Abstract
Background:
Breast carcinoma is the most frequent cancer among women with considerable invasive and metastatic behavior. CCND1, the oncogene encoding cyclin D1, is amplified in a substantial proportion of human cancers. Although cyclin D1 overexpression has been reported in up to 50% of human breast cancers, its prognostic impact on breast carcinoma is still controversial.
Materials and Methods:
In this cross-sectional investigation, 89 patients with breast invasive ductal carcinoma enrolled in the study. Tumor tissue samples were stained immunohistochemically for cyclin D1. The marker was semiquantitatively scored using the Allred scoring method and its relationship with ER, PR, and HER2-neu status as well as age, tumor grade and stage was then determined.
Results:
Cyclin D1 was strong (S), intermediate (I), weak (W), and negative (N) in 19.1%, 44.9%, 14.6%, and 21.3% of the cases, respectively. Estrogen receptor (ER), progesterone receptor (PR), and HER2- neu were positive in 60.7%, 58.4%, and 36% of the cases, respectively. There was a statistically significant reverse relationship between tumor grade and cyclin D1 (P = 0.009). The relationship between cyclin D1 and both hormone receptors was also statistically significant (P = 0.0001). There was no statistically significant relationship between cyclin D1 on one hand and age, stage, and HER2-neu on the other (P > 0.05).
Conclusion:
The reverse relationship between cyclin D1 overexpression and tumor grade as well as its positive relationship with ER and PR in invasive ductal carcinoma suggest that cyclin D1 may directly or indirectly result in maturation and differentiation of tumor cells.
Keywords: Breast carcinoma, cyclin D1, estrogen receptor, progesterone receptor, tumor grade
INTRODUCTION
Breast carcinoma is the most frequent cancer among women with considerable invasive and metastatic behavior. The recent increased knowledge in molecular mechanisms of this cancer and consequent targeted treatments have improved its outcome.[1]
Cyclin D1 is one of the main regulatory molecules of the cell cycle.[2] It belongs to the family of D-type cyclins, which regulate cell cycle progression from G1 to S phase by regulating the activity of cyclin-dependent kinases (CDKs).[3] Cyclin D1 and its close relatives, cyclin D2 and cyclin D3, all appear to associate with the same kinase partners.[3] Binding of cyclin D1 to CDK4 and CDK6 induces hyperphosphorylation of retinoblastoma protein (Rb). Hyperphosphorylated Rb loses its ability to bind to the E2F family of transcription factors. This leads to the activation of E2F and transcription of several genes required for the G1 to S phase transition, thereby promoting cellular proliferation.[4] Recent findings have revealed further roles of cyclin D1 in promoting cell cycle progression through CDK-independent mechanisms such as interaction with and modulation of transcription factor activities.[5]
The well-known oncogene CCND1, which encodes cyclin D1 is amplified in a substantial proportion of human cancers including parathyroid carcinoma, colon cancer, lymphoma, melanoma, prostate cancer and breast cancer.[5] Cyclin D1 overexpression has been reported in up to 50% of human breast cancers.[4,5] However, the impact of cyclin D1 overexpression on behavior of breast cancer remains controversial.[2] Although cyclin D1 has a pivotal role in promoting cell cycle progression and its overexpression in breast cancer is expected to be associated with poor prognosis, recent studies have shown both positive and negative prognostic impacts.[4]
Besides inducing cell cycle progression through regulation of CDKs activity, cyclin D1 promotes other regulatory molecules by CDK-independent mechanisms.[4] Cyclin D1 represses the transcriptional activity of signal transducer and activator of transcription 3 (STAT3) in vitro. The resultant loss of antiapoptotic activity of STAT3 is apoptosis.[4] Cyclin D1 is the intermediary molecule in other cell cycle pathways such as nuclear factor-κB (NFκB), Rac1 and 5′ adenosine monophosphate-activated protein kinase (AMPK) signaling pathways. Decline in Rac1 levels causes inhibition of NFκB signaling and induces downregulation of cyclin D1.[6,7] Active AMPK leads to loss of cyclin D1 messenger ribonucleic acid and protein.[8]
Only a minority of breast cancers with cyclin D1 overexpression show amplification of the cyclin D1 gene, indicating that pathogenic transcriptional activation of this gene by factors such as estrogen receptor (ER) could be another important mechanism triggering its overexpression.[5] Using antibody against cyclin D1 protein, overexpression of the protein can be detected even in the absence of any apparent increase in copy numbers. Therefore, immunohistochemical staining with the specific monoclonal antibody provides an accurate method for detection of deregulated cyclin D1 expression.[3]
ER is another regulatory molecule with critical roles in proliferation of cancer cells in reproductive organs such as breast and uterus. ER status accompanies specific histologic characteristics of breast cancer. Most ER positive breast cancers are low-grade and have lower metastatic potential while ER negative ones demonstrate poor differentiation.[1] ER expression is in turn influenced by the expression of other genes such as MTA1. Silencing MTA1 gene results in ER expression in ER negative cells.[1]
Genetically modified mouse models reveal the necessity of cyclin D1 expression for postnatal mammary development in response to the sex steroids. Indeed, cyclin D1 expression in mammary epithelial cells is induced by estrogen and progesterone.[5]
The present study aims to find the frequency of cyclin D1 overexpression and the relationship between cyclin D1 overexpression and some well-known clinicopathologic prognostic determinants in breast invasive ductal carcinoma.
