Summary
Background
Tinnitus, occurring at least once in a lifetime in about 10–20% of the population, is an important clinical problem with complex etiology. Rare causes of tinnitus include cranial dural arteriovenous fistulas (DAVFs), which are usually small lesions consisting of abnormal connections between branches of dural arteries and venous sinuses or veins.
Case Report
Authors present a case of a 44-year-old woman with persistent, unilateral, treatment-resistant pulsatile tinnitus caused by a small dural arteriovenous fistula revealed in computed tomography angiography.
Conclusions
Computed tomography angiography is a useful diagnostic method that in some cases allows for establishing the cause of unilateral, pulsatile tinnitus.
Keywords: cranial dural arterio-venous fistula (DAVF), pulsatile tinnitus, sigmoid sinus, computed tomography angiography (CTA), VRT reconstructions
Background
According to the definition proposed by McFadden, tinnitus is defined as perception of sound originating in the head or ears. Frequency of tinnitus defined as at least one 5-minute-long episode is estimated at about 10–20% depending on the study. Tinnitus is a symptom of a variety of diseases and therefore requires appropriate, often multidisciplinary diagnostic approach [1].
Rare causes of tinnitus include cranial dural arteriovenous fistulas (DAVFs). They are usually small lesions consisting of numerous, minute abnormal connections between dural arterial branches and venous sinuses or veins [2] and are characterized by absence of pathological tangling of vessels – the so-called “nidus.” DAVFs belong to a group of rare vascular malformations, constituting about 10–15% of intracranial arteriovenous malformations [3].
In this publication authors present a case of a woman with persistent, unilateral, pulsatile tinnitus caused by a small dural arteriovenous fistula visualized in computed tomography angiography.
The goal of this work is to establish the efficacy of computed tomography angiography in the diagnostics of rare causes of tinnitus.
Case Report
We describe a case of a 44-year-old woman who presented at her GP’s office with a 10-month history of persistent, unilateral, pulsatile tinnitus in her left ear. Her medical history included an episode of pulmonary embolism (in 2009), osteoarthritis of cervical and lumbar vertebra, premature menopause and elevated aminiotransferase levels. Family and social history as well as history of chronic drug use were unremarkable. Initially, the patient was referred to otolaryngological ward, where otolaryngological basis for her problems was excluded. An ambulatory angiology consultation was subsequently carried out. Ultrasound of carotid arteries performed during the consultation revealed dilatation of left occipital and temporal arteries with low-resistance, high-velocity flow. Carotid artery ultrasound prompted a suspicion of left occipital artery arteriovenous fistula. Patient was referred to the Department of Internal Diseases for further diagnostics. Physical examination performed on admission to the hospital revealed a machinery (systolic and diastolic) murmur in the region of mastoid process of left temporal bone. Physical examination was otherwise unremarkable. In the course of hospitalization, computed tomography angiography of carotid and vertebral arteries was performed (with a 128-slice scanner) using a broadened protocol to include cerebral arteries. It showed bilaterally patent common, internal and external carotid arteries, vertebral arteries and the circle of Willis. Arterial phase of the examination revealed filling of a dilated internal carotid artery with contrast (Figure 1). Moreover, it visualized an asymmetrical dilatation of left occipital artery originating from the left external carotid artery together with minute arterial branches penetrating through the lambdoid suture into the sigmoid sinus (Figure 2). Computed tomography angiography imaging was the basis for suspicion of dural arteriovenous fistula. Patient was discharged from the Department of Internal Diseases and referred to the Department of Neurosurgery in order to verify the diagnosis using classical carotid artery angiography and potential qualification for fistula closing. Classical carotid artery angiography performed at the Department of Neurosurgery confirmed the diagnosis of dural arteriovenous fistula between the branches of left occipital artery and sigmoid sinus. Embolization of a fistula was performed, leading to resolution of tinnitus. Patient was discharged home with a follow-up in angiology and neurosurgery clinics.
