Summary
Background
To describe cerebellar abnormalities in a family composed by a father and two affected sibs with Adams Oliver syndrome (AOS) (OMIM 100300).
Material/Methods
Brain MRI and MR angiography were performed at 1.5T.
Results
The siblings presented cerebellar cortex dysplasia characterized by the presence of cysts.
Conclusions
Abnormalities of CNS are an unusual manifestation of AOS. To our knowledge, this is the first report of cerebellar cortical dysplasia in a family with AOS.
Keywords: Adams Oliver syndrome, cerebellar cysts, cerebellar cortical dysplasia, MRI
Background
AOS is a rare condition which involves skin, limbs, cardiovascular system and CNS. It is mainly characterized by aplasia cutis, marmorata telangiectatica congenita and/or bony defects of the underlying skull, associated with various degree of terminal limb defects.
It was initially described as having an autosomal dominant inheritance with marked variability in expression and lack of penetrance in some cases. However, there are familiar cases suggesting an autosomal recessive inheritance; sporadic cases are also reported [1–7].
CNS abnormalities are more commonly seen in those cases where an autosomal recessive pattern of inheritance is reported (severe variant of AOS) [1,5].
Aplasia cutis congenita is typical of AOS [1–4,6,8,9]. It consists of a congenital absence of skin, usually located over the parietal scalp, although it can be located anywhere in the body. The defect may be limited to the epidermis but it can frequently involve the underlying bone too. Dilated scalp veins are also frequently evident [5,10], as in our patients. In infancy, calvarial defects can be unapparent, presenting simply as an enlarged anterior fontanelle [5].
Cutis marmorata telangiectatica, skin showing livid reddish macular areas (this concerns the upper dermal vascular plexus) and peripheral venous incontinence, have also been reported.
Abnormalities of limbs often include terminal transverse defects, such as: short fingers, small toenails, syndactyly, truncation defects affecting the distal phalanges or entire fingers [3,4,6,9]. Metatarsals/metacarpals or more proximal limb structures are only rarely affected [3].
Cardiovascular system can also be involved in AOS in 13.4% of patients [3,11].
Encephalocele, acrania, abnormal cerebral vasculature, microcephaly, arhinencephaly, pons and medulla hypoplasia, partial agenesis of the corpus callosum, intracranial calcifications [12,13], nodular heterotopia and polymicrogyria have been also described [1,2,6,9,14].
Herein we described a family in which the father and two children (sibilings) were affected by AOS.
MR brain examinations demonstrated the same cerebellar malformation in both children, never reported before in this syndrome.
Material and Methods
The two children and the father underwent MRI at 1.5T (Philips Gyroscan INTERA). The sequence included axial, coronal and sagittal Turbo Spin-Echo (TSE) T2-weighted, axial and coronal T1-weighted Inversion-Recovery (IR), axial Fluid-Attenuated-Inversion-Recovery (FLAIR)-weighted, integrated by MRA with Time-of-flight (TOF) and Phase-contrast (PC) sequences without gadolinium injection.
Patients
Father
A 46-year-old male with AOS, diagnosed at the age of 41 on the basis of typical clinical features including cutis marmorata, cutaneous syndactyly of the second and third toe and brachydactyly. Vascular malformations involved lungs, lower limbs and hemorrhoidal plexus. A chest X-ray and contrast-enhanced Computed Tomography (CT) of the chest revealed arteriovenous malformations. Echocardiogram showed ischemic cardiomyopathy. Cardiac catheterization revealed an occlusive stenosis of interventricular ramus of the left coronary artery. A Doppler Ultrasound of the lower limbs showed bilateral varices and a diffuse deep venous incontinence. Brain MRI was normal.
Patient 1
A 15-year-old male was the first child of unrelated parents.
He was born SGA at 40 weeks by cesarean section, after a pregnancy characterized by placental ageing detected during the fourth week.
He showed normal growth and psychomotor development during the first years of life. At the age of 11 years he was admitted to our Pediatric Department with asthenia and pains in the lower limbs. A USD revealed superficial and deep venous incontinence.