MATERIALS AND METHODS
In this descriptive-analytical and cross-sectional investigation, 89 patients with breast invasive ductal carcinoma hospitalized in Alzahra Hospital, Isfahan, Iran from 2003 to 2008 enrolled in the study. Patients had undergone mastectomy and axillary lymph node dissection and the status of ER, progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2)-neu had been determined immunohistochemically using the formalin fixed and paraffin embedded tissue samples of the primary tumor.
The studied variables included age, tumor grade, tumor stage, ER, PR, HER2-neu and cyclin D1 status.
Grading was carried out based on the Nottingham modification of Bloom and Richardson system, which considers the three parameters of tubule formation, nuclear pleomorphism and mitotic count as determining factors.[9] Staging was carried out based on the TNM classification of malignant tumours (TNM staging system) for breast carcinoma.
Expression of ER and PR was considered as negative if lesser than 1% of nuclei were stained and as positive if 1% or higher of nuclei were stained. The antibodies used were against ER (monoclonal mouse anti-human; Clone: 1D5; isotype: IGg1, kappa; Dakocytomation, Denmark) and PR (monoclonal mouse anti-human; Clone: 1A6; isotype: IGg1, kappa; Dakocytomation, Denmark).
The antibody used for HER2-neu study was polyclonal rabbit antihuman antibody against c-erbB-2 oncoprotein, Dakocytomation, Denmark. HER2-neu expression was scored as 3+ (positive) when strong complete staining of the membrane was present in more than 30% of the neoplastic cells, 2+ (positive) if weak to moderate complete staining of the membrane was seen in more than 10% of the neoplastic cells, 1+ (negative) when faint staining was detected in a portion of the circumference of the cytoplasmic membrane in more than 10% of the neoplastic cells and 0 (negative) when no membranous staining was identified or membrane staining was observed in less than 10% of the tumor cells.[10]
The cyclin D1 antibody used was monoclonal mouse anti-human antibody, clone cyclin D1 antibody (DCS-6) (isotype: IgG2a, kappa), Dakocytomation, Denmark. The staining was carried out according to the manufacturer's instructions. Four micron sections were prepared from each formalin-fixed and paraffin-embedded tissue sample to be stained with antibody against cyclin D1 using the envision method. Sections were placed on poly-l-lysine slides and dried in an oven at 60° c for 60 min. Subsequently, they were deparaffinized, rehydrated and rinsed in tap water before antigen retrieval. After inactivation of endogenous catalase by 3% hydrogen peroxide, the sections were incubated with primary antibody for 1 h and secondary antibody for 30 min. After each step of antibody treatment, slides were drained with phosphate buffer to eliminate excess antibody. Diaminobenzidine was used as the chromogen. The slides were counterstained with hematoxylin. The validity of immunohistochemistry staining was provided by using positive and negative controls in each set of staining.
The intensity and distribution of cyclin D1 immunoreactivity were semiquantitatively scored using the Allred scoring method. The intensity of immunohistochemical reaction by light microscopy was recorded as 0 (negative) when no staining of the nuclei was seen even at high magnification, 1 (weak) if staining was visible only at high magnification, 2 (moderate) when staining was readily visible at low magnification and 3 (strong) if staining was strikingly positive even at low power magnification. The proportion of tumor cell nuclei showing positive staining was also scored as either 0 (none), 1 (<1/100), 2 (1/100-1/10), 3 (1/10-1/3), 4 (1/3-2/3) and 5 (>2/3).
