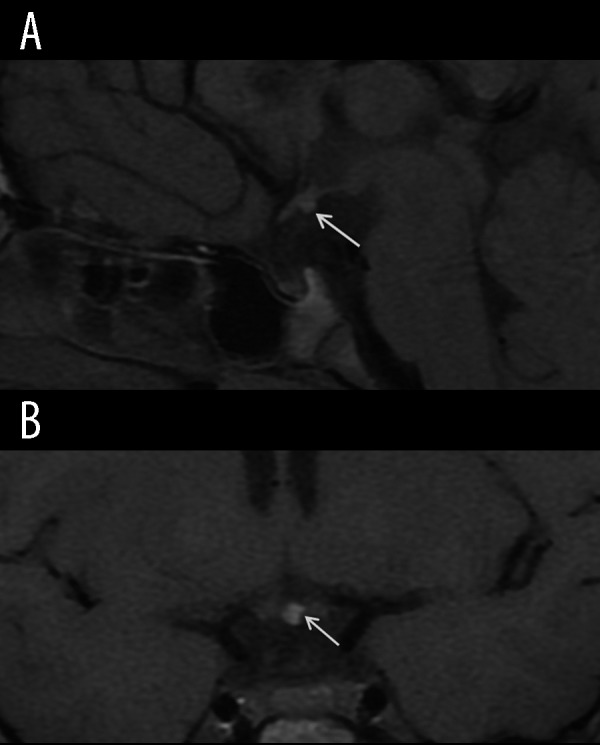
Ectopic posterior pituitary lobe. Sagittal (A) and coronal (B) T1-weighted images show hyperintense posterior pituitary lobe in the ectopic location within hypothalamus (arrows).
Melanin
Typically, melanin exhibits high signal on T1-weighted images and low signal on T2-weighted images, which is related to its paramagnetic properties.
Intracranially, melanin is most often identified in metastatic melanoma. Metastases to the head may be located within any intra- or extracranial structure (Figure 14) [18]. High signal of melanoma metastases on T1-weighted images may result not only from the presence of melanin, but also bleeding (methemoglobin phase), which often coexists with such lesions. Metastases of amelanotic melanoma do not shorten the T1-relaxation time and remain hypointense on T1-weighted images and hyperintense on T2-weighted images just like other brain tumors [5,6,18].
Figure 14.
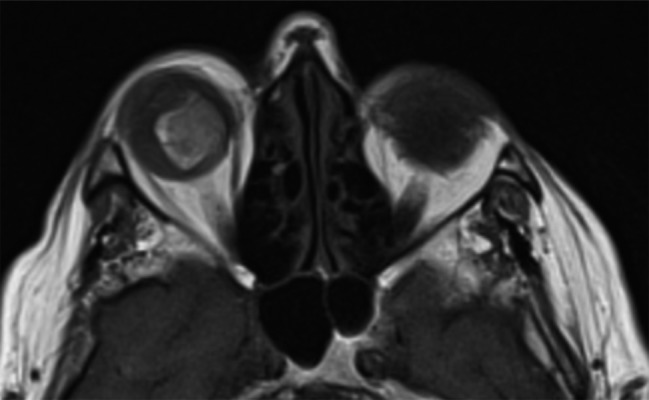
Metastatic melanoma to the right eyeball, axial unenhanced T1-weighted image.
Rarely, intracranial presense of melanin is associated with such pathologies as primary diffuse meningeal melanomatosis (aggressive form of primary intracranial melanoma that involves mainly the pia matter) or neurocutaneous melanosis (congenital disorder that involves coexistence of lesions containing melanin within pia matter and multiple giant skin lesions) [8].
Minerals
Mineral substances that may give high signal on T1-weighted images include calcium, copper, iron and manganese.