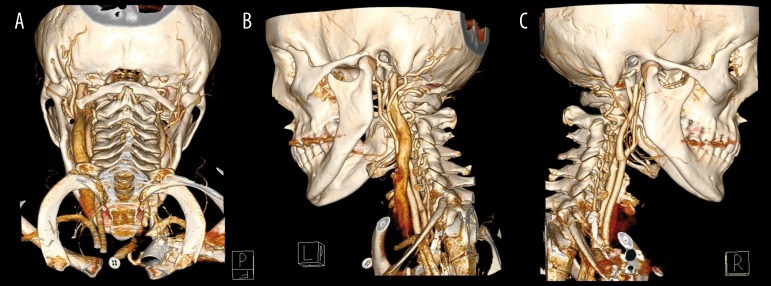
Figure 1.
CTA examination, VRT reconstructions show dilated left internal jugular vein and dilated left occipital artery: (A) posterior view, (B) left lateral view, (C) right lateral view.
Figure 2.
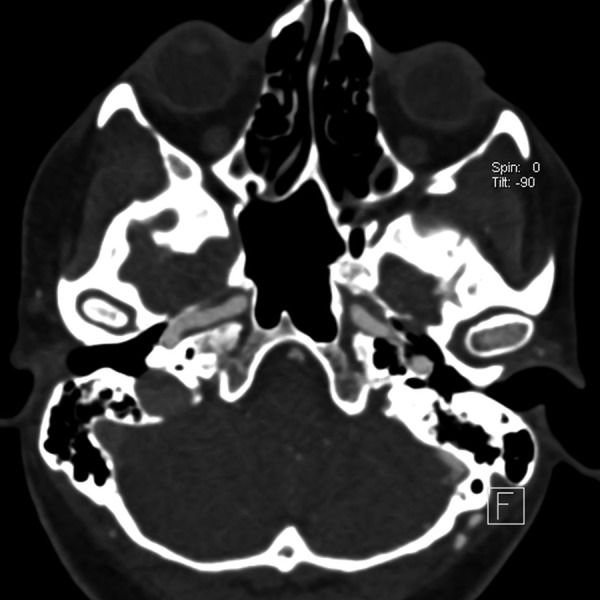
CTA examination, axial scan shows asymmetrical dilatation of left occipital artery and contrast enhancement of left sigmoid sinus.
Discussion
Due to the multitude of disorders that may be associated with it, tinnitus constitutes a difficult diagnostic problem, often requiring multidisciplinary management. Table 1 presents a compilation of disorders that may be related to tinnitus. Objective tinnitus can be recorded, while subjective tinnitus is a phantom phenomenon, which is only heard by the patient. Vascular tinnitus is typically unilateral and pulsatile, while tinnitus of mechanical origin is not characterized by pulsation [1]. In the presented case, unilateral and pulsatile character of tinnitus indicated its vascular nature.
Table 1.
Causes of tinnitus.