The intensity and proportion scores were then added to obtain a total score ranging from 0 to 8 and the tumors were categorized into four groups based on their total score:
Negative expression: Total score of 0
Weak expression: Total score of 1 or 2
Intermediate expression: Total score of 3-5
Strong expression: Total score of 6-8.
Only nuclear staining was considered specific.[11]
Data was collected in prepared checklist and analyzed by version 16 of statistical package for the social sciences (SPSS) software (SPSS Corp, Chicago, USA) using ANOVA and Chi-square tests.
RESULTS
The mean age of patients (±SD) was 51.38 ± 11.37 years (range: 26-83 years). 18%, 41.6% and 40.4% of the tumors were Grade I, II and III, respectively. 7.9%, 40.4%, 18%, 30.3%, 1.1% and 2.2% of the cases fitted into stages 1, 2A, 2B, 3A, 3B and 3C, respectively.
ER, PR and HER2-neu were positive in 60.7%, 58.4% and 36% of the cases, respectively.
Cyclin D1 was strong (S), intermediate (I), weak (W) and negative (N) in 19.1%, 44.9%, 14.6% and 21.3% of cases, respectively [Figures 1 and 2].
Figure 1.
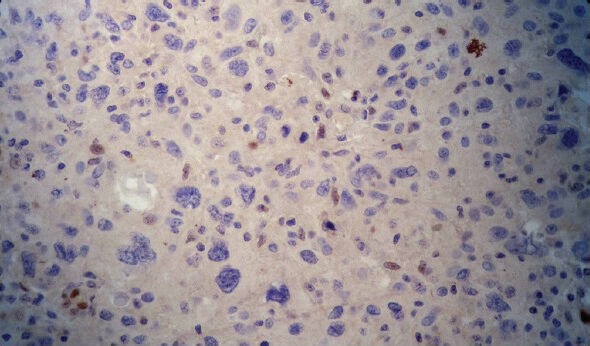
Negative cyclin D1 status was mostly seen in grade III
Figure 2.
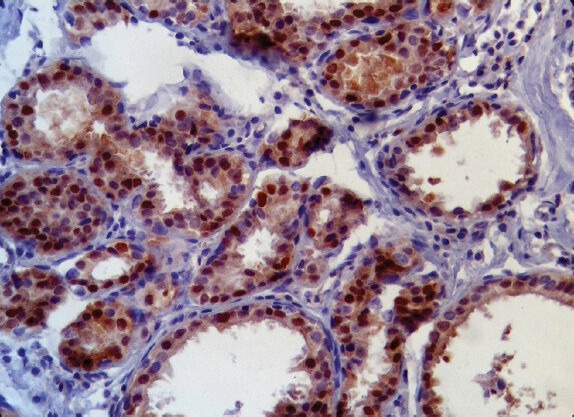
Strong cyclin D1 status was more frequent in grades I & II
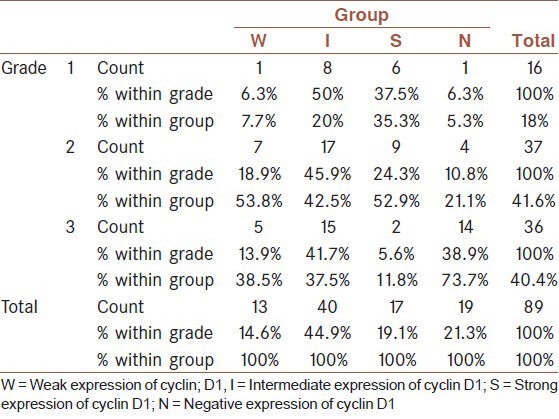
There was a statistically significant reverse relationship between cyclin D1 and tumor grade (P = 0.009). The N status of cyclin D1 was mostly seen in grade 3 while S, I and W states were most frequent in Grade 2 [Table 1].
Table 1.
