Abstract
Background:
Helicobacter pylori (H. pylori) resistance to antibiotics has become a global problem and is an important factor in determining the outcome of treatment of infected patients. The purpose of this study was to determine the H. pylori resistance to clarithromycin, metronidazole, and amoxicillin in gastrointestinal disorders patients.
Materials and Methods:
In this study, a total of 260 gastric antrum biopsy specimens were collected from patients with gastrointestinal disorders who referred to Endoscopy Section of the Isfahan Hospitals. The E-test and Modified Disk Diffusion Method (MDDM) were used to verify the prevalence of antibiotic resistance in 78 H. pylori isolates to the clarithromycin, metronidazole, and amoxicillin.
Results:
H. pylori resistance to clarithromycin, metronidazole, and amoxicillin were 15.3, 55.1, and 6.4%, respectively. In this study, we had one multidrug resistance (MDR) isolates from patient with gastritis and peptic ulcer disease.
Conclusion:
Information on antibiotic susceptibility profile plays an important role in empiric antibiotic treatment and management of refractive cases. According to the results obtained in this study, H. pylori resistance to clarithromycin and metronidazole was relatively high. MDR strains are emerging and will have an effect on the combination therapy.
Keywords: Amoxicillin, clarithromycin, Helicobacter pylori, metronidazole
INTRODUCTION
Helicobacter pylori (H. pylori) is a gram negative, spiral, rod-shaped, and flagellated bacteria that colonize in human stomach. This bacterium is one of the most common infectious diseases in the world that colonizes about 50% of the world's population. H. pylori causes gastritis, 90% of stomach ulcers, 75% of duodenal ulcers, and risk factor for gastric lymphoma and adenocarcinoma in Asia, Europe, and North America.[1,2,3,4] This infection is usually acquired in childhood by transmission within families and in most cases remains for all lifetime unless removed by antibiotic treatment. Prevalence of H. pylori infection varies from 25% in developed countries to over 80% in developing countries.[5,6,7]
Prevalence of H. pylori antibiotic resistance is different in various geographical areas based on the date. Although H. pylori is sensitive to the antimicrobial agents in vitro, but removing the organism in the body is difficult. Treatment of infection can be done with multidrug includes a combination of clarithromycin, metronidazole, or amoxicillin plus a proton pump inhibitor (PPI), for example, omeprazole. Clarithromycin and metronidazole antibiotics are frequently used for the treatment of H. pylori infection. But the appearance of antibiotic resistance during treatment of H. pylori infection is the major cause of failure in the eradication of infection.[8,9,10]
The purpose of this study was to determine the prevalence of clarithromycin, metronidazole, and amoxicillin resistance among the H. pylori isolates obtained from patients with gastrointestinal disorders.
MATERIALS AND METHODS
Patients and samples
Gastric antrum biopsy specimens were prepared from 260 patients who referred to Endoscopy Section of the Isfahan Hospitals from March 2011 to July 2012. The patients suffered from gastritis, gastric ulcer, duodenal ulcer, and gastric cancer. The patients who received antibiotics were excluded from this study. A part of the biopsy sample was tested for a rapid urease test and the other section of sample transported in Brain Heart Infusion Broth (Merck, Germany) supplemented with 2% of glucose (Merck, Germany) for culturing and biochemical tests.
Bacteria and culture conditions
Biopsy specimens in Brain Heart Infusion Broth, were crushed between two sterile glass slides and then cultured on Brucella agar supplemented with 5% human blood, 7% fetal calf serum (FCS; Bahar Afshan-Iran), vancomycin (2 mg/l; Merck, Germany), polymyxin B (0.05 mg/l; Merck, Germany), L-cysteine 2% (Merck, Germany), and trimethoprim (1 mg/l; Merck, Germany), and amphotericin B (5 mg/l). Then the plates in the microaerophilic atmosphere (6% O2, 10% CO2, and 84% N2) and at 37°C were incubated for 3-5 days in MART system (Anoxomat, Lichtenvoorde, Netherlands). Growth bacteria were identified as H. pylori based on colony morphology, gram staining, and positive biochemical reactions such as urease, catalase, and oxidase. Positive clinical isolates were stored at -80°C in Brucella broth supplemented with 20% glycerol and 7% fetal calf serum (FCS) until susceptibility.