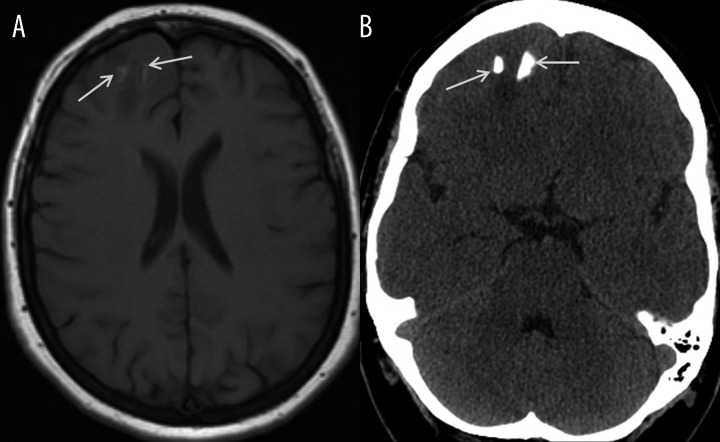
Calcifications usually have low signal in all MR sequences and are easily detected with CT examination. Calcifications may exhibit high signal on T1-weighted images if they contain diamagnetic calcium salts that, together with paramagnetic cations such as ferrum and manganese, cause shortening of T1 relaxation time [5,6,8]. Physiological calcifications most frequently occur in falx cerebri, pineal gland, choroid plexus, while pathological calcifications are characteristic for some tumors, e.g. meningiomas, oligodendrogliomas. Hyperintense calcifications within the tumors are difficult to distinguish from foci of intratumoral hemorrhages, which also give high signal due to the presence of methemoglobin. Presence of calcifications may be easily confirmed in CT examination (Figure 15). Excessive intracerebral calcium deposition may accompany endocrine disorders such as: hypo- or hyperparathyroidism, hypothyroidism or inflammatory processes such as toxoplasmosis, cytomegaly or rubella infections [5,6,8].
Figure 15.
Calcifications within oligodendroglioma. Unenhanced T1-wieghted MR image (A) demonstrates hyperintense foci within the tumor in the right frontal area (arrows) requiring differentiation between hemorrhage and calcifications. Unenhanced CT image (B) confirms presence of calcifications (arrows).
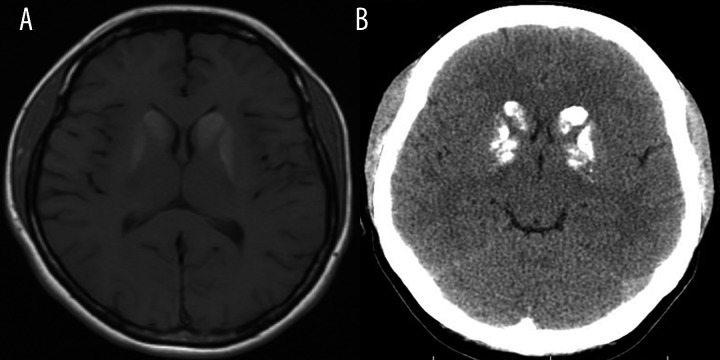
Fahr’s syndrome, also known as bilateral striato-pallido-dentate calcinosis, is an example of pathology associated with excessive cerebral calcium deposition. It is a rare neurodegenerative disorder characterized by bilateral, symmetrical deposition of calcium (and other mineral compounds such as zinc, aluminum, ferrum and magnesium) in the basal ganglia, thalamus, dentate nuclei of the cerebellum and in the semioval center [6] in the absence of disturbances in calcium-phosphorus metabolism [19]. The most common clinical symptoms of the disease include cognitive impairment (subcortical dementia), extrapyramidal movement disorders and schizophreniform psychosis (in young adults). Bilateral calcifications visible in MR examination in Fahr’s disease are usually hyperintense on T1-weighted images (“dense” calcifications) and hypo- or hyperintense on T2-weighted images (Figure 16) [5,6,8,19].
Figure 16.
Fahr’s disease. Unenhanced T1-weighted image (A) reveals high signal intensity of the heads of both caudate nuclei and putamina. Unenhanced CT image (B) confirms presence of calcification in the region of basal ganglia.