The clinical features included: cutis marmorata, short neck, dorsal scoliosis, cutaneous syndactyly of the second and third toe bilaterally (Figure 1), brachydactyly. Those elements suggested a diagnosis of AOS.
Figure 1.
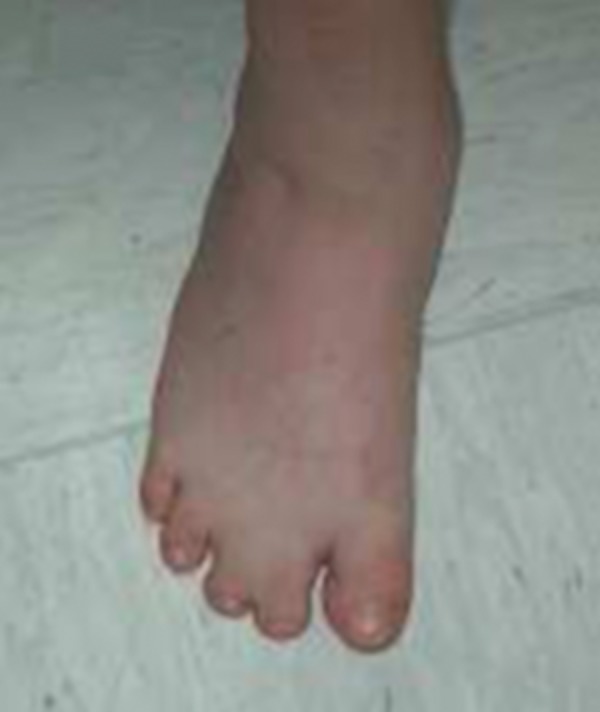
Patient 1: Cutaneous syndactyly of the second and third toe and brachydactyly.
Both neurological examination and EEG recording performed when he was 12 years old, were normal.
He was subjected to brain MRI (1.5 T) and brain MRA without gadolinium injection.
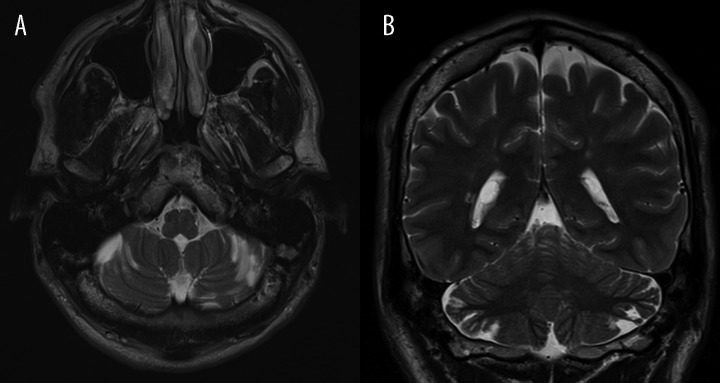
Some peripheral and variable-sized, CSF-intensity lesions were located in the cerebellar hemispheres (Figure 2A), without any surrounding hyperintensity on FLAIR. The hemispheric abnormalities were more widespread on the surface of the superior and inferior semilunar lobules.
Figure 2.
(A) Patient 1: Axial TSE T2-weighted section. Presence of some cerebellar small cortico-subcortical longitudinal areas, isointense with CSF, orientated along the sulcis. (B) Patient 1: Coronal TSE T2-weighted section: Short, thin, atrophic folia of the superior semilunar lobules with dilatation of CSF spaces. Note the little periventricular hyperintensities of the supratentorial white matter.
Small, short and disorganized folia with abnormal arborization of the white matter were also appreciable. The CSF-intensity lesions were continuous with the disorganized folia (Figure 2B). The volume of the cerebellum and the orientation of the fissures were apparently normal.
A right temporal arachnoid cyst, isointense to CSF was found.
Few little T2-hyperintense lesions were evident in the white matter near the frontal horns and trigones, indicative of gliosis and typical for a hypoxic-ischemic encephalopathy (HIE).
There were some T2-hyperintense vessel dilations, possibly of veins, in the skin of the scalp.
MRA showed no arterial or venous malformations.