| Objective tinnitus | Vascular | Arteriovenous fistulas: congenital, acquired, Paget’s disease |
| Arteriovenous fistulas: congenital, acquired | ||
| Venous anomalies: abnormal size/location of internal jugular bulb | ||
| Pressure exerted by the sternocleidomastoid muscle on internal jugular vein | ||
| Jugular vein stenosis | ||
| Abnormal course of the vessels in the pontocerebellar angle | ||
| Intracranial tumors: meningioma, angioneuromyoma, aneurysm, hemangioma | ||
| Mechanical | Clonic spasms of soft palate muscles | |
| Clonic spasms of inner ear muscles | ||
| Disorders of auditory tube patency | ||
| Temporomandibular joint disease | ||
| Foreign body moving within the external auditory canal | ||
| Subjective tinnitus | Originating in the outer ear | Mechanical occlusion of external auditory canal |
| Originating in the middle ear | Tympanic cavity effusion | |
| Otosclerosis | ||
| Originating in the inner ear | Acoustic trauma | |
| Drug ototoxicity: salicylates, ototoxic antibiotics | ||
| Viral (mumps, flu) and bacterial (scarlet fever) infections | ||
| Ménière’s disease | ||
| Trauma: barotrauma, temporal bone fracture | ||
| Middle ear damage in the course of chronic diseases: diabetes, atherosclerosis, thyroid disease, anemia | ||
| Originating in the auditory nerve | Acoustic neuroma | |
| Auditory neuritis and meningitis | ||
| Originating in cortical structures | Demyelination diseases | |
| Central nervous system tumors | ||
| Other | Psychogenic | |
| Auditory hallucinations in the course of psychiatric disorders | ||
| Spinal osteoarthritis |
A diagnostic scheme in a patient with tinnitus should include physical examination (general and otolaryngological), audiological studies and additional tests (laboratory and imaging) [4]. Imaging modalities (ultrasound, angiography, CT and MRI) seem to possess the greatest significance for the diagnostics of objective vascular tinnitus, in case of objective mechanical tinnitus – physical otolaryngological examination is of greatest value, in subjective tinnitus originating in the middle and outer ear – physical otolaryngological examination and audiological studies, in case of subjective tinnitus originating in the inner ear – audiological studies and laboratory tests (assessing function of other systems against chronic diseases), while in case of subjective tinnitus originating at a level of auditory nerve and cortical structures – also imaging studies (CT and MRI) are of use [1,4,5]. Presented case of vascular tinnitus caused by DAVF was diagnosed based on imaging methods.
Cranial dural arteriovenous fistulas can (as in the presented case) be the cause of vascular tinnitus as well as a variety of other clinical symptoms such as, among other things, intracranial bleeding or neurological deficits. In some cases DAVFs are asymptomatic [6]. Initiating factors for DAVF development are not fully recognized [7]. There are reports in the literature describing their association with trauma, inflammation, surgical procedures or venous sinus thrombosis [8]. According to the hypothesis presented by Miyachi et al. for the formation of DAVFs, development of a local leakage may be initiated by an inflammatory reaction at a site of penetration of one of the emissary veins [9].
According to the current state of knowledge, it is thought that the pattern of venous drainage determines the severity of symptoms in patients with DAVFs. Cortical venous reflux (CVF) is associated with an increased risk of intracranial bleeding and non-hemorrhagic neurological deficits [3]. A classification of DAVFs according to Borden and Cognard [10,11] was developed based on the pattern of venous drainage (Table 2). Geibprasert et al. presented an alternative DAVF classification based on the characteristics of venous drainage patterns to various epidural spaces according to the evolution and embryological development of the draining system for the central nervous system and surrounding structures. On its basis we may distinguish 3 subgroups of arteriovenous leaks: VE – ventral epidural, including the location in the sigmoid sinus, DE – dorsal epidural, and LE – lateral epidural. The latter are always “aggressive” due to the presence of CVR [12]. In the presented case we dealt with type I DAVF according to Borden classification.
Table 2.
| A | ||
|---|---|---|
| Type | Venous drainage | Cortical venous reflux |
| I | Dural venous sinus | No |
| II | Dural venous sinus | Yes |
| III | Cortical veins | Yes |
| B | |||
|---|---|---|---|
| Type | Venous drainage | Direction of flow | Cortical venous reflux |
| I | Dural venous sinus | Antegrade | No |
| IIa | Dural venous sinus | Retrograde | No |
| IIb | Dural venous sinus | Antegrade | Yes |
| IIa+b | Dural venous sinus | Retrograde | Yes |
| III | Undilated cortical veins | – | Yes |
| IV | Dilated cortical veins | – | Yes |
| V | Paraspinal veins | – | Yes |
Classification of DAVFs is helpful in the assessment of risk, although it should be remembered that DAVFs are dynamic lesions and even those initially considered benign may develop venous drainage through cerebral cortex due to venous strictures, thrombosis or increased arterial flow. It is also possible that untreated lesions become spontaneously closed (in 12.5% of cases according to Kim et al.), which most often occurs when DAVFs are located in the area of transverse and cavernous sinus [3,13].