Antibiotic susceptibility test
Modified Disk Diffusion Method (MDDM) was selected to assess the sensitivity of H. pylori strains to three antibiotic clarithromycin (15 mg), metronidazole (5 mg), and amoxicillin (10 mg) (HIMEDIA, India). For this purpose, suspensions of bacteria were prepared in the sterile saline (2 ml) equivalent to standard 3 McFarland. The suspension was spread on Brucella agar supplemented with 5% human blood and 7% FCS (Bahar Afshan, Iran). When plates dried, antibiotic disks were placed and incubated in microaerophilic atmosphere at 37°C for 3-5 days. Susceptibility results were recorded as resistant according to the following interpretive criteria by Clinical and Laboratory Standards Institute (CLSI) guideline; for clarithromycin, no zone of growth inhibition; for metronidazole, a zone <16 mm, and for amoxicillin a zone <11 mm.[9]
Minimum inhibitory concentration (MIC)
For this purpose, suspensions of pure bacteria were prepared in sterile saline (2 ml) equivalent to standard 3 McFarland. The suspension was spread on Brucella agar supplemented with 5% human blood and 7% FCS (Bahar afshan, Iran). The MIC of H. pylori to antibiotics was evaluated by E-test strips. H. pylori strains were considered resistance to clarithromycin, metronidazole, and amoxicillin if they were MIC ≥1 μg/ml, MIC >4 μg/ml and MIC >1 μg/ml (Abbiodisk, Solana, Sweden).[11]
RESULTS
In the present study, during March 2011-July 2012, 260 antral biopsies of patients with gastritis and peptic ulcer diseases were isolated. The patients age was between 14 and 89 years (45.8 ± 17.8 (14-89)). Male/female ratio was 121/139; history of infection in family members was 127 (48.8%); previous infection with H. pylori was 82 (31.5%); gastritis and peptic ulcer diseases were present in 91 (35%) and 34 (13%), respectively; and current infection with H. pylori and positive urease test were 78 (30%) (*data presented as mean ±standard deviation (SD) and number (present)).
Among all patients who underwent endoscopy, involved 139 women whose ages were 14-88 years (average age: 51) and 121 men whose ages were 18-89 years old (average age: 53.5).
Of 139 female patients, 52 (37.4%) had gastritis and 19 (13.6%) had peptic ulcer disease. Of 121 male patients, 39 (32.2%) had gastritis and 15 (12.3%) had peptic ulcer disease.
In our study, susceptibility tests were performed by MDDM. Twelve of 78 (15.3%) H. pylori isolates resistant to clarithromycin, 43 of 78 (55.1%) H. pylori isolates resistant to metronidazole, and five of 78 (6.4%) H. pylori isolates were resistant to amoxicillin.
In this study, of 12 clarithromycin resistant patients, seven (58.3%) were females and five (41.6%) males. Of 43 metronidazole resistant patients, 32 (74.4%) were females and 11 (25.5%) males. Of five amoxicillin resistant patients, four (80%) were females and one (20%) was male. Also of 12 clarithromycin resistant patients, eight of 12 (66.6%) were diagnosed with gastritis and four of 12 (33.3%) were diagnosed with peptic ulcer disease. Of 43 metronidazole resistant patients, 31 of 43 (72%) were diagnosed with gastritis and 12 of 43 (28%) were diagnosed with peptic ulcer disease; and of five amoxicillin resistant patients, four of five (80%) were diagnosed with gastritis and one of five (20%) were diagnosed with peptic ulcer disease.