The relationship between cyclin D1 and tumor grade
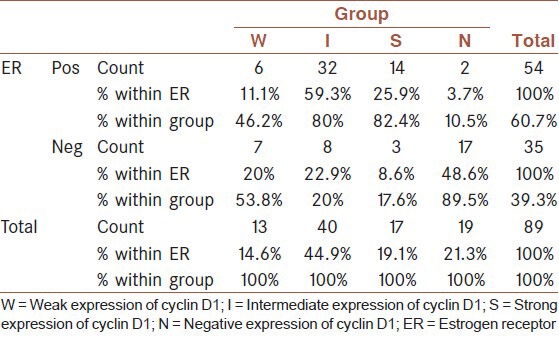
The relationship between cyclin D1 and ER was also statistically significant (P = 0.0001). The S and I states were mostly seen in patients with positive ER while the N status was most frequent in patients with negative ER [Table 2].
Table 2.
The relationship between cyclin D1 and estrogen receptor
A statistically significant relationship was found between cyclin D1 and PR (P = 0.0001). The S, I and W states were mostly seen in patients with positive PR while the N status was most frequent in patients with negative PR [Table 3].
Table 3.
The relationship between cyclin D1 and progesterone receptor
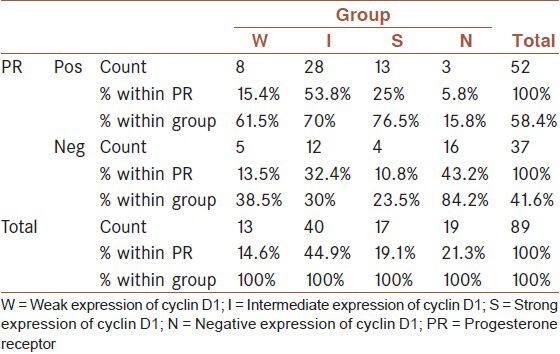
There was no statistically significant relationship between cyclin D1 on one hand and age, stage and HER2-Neu on the other (P > 0.05).
DISCUSSION
The present study was conducted to determine the relationship between cyclin D1 overexpression and well-known clinicopathologic prognostic determinants in breast invasive ductal carcinoma.
It has been shown in previous studies that cyclin D1-deficient mice are susceptible to mammary carcinomas induced by c-myc or Wnt-1, but not those induced by c-neu and v-Ha-ras. These findings suggest a pivotal role for cyclin D1 in a subset of breast cancers.[12]
The oncogene CCND1 is amplified in 10-20% of breast carcinomas while the overexpression of its product cyclin D1 is more frequent and seen in about 34-81% of breast carcinomas.[3,13] In our study, cyclin D1 expression was seen in 78.6% of the cases. The frequency of strong (S), intermediate (I), weak (W) and negative (N) states of cyclin D1 were found to be 19.1%, 44.9%, 14.6% and 21.3%, respectively. No statistically significant relationship was found between age and cyclin D1.
Many researches have shown that overexpression of cyclin D1 in tumors is related to an unfavorable outcome, but others have yielded different results.[14] In our study, there was no statistically significant relationship between cyclin D1 overexpression and tumor stage. This finding may be explained by the fact that cyclin D1 overexpression cannot always be attributed to gene amplification. Activation of other mitogenic pathways such as β-catenin, STAT 5, STAT 3, nuclear factor kappa b, c-jun, e2f-1, ppar y, calveolin-1 and ras signaling may provide alternate routes to disturb regulation of cyclin D1.[15] Moreover, the promotion provided by cyclin D1 to proceed the cell through the cell cycle notwithstanding, degradation of cyclin D1 is essential for replication of deoxyribonucleic acid (DNA). Cyclin D1 level rises early in G1 phase of the cell cycle and continues to accumulate followed by a rapid decline by the entrance to the S phase. Some studies have demonstrated that acute overexpression of cyclin D1 in fibroblasts prevents S-phase entry. Cyclin D1 has been shown to repress DNA replication by binding to proliferating cell nuclear antigen and CDK2.[16]
In the present study, a statistically significant reverse relationship was found between cyclin D1 and tumor grade as evidenced by the observation that negative cyclin D1 status was mostly seen in grade III while strong, and intermediate states of cyclin D1 were more frequent in grades I and II. This observation is the same as the finding of some other studies in this field.[17] The grade of invasive ductal carcinoma is estimated by histological evaluation of tubular formation, mitosis and pleomorphism. Low grade tumors are well-differentiated and show histological features closer to their original tissue. The reverse relationship observed between cyclin D1 overexpression and tumor grade suggests that higher expression of cyclin D1 may directly or indirectly result in maturation of tumor cells. Some of the past researches support this opinion.[18]
We found a statistically significant relationship between cyclin D1 expression and ER. S and I states of cyclin D1were mostly seen in patients with positive ER while N status was most frequent in patients with negative ER. A statistically significant relationship was also found between cyclin D1 expression and PR. S and I states were most frequent in patients with positive PR while N status was mostly seen in patients with negative PR. These findings further confirm the results of some previous studies in this field, which have stated a positive relationship between hormone receptor status and cyclin D1 overexpression in breast carcinoma.[19] This is in favor of the effect of cyclin D1 on cell maturation and differentiation. There was no statistically significant relationship between cyclin D1 overexpression and HER2-neu status. However, overexpression of HER2-neu has been reported to be associated with the high expression of cyclin D1.[19] Further studies are needed to resolve this discrepancy.