Most commonly, DAVFs drain into the transverse and sigmoid sinuses. At the same time, they usually present with most benign clinical course and the most frequent symptoms include headaches and tinnitus. Persistent pulsatile tinnitus, which is considered a benign symptom of DAVFs, may be an indication for surgical treatment of DAVF [3,6,14]. Such case was presented in this publication.
Diversity and lack of specificity of both clinical and radiological symptoms is the cause of a delay in diagnosis of DAVF or failure to correctly identify the lesion. Fundamental significance for imaging diagnostics of DAVFs is ascribed to computed tomography (CT), magnetic resonance (MRI) and computed tomography angiography (CTA), magnetic resonance angiography (MRA) and digital subtraction angiography (DSA). Initial assessment includes CT and/or MRI imaging. The role of non-contrast CT examination is limited to excluding possible complications: intracranial hemorrhage and brain edema caused by venous congestion. Besides, the bone window may visualize dilated cranial vascular canals derived from intraosseous supplying arteries. Administration of contrast allows for visualizing dilated, tortuous draining veins near the brain surface, dilated dural sinuses and deficits of their contrasting in case of thrombosis. MRI examination, beside dilatation of vessels and their contrast enhancement, may reveal signs of elevated venous pressure in case of more aggressive lesions such as: hyperintense white matter, intracranial bleeding or cerebral venous stroke [2,3]. Any suspicious focus of no flow in the area of venous sinuses should be verified using dynamic studies: CTA, MRA or DSA.
DSA remains the “gold standard” for the assessment of morphology and hemodynamics of DAVFs, as high special resolution and selective cannulation allow for, among other things, identification of all sources of blood supply and detailed assessment of venous drainage with determination of the direction of flow or potential venous cortical reflux – information significant for making further therapeutic decisions. However, there are some limitations to this method: it is relatively expensive and time-consuming, poses a risk of “silent” embolisms and transient/permanent worsening of neurological condition [15,16].
CTA seems particularly useful for precise planning of DAVF treatment, determining its anatomical relationships to the brain and the cranium. It is considered a first-line examination in the diagnostics of pulsatile tinnitus, as it may visualize its structural (developmental abnormalities of the inner ear), neoplastic (e.g. paraganglioma) or vascular causes. It also reveals strictures or presence of dural venous sinus diverticulum not associated with DAVF [17].
Although CTA and MRA do not allow for unequivocal exclusion of DAVF, these modalities gain increasingly more importance for visualization of such types of lesions due to current technical means. Recent publications on 4D CTA using a 320-slice scanner (compared to DSA) demonstrated great potential of this method for establishing proper diagnosis, classification and aiding in management and monitoring of DAVFs [15,18]. Exceptions included small, low-flow fistulas, visualization of which seemed to require invasive angiography [15]. Another study revealed that time-resolved MRA (trMRA) using a 3-Tesla scanner (also compared with DSA) appears to be an equally useful method of DAVF detection, even as a screening study [16].
Due to modern treatment methods, the great majority of dural arteriovenous fistulas are treatable and patients gain significant clinical improvement [19]. Also in case of the patient presented in this publication treatment of DAVF led to substantial improvement of her clinical condition.
Conclusions
Computed tomography angiography using a 128-slice scanner may visualize a small cranial dural arteriovenous fistula.
Computed tomography angiography is an examination that allows for establishing the cause of pulsatile tinnitus, i.a. dural arteriovenous fistula.