Patient 2
She was the younger sister of patient no. 1. She was born at 36 weeks of gestation with cesarean section. Pregnancy was complicated by placentar ageing, oligohydramnios and a reduced fetal growth detected at 24 weeks. A low birth weight (2,130 g.) and head circumference at birth below the 3rd percentile (29 cm) were reported. At birth, a cutaneous lesion of the scalp along the sutures, telangiectasias on the abdomen and inferior limbs, cutis marmorata were noted. The toes were apparently short and there was a proximal cutaneous syndactyly of the second and the third toe. Those components suggested the diagnosis of AOS. Cerebral ultrasonography (US) revealed hyperechogenicity of the periventricular parenchyma.
She showed normal psychomotor development. The patient was admitted to our Pediatric Department when she was 3 years old. A clinical examination revealed microcephaly, cutis marmorata and skin atrophy at the skull vertex due to aplasia cutis (Figure 3A, 3B).
Figure 3.
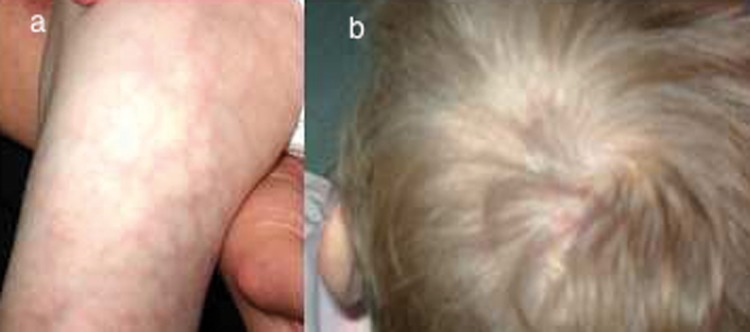
Patient 2: Cutis marmorata telangiectatica (A). Cutaneus atrophy at the skull vertex, which resulted from aplasia cutis (B).
Brain MRI showed some longitudinal CSF-intensity areas of the cerebellum attributable to cysts, short and disorganized folia with abnormal arborization of the white matter on the surface of the superior and inferior semilunar lobules, as in her brother (Figure 4).
Figure 4.
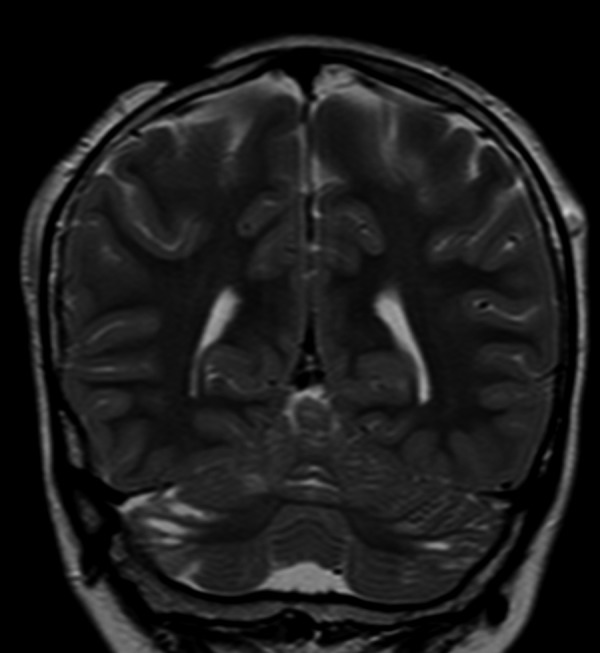
Patient 2: Coronal TSE T2-weighted section. Small CSF areas bilaterally located on the surface of the superior and inferior semilunar lobules. A left parietal subcutaneous vascular ectasia and a zone of aplasia cutis in the right parietal region are also evident.
Some dilated veins in the skin of the scalp were found, but no cerebral vascular malformations.