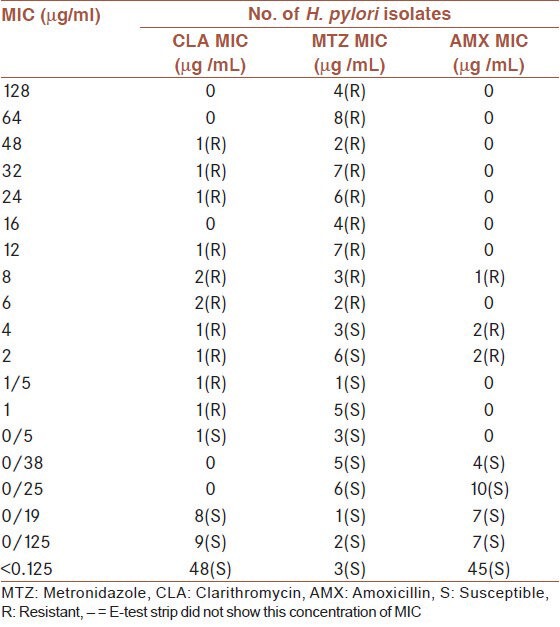
As shown in Table 1, in this study the MIC tested for isolates of three antibiotics were 0.125-256 μg/ml according to E-test strip (Abbiodisk, Solana, Sweden). The MIC 12 of isolates resistant to clarithromycin, had MIC = 1-48 μg/ml and 43 isolates resistant to metronidazole, had MIC = 6-128 μg/ml and five isolates resistant to amoxicillin had MIC = 2, 4 and 8 μg/ml [Table 1].
Table 1.
Minimal inhibitory concentration (MIC) of the three antibiotics (CLA, AMO, MET) for 78 isolates of H. pylori by E-test
DISCUSSION
H. pylori antibiotic resistance is rising in the world, so awareness about the prevalence of antibiotic resistant strains and determining the outcome of treatment is important.[12,13] In Iran with extremely high rate of H. pylori infection (more than 80%), antibiotic resistance to various antimicrobials is considered as the major cause of the H. pylori treatment failure.[7,8]. On the other hand, the low eradication rate and a considerable reinfection rate (20%) indicated the significance of controlling H. pylori infection as an important health problem. Prevalence of antibiotic resistance of H. pylori varies in different geographical areas and is associated with the consumption of antibiotics in those areas.[9] In this study, we investigated the H. pylori resistance to metronidazole, clarithromycin, and amoxicillin in 260 biopsy specimens in patients with gastritis and peptic ulcers disorder by MDDM and E-tests. In this study, of 12 clarithromycin resistant patients, eight of 12 (66.6%) were diagnosed with gastritis and four of 12 (33.3%) were diagnosed with peptic ulcer disease. Of 43 metronidazole resistant patients, 31 of 43 (72%) were diagnosed with gastritis and 12 of 43 (28%) were diagnosed with peptic ulcer disease; and of five amoxicillin resistant patients, four of five (80%) were diagnosed with gastritis and one of five (20%) were diagnosed with peptic ulcer disease. Among the macrolide antibiotics, clarithromycin has the main role for H. pylori eradication.[14] Clarithromycin is a bacteriostatic antibiotic that reversibly connected to domain V of the 23SrRNA genes in the bacterial ribosome subunit 50s and avoid of protein elongation.[15,16] The rate of resistance to clarithromycin was different in various times and location of Iran. The resistance rate to clarithromycin in this study was 15.3% and is comparable with the other studies from Aslani et al., (14%), Siavoshi et al., (14.5%), Mohammadi et al., (16.7%), and Mirzaei et al., (14.6%).[17,18,19,20] However, the resistance rate to clarithromycin in our study was higher than those reported by Sirous et al., (0%), Fallahi et al., (4.16%), and Khashei et al., (6.25%);[2,13,21] and lower than those reported by Falsafi et al., (21%), Haghi Tomatari et al., (23%), and Kargar et al., (22.6%).[9,14,22] It showed an increasing trend in Isfahan (15.3%). Clarithromycin resistance was greater in Europe 11.1%, Asia 18.1%, and America 29.3%. Among European countries, Spain with 49.2% had highest resistance to clarithromycin and Sweden with 1.5% had the lowest one. Among Asian countries, Japan with 40.7%, showed the highest and Malaysia with 2.1% the lowest resistance to clarithromycin.[12] Several countries reported the rate of clarithromycin resistance as follows: Hong Kong 4.5%, Korea 5–6%, and Brazil 8%.