Another example of a pathology related to excessive deposition of minerals is Wilson’s disease (hepatolenticular degeneration). In this disease, inherited in an autosomal recessive manner, body metabolism of copper is disturbed and it is deposited in various organs, i.a. in the liver, brain, heart and cornea. An orange-brown Kayser-Fleischer corneal ring is a typical sign of Wilson’s disease. Neurological symptoms occur in about 40% of patients – speech disturbances, tremors, dystonias, impaired motor and gait coordination [20]. Typical signs of Wilson’s disease in MR imaging includes symmetrical T1-hypointensity and T2-hyperintensity within the putamen, globus pallidus and thalamus, rarely within cerebellum (vermis, dentate nucleus), mesencephalon, pons, cortex and subcortical regions. Signal increase on T1-weighted images occurs rarely, mainly in the globus pallidus and thalamus (Figure 17). Cortical signal changes in the course of Wilson’s disease may disappear after treatment [5,6,8].
Figure 17.
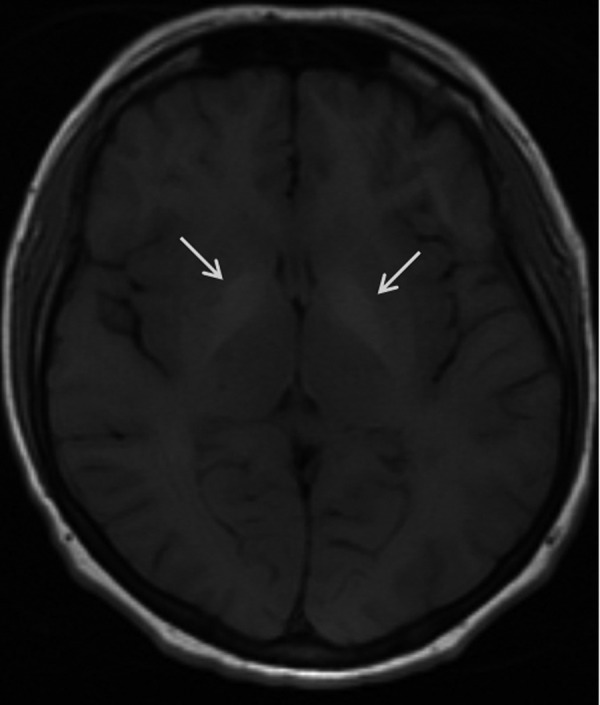
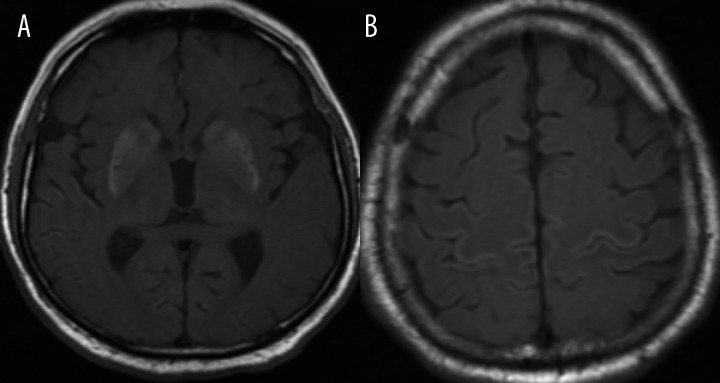
Wilson’s disease. Axial T1-weighted image shows bilateral regions of increased signal intensity within globi pallidi (arrows) due to pathological copper accumulation.
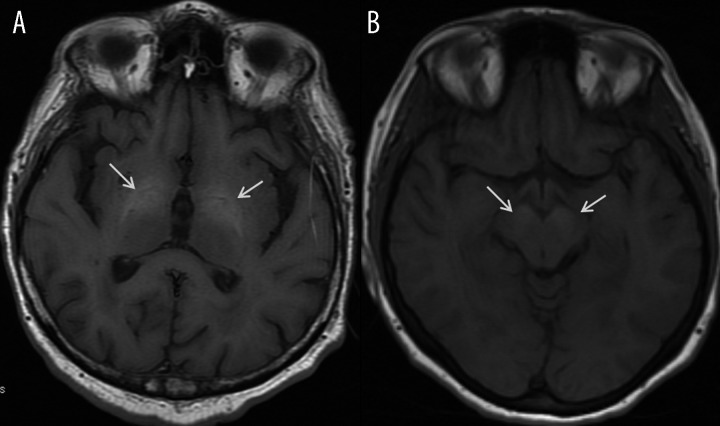
Patients with hepatic encephalopathy present with radiological picture similar to that of Wilson’s disease. It is a neuropsychiatric syndrome, potentially reversible, which accompanies acute or chronic liver failure and is caused by excessive accumulation of neurotoxins such as ammonia, manganese, bilirubin and aromatic amino acids in the brain [5,6,8]. Manganese is particularly toxic, as it is deposited in the globus pallidus and substantia nigra, leading to development of Parkinson’s syndrome. Manganese is also responsible for symmetrical increase of signal in the basal ganglia (globus pallidus in particular), mesencephalon (Figure 18), hypothalamus and anterior pituitary on T1-weighted images [5,6,8,21,22]. After liver transplantation the cerebral T1-hyperintensity may return to normal [23].