Discussion
The pathophysiologic mechanism of AOS still remains unknown, even though it has been speculated that the typical vascular skin changes may probably indicate a congenital abnormal development of small vessels, or abnormal pericyte recruitment to vessels [1,3,9,13,15]. It has also been hypothesized that AOS results from the interruption of early embryonic blood supply in subclavian arteries, and that the gene predisposing to this interruption is inherited in an autosomal dominant fashion [8]. Other theories are based on developmental disorders of neural tube closure, amniotic adhesions or intrauterine external compressions [16].
However, the most prominent theories for the etiology of AOS involve either vascular disruption or abnormal morphogenesis because some signs of this syndrome are more difficult to explain only with a vascular mechanism [1].
Distal limb deficiencies, body wall defects, digital and intracranial abnormalities have been described in the Amniotic Band syndrome (ABS) too. In this syndrome constriction rings are typical but it remains controversial whether they are caused by external constriction of amniotic bands or by genetic factors and whether AOS and ABS should be considered separate entities or not.
A variety of cranial and brain malformations has been reported in AOS [14], but the dysplasia of the cerebellar cortex with cysts has not been previously noted.
Temtamy et al. described retrocerebellar cyst and mild cerebellar hypoplasia of the vermis and of the right hemisphere in a case of AOS, associated with microcephaly, lacunar skull defects, cutis marmorata and limb defects. Previous studies reported that hypoplasia or aplasia of cerebellar arteries of probable early intrauterine origin would be the cause of this malformation [17].
Focal minor cerebellar cortical dysplasias are often observed in healthy newborns and are considered a common feature of human cerebellar development [2,18,19].
Cerebellum is developed over a very long period of time, from the initial appearance of the rhombic lips at 6 weeks of gestational age to the disappearance of the external granule cell layer at 12–18 postnatal months [2,10,15,20–24].
Cerebellar cortical dysplasias are caused by disturbance of this cortical layering. The mechanism underlying these abnormalities is not clear; however, the external granular cells and Purkinje cells may be involved. Meningeal cells could influence this process of foliation and fissuration too [2,10,11,21,25,26]. Microscopically, the folia are scrambled together with fusion of molecular layers surrounding small cavities with meningeal vessels, with a granular layer deficit and Purkinje cells scattered at the boundaries of these layers [21,27].
MRI findings of this kind of cerebellar cortical dysplasia include: disordered alignment of the folia that can be thickened or normal, irregular gray/white matter junction, lack of the normal white matter arborization, abnormal fissure orientation and cysts [2,10,18,20,21,23,27,28].
Small parenchymal cerebellar cysts have been described in association with brain cortical dysgenesis in Fukuyama congenital muscular dystrophy (FCMD) and other muscular dystrophies [11,18,19,28–31].
Some pathological studies on FCMD revealed that the cerebellar cysts can exist within or near some folia abnormalities, which usually fuse with each other and obstruct the sulci in their superficial parts. In such cases the cysts are partially lined by leptomeningeal tissue and surround a nearly normal molecular layer [22,29].
Our patients had dysplastic hemispheric short and thin folia, longitudinal “cyst-like” loculations on the surface of the superior and inferior semilunar lobules, without abnormal orientation of the fissures. These superficial “cyst-like” loculations were quite different from the intraparenchymal cysts of FCMD especially in terms of location, probably because they could communicate with adjacent subarachnoid spaces because related to the dysplastic cortex.
Demaerel et al. describes 15 patients without dystrophy but with vermian abnormalities and longitudinal “cyst-like” abnormalities different from the intraparenchymal cysts of FCMD [2,18], similar to those in our patients.
Conclusions
The cases reported here show the peculiarity of the unusual, previously not reported association of AOS with a form of cerebellar cystic cortical dysplasia in a family. Our results suggest a need for an accurate evaluation of the posterior fossa in patients with AOS, in order to identify such cerebellar findings that, in some cases, could be difficult to visualize.