[23,24,25,26] Clarithromycin is an expensive antibiotic, which was used limited in Iran until 2000. It seems that production of antibiotics and its excessive use in treatment of respiratory tract infections in children has an important role in H. pylori resistance to clarithromycin in our area. In this study, clarithromycin resistance in H. pylori isolates of patients with gastritis was higher than patients with peptic ulcer (eight versus four). Resistance of bacteria to nitroimidazoles which include metronidazole have been between 20 and 95%, which is the highest rate of resistance among the other antibiotics.[27] Metronidazole resistance occurs by rdxA gene (which encodes an oxygen-insensitive NADPH nitroreductase), inactivation of frxA (NADPH flavin oxidoreductase), fdxB (ferrodoxin-like protein). The mechanism of intrinsic metronidazole resistance is related to the decreasing drug uptake or increased drug efflux.[9,27] H. pylori resistance to metronidazole in developed countries is about 35% and in some areas almost all strains resistant to metronidazole.[14,16] We found that the H. pylori resistance to metronidazole was 55.1% in agreement with other studies, Fallahi et al., (54.16%), Sirous et al., (51.5%), Siavoshi et al., (55.6%), Mohamadi et al., (57%), and Mirzaei et al., (56.3%).[2,13,19,20,28] In this study, resistance to metronidazole was higher than those reported by Khashei et al., (30%).[21] It was lower than those reported by Rafeey et al., (95%) and Haghi Tomatari et al., (64%).[9,29]
Resistance of H. pylori to metronidazole in adults in different parts of the world was as follows: France 31.5%, USA 33.9%, Brazil 53%, Korea 41.9%, and Singapore 31.7%.[30] Metronidazole is an antibiotic which is used commonly in Iran to treat anaerobic bacterial infections, parasitic and genital infections. So the overuse of antibiotics can increase the resistance of bacteria to this antibiotic. In agreement with clarithromycin, metronidazole resistance in H. pylori isolates of patients with gastritis was higher than patients with peptic ulcer (31 versus 12).
Amoxicillin is a bactericidal antibiotic that belongs to the penicillin group of antibiotics. This antibiotic binds to penicillin binding proteins (PBPs) and interferes with bacterial cell wall synthesis.[27] Frequent use of amoxicillin and transfer of horizontal gene in H. pylori and mutation in Pbp-1A gene is involved in resistance to this antibiotic.[13,28] Increasing rate of H. pylori resistance to amoxicillin in different geographical areas might be due to obtaining the antibiotics without prescription.[31] In our study, H. pylori resistance to amoxicillin was 6.4%. Similar results in agreement with our study have shown by Siavoshi et al., (7.3%) and Fallahi et al., (8.3%).[2,18] Resistance to amoxicillin in this study was higher than those reported by Khashei et al., (2.5%), Haghi Tomatari et al., (2.5%), and Mirzaei et al., (4.2%)[9,20,21] and lower than those reported by Kohanteb et al., (20.8%) and Farshad et al., (20%).[32,33] Resistance of H. pylori to amoxicillin in adults in different parts of the world was as follows: France (0%), Portugal (0%), Sweden (0%), Japan (0%), and Poland (0%).[30,34,35] In this studyΈ we had one MDR isolates from patient with gastritis and peptic ulcer disease which is an alarm for more attention.