Figure 18.
Hepatic encephalopathy in a 66-years-old man. Axial T1-weighted images show bilateral symmetrical regions of hyperintensity within globi pallidi (arrows) (A) and substantia nigra in the midbrain (arrows) (B).
Toxic accumulation of manganese, which causes characteristic increase in signal in the basal ganglia, mesencephalon and anterior pituitary on T1-weighted images was also described in people exposed to excessive amounts of manganese (e.g. welders), as well as in drug addicts using ephedrine solution contaminated with manganese (Figure 19) [25].
Figure 19.
Manganese intoxication in a 32-year-old intravenous drug abuser. Axial T1-weighted images reveal diffuse brain injury due to abnormal manganese accumulation after 15 years of addiction. Significantly increased signal can be noted within the anterior lobe of the pituitary gland (white arrow), superior cerebellar peduncles (black arrows) (A) as well as basal ganglia and hemispheric white matter (B).
Conclusions
Hyperintense cerebral changes on T1-weighted images are formed due to accumulation of substances characterized by short longitudinal relaxation time including: gadolinium contrast, intra- and extracellular methemoglobin, melanin, fatty and protein-rich substances and minerals, i.a. calcium, copper and manganese. Knowledge of localization and morphology of pathologies that cause characteristic T1-hyperintensity allows for narrowing the differential diagnosis and in many cases establishing the final diagnosis based solely on MR examination.
References
- 1.Sklinda K. Œrodki cieniujące i kontrastowe stosowane w neuroradiologii. In: Walecki J, editor. Diagnostyka obrazowa Układ nerwowy ośrodkowy. Wydawnictwo Lekarskie PZWL; Warszawa: 2013. pp. 35–42. [in Polish] [Google Scholar]
- 2.Simona JH, Lib D, Traboulseec A, et al. Standardized MR Imaging Protocol for Multiple Sclerosis: Consortium of MS Centers Consensus Guidelines. Am J Neuroradiol. 2006;27:455–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Thomsen HS, Morcos SK, Almen T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23:307–18. doi: 10.1007/s00330-012-2597-9. [DOI] [PubMed] [Google Scholar]
- 4.Bradley WG., Jr MR appearance of hemorrhage in the brain. Radiology. 1993;189(1):15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 5.Warakaulle DR, Anslow P. Differential Diagnosis of Intracranial Lesions with High Signal on T1 or Low Signal on T2-weighted MRI. Clin Radiol. 2003;58(12):922–33. doi: 10.1016/s0009-9260(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 6.Cakirer S, Karaarslan E, Arslan A. Spontaneously T1-hyperintense lesions of the brain on MRI: a pictorial review. Curr Probl Diagn Radiol. 2003;32(5):194–217. doi: 10.1016/s0363-0188(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 7.Leach JL, Fortuna RB, Jones BV, et al. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radio Graphics. 2006;26:19–43. doi: 10.1148/rg.26si055174. [DOI] [PubMed] [Google Scholar]
- 8.Ginat DT, Meyers SP. Intracranial Lesions with High Signal Intensity on T1-weighted MR Images: Differential Diagnosis. Radio Graphics. 2012;32:499–516. doi: 10.1148/rg.322105761. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz H, Hakyemez B, Koroglu M, et al. Intracranial lipomas: importance of localization. Neuroradiology. 2006;48(1):1–7. doi: 10.1007/s00234-005-0001-z. [DOI] [PubMed] [Google Scholar]