Abbrevations
- AOS
Adams Oliver syndrome
- FCMD
Fukuyama congenital muscular dystrophy
- ABS
Amniotic Band syndrome (ABS)
References
- 1.Amor DJ, Leventer RJ, Hayllar S, et al. Polimicrogyria Associated with scalp and Limb Defects: Variant of Adams-Oliver Syndrome. Am J Med Genet. 2000;93:328–34. doi: 10.1002/1096-8628(20000814)93:4<328::aid-ajmg13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Demaerel P, Wilms G, Marchal G. Rostral vermian cortical dysplasia: MRI. Neuroradiology. 1999;41:190–94. doi: 10.1007/s002340050732. [DOI] [PubMed] [Google Scholar]
- 3.Kocer U, Aksoy HM, Tiftikcioglu YO, et al. Coexistence of aplasia cutis congenita of the scalp with symbrachydactyly of bilateral feet: an unreported form of Adams-Oliver syndrome. Eur J Plast Surg. 2001;24:310–14. [Google Scholar]
- 4.Kuster W, Lenz W, Kaariainen H, et al. Congenital scalp defects with distal limb anomalies (Adams Oliver syndrome): report of ten cases and review of the literature. Am J Med Genet. 1988;31(1):99–115. doi: 10.1002/ajmg.1320310112. [DOI] [PubMed] [Google Scholar]
- 5.McGoey RR, Lacassie Y. Adams-Oliver syndrome in sibiling with central nervous system findings, epilepsy, and developmental delay: refining the features of a severe autosomal recessive variant. Am J Genet A. 2008;146(A):488–91. doi: 10.1002/ajmg.a.32163. [DOI] [PubMed] [Google Scholar]
- 6.Orstavik KH, Stromme P, Spetalen S, et al. Aplasia cutis congenita associated with limb eye and brain anomalies in sibs: a variant of Adams-Oliver syndrome. Am J Med Genet. 1995;59:92–95. doi: 10.1002/ajmg.1320590118. [DOI] [PubMed] [Google Scholar]
- 7.Yéè S, Perrot P, Bellier-Waast F. The Adams-Oliver syndrome. A case report. Chir Main. 2010;29(4):274–76. doi: 10.1016/j.main.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Der Kaloustian VM, Hoyme HE, Hogg H. Possible common pathogenetic mechanisms for Poland sequence and Adams-Oliver syndrome. Am J Med Genet. 1991;38(1):69–73. doi: 10.1002/ajmg.1320380116. [DOI] [PubMed] [Google Scholar]
- 9.Savarirayan R, Thompson EM, Abbott KJ, et al. Cerebral cortical dysplasia and digital constriction rings in Adams-Oliver syndrome. Am J Med Genet. 1999;386:15–19. doi: 10.1002/(sici)1096-8628(19990903)86:1<15::aid-ajmg4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Takanashy J, Sugita K, Barkovich AJ, et al. Partial Midline Fusion of the Cerebellar Hemispheres with Vertical folia: A New Cerebellar Malformation? Am J Neuroradiol. 1999;20:1151–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Necchi D, Scherini E, et al. The malformation of the cerebellar fissure prima: a tool for studyng histogenetic processes. Cerebellum. 2002;1(2):137–42. doi: 10.1080/147342202753671277. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian M, Collins AL. Aplasia cutis congenital, terminal limb defect and periventricular leukomalacia in one sibiling with minor findings in other-probable autosomal recessive Adams-Oliver Syndrome. Eur J Med Genet. 2009;52(4):234–38. doi: 10.1016/j.ejmg.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 13.De Wit MC, de Coo IF, Schot R. Periventricular nodular heterotopia and distal limb deficiency: a recurrent association. Am J Med Genet A. 2010;152A(4):954–59. doi: 10.1002/ajmg.a.33258. [DOI] [PubMed] [Google Scholar]
- 14.Brancati F, Garaci, Mingarelli R, et al. Abnormal neuronal migration defect in the severe variant subtype of Adams-Oliver syndrome. Am J Med Genet A. 2008;146A(12):1622–23. doi: 10.1002/ajmg.a.32357. [DOI] [PubMed] [Google Scholar]