References
- 1.Szymiec E. Szumy uszne. Przew Lek. 2002;5(10):99–102. [in Polish] [Google Scholar]
- 2.Gupta A, Periakaruppan A. Intracranial dural arteriovenous fistulas: A Review. Indian J Radiol Imaging. 2009;19(1):43–48. doi: 10.4103/0971-3026.45344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi D, Chen J, Pearl M, et al. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. Am J Neuroradiol. 2012;33(6):1007–13. doi: 10.3174/ajnr.A2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan Y. Tinnitus: etiology, classification, characteristics, and treatment. Discov Med. 2009;8(42):133–36. [PubMed] [Google Scholar]
- 5.Kaczmarek JP, Szymiec E, Dabrowski P, et al. Unilateral tinnitus – diagnostics and treatment. Otolaryngol Pol. 2002;56(3):361–64. [PubMed] [Google Scholar]
- 6.Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on localization and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24(6):1637–53. doi: 10.1148/rg.246045026. [DOI] [PubMed] [Google Scholar]
- 7.Ushikoshi S, Kikuchi Y, Miyasaka K. Multiple dural arteriovenous shunts in a 5-year-old boy. Am J Neuroradiol. 1999;20(4):728–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Sencer A, Kiris T. Intracranial dural arteriovenous fistulas: a brief review on classification and general features. Turk Neurosurgery. 2006;16(2):57–64. [Google Scholar]
- 9.Miyachi E, Izumi T, Matsubara N, et al. Mechanism of the formation of dural arteriovenous fistula: the role of the emissary vein. Interv Neuroradiol. 2011;17(2):195–202. doi: 10.1177/159101991101700209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166–79. doi: 10.3171/jns.1995.82.2.0166. [DOI] [PubMed] [Google Scholar]
- 11.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–80. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 12.Geibprasert S, Pereira V, Krings T, et al. Dural arteriovenous shunts: a new classification of craniospinal epidural venous anatomical basesand clinical correlations. Stroke. 2008;39(10):2783–94. doi: 10.1161/STROKEAHA.108.516757. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Brugge K, Krings T, et al. Spontaneous angiographic conversion of intracranial dural arteriovenous shunt: long-term follow-up in nontreated patients. Stroke. 2010;41(7):1489–94. doi: 10.1161/STROKEAHA.110.581462. [DOI] [PubMed] [Google Scholar]
- 14.Shownkeen H, Yoo K, Leonetti J, et al. Endovascular treatment of transverse-sigmoid sinus dural arteriovenous malformations presenting as pulsatile tinnitus. Skull Base. 2001;11(1):13–23. doi: 10.1055/s-2001-12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willems PW, Brouwer PA, Barfett JJ, et al. Detection and classification of cranial dural arteriovenous fistulas using 4D-CT angiography: initial experience. AJNR Am J Neuroradiol. 2011;32(1):49–53. doi: 10.3174/ajnr.A2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farb RI, Agid R, Willinsky RA, et al. Cranial dural arteriovenous fistula: diagnosis and classification with time-resolved MR angiography at 3T. Am J Neuroradiol. 2009;30:1546–51. doi: 10.3174/ajnr.A1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narvid J, Do HM, Blevins NH, et al. CT angiography as a screening tool for dural arteriovenous fistula in patients with pulsatile tinnitus: feasibility and test characteristics. Am J Neuroradiol. 2011;32(3):446–53. doi: 10.3174/ajnr.A2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouwer PA, Bosman T, van Walderveen MA, et al. Dynamic 320-section CT angiography in cranial arteriovenous shunting lesions. Am J Neuroradiol. 2010;31:767–70. doi: 10.3174/ajnr.A1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giller CA, Barnett DW, Thacker IC. Multidisciplinary treatment of a large cerebral dural arteriovenous fistula using embolization, surgery, and radiosurgery. Proc (Bayl Univ Med Cent) 2008;21(3):255–57. doi: 10.1080/08998280.2008.11928405. [DOI] [PMC free article] [PubMed] [Google Scholar]