CONCLUSION
The reverse relationship between cyclin D1 overexpression and tumor grade as well as the positive relationship between the overexpression of this marker and hormone receptor status in breast carcinoma suggest a regulatory role of cyclin D1 overexpression in cell maturation and differentiation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jiang Q, Zhang H, Zhang P. ShRNA-mediated gene silencing of MTA1 influenced on protein expression of ER alpha, MMP-9, CyclinD1 and invasiveness, proliferation in breast cancer cell lines MDA-MB-231 and MCF-7 in vitro. J Exp Clin Cancer Res. 2011;30:60. doi: 10.1186/1756-9966-30-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin NP, Sims AH, Lundgren KL, Lehn S, Landberg G. Cyclin D1, Id1 and EMT in breast cancer. BMC Cancer. 2011;11:417. doi: 10.1186/1471-2407-11-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–7. [PubMed] [Google Scholar]
- 4.Ishii Y, Pirkmaier A, Alvarez JV, Frank DA, Keselman I, Logothetis D, et al. Cyclin D1 overexpression and response to bortezomib treatment in a breast cancer model. J Natl Cancer Inst. 2006;98:1238–47. doi: 10.1093/jnci/djj334. [DOI] [PubMed] [Google Scholar]
- 5.Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20:2513–26. doi: 10.1101/gad.1446006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J, Benbrook DM, Yu H, Ding WQ. Clioquinol suppresses cyclin D1 gene expression through transcriptional and post-transcriptional mechanisms. Anticancer Res. 2011;31:2739–47. [PubMed] [Google Scholar]
- 7.Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, et al. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657–68. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer. Br J Cancer. 1957;11:359–77. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunelli M, Manfrin E, Martignoni G, Bersani S, Remo A, Reghellin D, et al. HER-2/neu assessment in breast cancer using the original FDA and new ASCO/CAP guideline recommendations: Impact on selecting patients for herceptin therapy. Am J Clin Pathol. 2008;129:907–11. doi: 10.1309/MD79CDXN1D01E862. [DOI] [PubMed] [Google Scholar]
- 11.Reis-Filho JS, Savage K, Lambros MB, James M, Steele D, Jones RL, et al. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: An immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol. 2006;19:999–1009. doi: 10.1038/modpathol.3800621. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland RL, Musgrove EA. Cyclin D1 and mammary carcinoma: New insights from transgenic mouse models. Breast Cancer Res. 2002;4:14–7. doi: 10.1186/bcr411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SY, Caamano J, Cooper F, Guo X, Klein-Szanto AJ. Immunohistochemistry of cyclin D1 in human breast cancer. Am J Clin Pathol. 1994;102:695–8. doi: 10.1093/ajcp/102.5.695. [DOI] [PubMed] [Google Scholar]
- 14.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–43. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 15.Bilalović N, Vranić S, Basić H, Tatarević A, Selak I. Immunohistochemical evaluation of cyclin D1 in breast cancer. Croat Med J. 2005;46:382–8. [PubMed] [Google Scholar]
- 16.Naidu R, Wahab NA, Yadav MM, Kutty MK. Expression and amplification of cyclin D1 in primary breast carcinomas: Relationship with histopathological types and clinico-pathological parameters. Oncol Rep. 2002;9:409–16. [PubMed] [Google Scholar]
- 17.Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol. 2005;23:4215–24. doi: 10.1200/JCO.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 18.Alao JP. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A, Park WC, Yim HW, Lee MA, Park G, Lee KY. Expression of c-erbB2, cyclin D1 and estrogen receptor and their clinical implications in the invasive ductal carcinoma of the breast. Jpn J Clin Oncol. 2007;37:708–14. doi: 10.1093/jjco/hym082. [DOI] [PubMed] [Google Scholar]