- 15.Patel S, Barkovich AJ. Analysis and Classification of Cerebellar Malformations. Am J Neuroradiol. 2002;23:1074–87. [PMC free article] [PubMed] [Google Scholar]
- 16.Trobs RB, Barenbeg K, Hemminghaus M, et al. Herniation of the brain after conservative treatment of a large congenital skull defect in an infant with Adams-Oliver syndrome. J Pediatr Surg. 2010;45(10):2064–67. doi: 10.1016/j.jpedsurg.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Temtamy SA, Aglan MS, Ashour AM, et al. Adams-Oliver syndrome: further evidence of an autosomal recessive variant. Clinical Dysmorphology. 2007;16:141–49. doi: 10.1097/MCD.0b013e3280f9df22. [DOI] [PubMed] [Google Scholar]
- 18.Soto Ares G, Delmaire C, Deries B, et al. Cerebellar cortical dysplasia: MR findings in a complex entity. Am J Neuroradiol. 2000;21:1511–19. [PMC free article] [PubMed] [Google Scholar]
- 19.Soto Ares G, Desvime L, Jorriot S, et al. Neuropathologic and MR Imaging Correlation in a Neonatal Case of Cerebellar Cortical Dysplasia. Am J Neuroradiol. 2002;23:1101–4. [PMC free article] [PubMed] [Google Scholar]
- 20.Demaerel P. Abnormalities of cerebellar foliation and fissuration: Classification, neurogenetics and clinicoradiological correlations. Neuroradiology. 2002;44:639–46. doi: 10.1007/s00234-002-0783-1. [DOI] [PubMed] [Google Scholar]
- 21.Altman NR, Naidich TP, Braffman BH. Posterior fossa malformations. Am J Neuroradiol. 1992;13:691–724. [PMC free article] [PubMed] [Google Scholar]
- 22.Aida N, Tamagawa K, Takada K, et al. Brain MR in Fukuyama Congenital Muscolar Dystrophy. Am J Neuroradiol. 1996;17:605–13. [PMC free article] [PubMed] [Google Scholar]
- 23.Soto Ares G, Deries B, Delmaire C, et al. Cerebellar cortical dysplasia: MRI aspects and signifiance. J Radiol. 2004;85:729–40. doi: 10.1016/s0221-0363(04)97675-5. [DOI] [PubMed] [Google Scholar]
- 24.van der Knaap MS, Smit LM, Barth PG, et al. Magnetic Resonance Imaging in Classification of Congenital Dystrophies with Brain Abnormalities. Ann Neurol. 1997;42:50–59. doi: 10.1002/ana.410420110. [DOI] [PubMed] [Google Scholar]
- 25.Sakata-Haga H, Sawada K, Hisano S, et al. Abnormalities of cerebellar foliation in rats prenatally exposed to ethanol. Acta Neuropathol. 2001;102:36–40. doi: 10.1007/s004010000345. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Jensen P, Goldowitz D. The Community Effect and Purkinje Cell Migration in the Cerebellar Cortex: Analysis of Scrambled Chimeric Mice. J Neurosci. 2002;22(2):464–70. doi: 10.1523/JNEUROSCI.22-02-00464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demaerel P, Lagae L, Caesar P, et al. MR of cerebellar cortical dysplasia. Am J Neuroradiol. 1998;19:984–86. [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki M, Oikawa H, Ehara S, et al. Disorganised unilateral cerebellar folia: a mild form of cerebellar cortical dysplasia? Neuroradiology. 2001;43:151–55. doi: 10.1007/s002340000340. [DOI] [PubMed] [Google Scholar]
- 29.Aida N, Yagishita A, Takada K, et al. Cerebellar MR in Fukuyama Congenital Muscolar Dystrophy: Polimicrogyria with Cystic Lesions. Am J Neuroradiol. 1994;15:1755–59. [PMC free article] [PubMed] [Google Scholar]
- 30.Barkovich AJ. Neuroimaging Manifestations and Classifications of Congenital Muscolar Dystrophies. Am J Neuroradiol. 1998;19:1389–96. [PMC free article] [PubMed] [Google Scholar]
- 31.Valanne L, Pihko H, Katevuo K, et al. MRI of the brain in muscle-eye-brain (MEB) disease. Neuroradiology. 1994;36:473–76. doi: 10.1007/BF00593687. [DOI] [PubMed] [Google Scholar]