- 10.Liu JK, Gottfried ON, Salzman KL, et al. Ruptured intracranial dermoid cysts: clinical, radiographic and surgical features. Neurosurgery. 2008;62(2):377–84. doi: 10.1227/01.neu.0000316004.88517.29. [DOI] [PubMed] [Google Scholar]
- 11.Bladowska J, Bednarek-Tupikowska G, Sokolska V, et al. MRI image characteristics of materials implanted at sellar region after transsphenoidal resection of pituitary tumours. Pol J Radiol. 2010;75(2):46–54. [PMC free article] [PubMed] [Google Scholar]
- 12.Siskas N, Lefkopoulos A, Ioannidis I, et al. Cortical laminar necrosis in brain infarcts: serial MRI. Neuroradiology. 2003;45:283–88. doi: 10.1007/s00234-002-0887-7. [DOI] [PubMed] [Google Scholar]
- 13.Bargallo N, Burrel M, Berengeuer J, et al. Cortical laminar necrosis caused by immunosuppressive therapy and chemiotherapy. Am J Neuroradiol. 2000;21(3):479–84. [PMC free article] [PubMed] [Google Scholar]
- 14.Armao D, Castillo M, Chen H, et al. Colloid cyst of the third ventricle: imaging-pathologic correlation. Am J Neuroradiol. 2000;21(8):1470–77. [PMC free article] [PubMed] [Google Scholar]
- 15.Sartoretti-Schefer S, Wichmann W, Aguzzi A, et al. MR Differentiation of Adamantinous and Squamous-Papillary Craniopharyngiomas. Am J Neuroradiol. 1997;18:77–87. [PMC free article] [PubMed] [Google Scholar]
- 16.Sumida M, Uozumi T, Makuda K, et al. Rathke cleft cyst: correlation of enhanced MR and surgical findings. Am J Neuroradiol. 1994;15(3):525–32. [PMC free article] [PubMed] [Google Scholar]
- 17.Bladowska J, Sokolska V, Czapiga E, et al. Advances in diagnostic imaging of the pituitary and the parasellar region. Adv Clin Exp Med. 2004;13:709–17. [Google Scholar]
- 18.Escott EJ. A Variety of Appearances of Malignant Melanoma in Head: Review. RadioGraphics. 2001;21:625–39. doi: 10.1148/radiographics.21.3.g01ma19625. [DOI] [PubMed] [Google Scholar]
- 19.Acou M, Vanslembrouck J, Deblaere K, et al. Fahr disease. JBR-BTR. 2008;91:19. [PubMed] [Google Scholar]
- 20.Lorincz MT. Neurologic Wilson’s disease. Ann NY Acad Sci. 2010;1184:173–87. doi: 10.1111/j.1749-6632.2009.05109.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonneville F, Cattin F, Marsot-Dupuch K, et al. T1 Signal Hyperintensity in the Sellar Region: Spectrum of Findings. Radio Graphics. 2006;26:93–113. doi: 10.1148/rg.261055045. [DOI] [PubMed] [Google Scholar]
- 22.Rovira A, Alonsoa J, Córdoba J. MR Imaging Findings in Hepatic Encephalopathy. Am J Neuroradiol. 2008;29:1612–21. doi: 10.3174/ajnr.A1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butterworth RF. Role of circulating neurotoxins in the pathogenesis of hepatic encephalopathy: potential for improvement following their removal by liver assist devices. Liver Int. 2003;23(Suppl 3):5–9. doi: 10.1034/j.1478-3231.23.s.3.1.x. [DOI] [PubMed] [Google Scholar]
- 24.Yangho KIM. Neuroimaging in manganism. Neuro Toxicology. 2006;27:369–72. doi: 10.1016/j.neuro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Stepens A, Stagg ChJ, Platkajis A, et al. White matter abnormalities in methcathinone abusers with an extrapyramidal syndrome. Brain. 2010;133:3676–84. doi: 10.1093/brain/awq281. [DOI] [PMC free article] [PubMed] [Google Scholar]