In conclusion, H. pylori resistance to clarithromycin, metronidazole, and amoxicillin in our study was higher than the former studies in Isfahan. It is indicative that the rate of resistance to those antibiotics is increasing. MDR strains is emerging and will have an effect on the combination therapy. Comparing this study with previous studies has indicated that H. pylori resistance may change with period even in the same population; however, in order to prevent antibiotic resistance and to determine the most effective anti H. pylori regimen, continuous surveillances is necessary.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088–94. doi: 10.3201/eid1006.030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fallahi GH, Maleknejad S. Helicobacter pylori culture and antimicrobial resistance in Iran. Indian J Pediatr. 2007;4:127–30. doi: 10.1007/s12098-007-0003-4. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–41. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay CY, Mitchell H, Dong Q, Goh KL, Dawes IW, Lan R. Population structure of Helicobacter pylori among ethnic groups in Malaysia: Recent acquisition of the bacterium by the Malay population. BMC Microbiol. 2009;9:126. doi: 10.1186/1471-2180-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghasemian Safaei H, Tavakkoli H, Mojtahedi A, Salehei R, Soleimani B, Pishva E. Correlation of cagA positive Helicobacter pylori Infection with clinical outcomes in Al-zahra hospital, Isfahan, Iran. J Res Med Sci. 2008;13:196–201. [Google Scholar]
- 6.Hosseini E, Poursina F, de Wiele TV, Safaei HG, Adibi P. Helicobacter pylori in Iran: A systematic review on the association of genotypes and gastroduodenal diseases. J Res Med Sci. 2012;17:280–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Kumala W, Rani A. Patterns of Helicobacter pylori isolate resistance to fluoroqinoloes, amoxicillin, clarithromycin and metronidazoles. Southeast Asian J Trop Med Public Health. 2006;37:970–4. [PubMed] [Google Scholar]
- 8.Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621–8. doi: 10.1128/aac.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haghi Tomatari F, Mohabbati Mobarez A, Amini M, Hosseini D, Talebi Bezmin Abadi A. Helicobacter pylori resistance to metronidazole and clarithromycin in dyspeptic patients in Iran. Iran Red Crescent Med J. 2010;12:409–12. [Google Scholar]
- 10.Debets-Ossenkopp YJ, Sparrius M, Kusters JG, Kolkman JJ, Vandenbroucke-Grauls CM. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett. 1996;142:37–42. doi: 10.1111/j.1574-6968.1996.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 11.Solna, Sweden: AB Biodisk; 2000. E-test technical guide 8: Susceptibility testing of Helicobacter pylori. [Google Scholar]
- 12.De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, et al. Worldwide H. pylori antibiotic resistance: A systematic. J Gastrointestin Liver Dis. 2010;19:409–14. [PubMed] [Google Scholar]
- 13.Sirous M, Mehrabadi JF, Daryani N, Eshraghi S, Hajikhani S, Shirazi M. Prevalence of antimicrobial resistance in Helicobacter pylori isolates from Iran. Afr J Biotechnol. 2010;9:5962–5. [Google Scholar]
- 14.Falsafi T, Mobasheri F, Nariman F, Najafi M. Susceptibilities to different antibiotics of Helicobacter pylori strains isolated from patients at the pediatric medical center of Tehran, Iran. J Clin Microbiol. 2004;42:387–9. doi: 10.1128/JCM.42.1.387-389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia HX, Fan XG, Talley NJ. Clarithromycin resistance in Helicobacter pylori and its clinical relevance. World J Gastroenterol. 1999;5:263–6. doi: 10.3748/wjg.v5.i3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Q, Li Y, Zhang ZJ, Liu Y, Gao H. New mutation points in 23S rRNA gene associated with Helicobacter pylori resistance to clarithromycin in northeast China. World J Gastroenterol. 2004;10:1075–7. doi: 10.3748/wjg.v10.i7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shokrzadeh L, Jafari F, Dabiri H, Baghaei K, Zojaji H, Alizadeh A, et al. Antibiotic susceptibility profile of Helicobacter pylori isolated from the dyspepsia patients in Tehran, Iran. Saudi J Gastroenterol. 2011;17:261–4. doi: 10.4103/1319-3767.82581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siavoshi F, Pourkhajeh AH, Merat S, Asl-Soleimani H, Heydarian E, Khatibian M, et al. Susceptibility of various strains of Helicobacter pylori to selected agents. Arch Iran Med. 2000;3:60–3. [Google Scholar]
- 19.Mohammadi M, Doroud D, Mohajerani N, Massarrat S. Helicobacter pylori antibiotic resistance in Iran. World J Gastroenterol. 2005;11:6009–13. doi: 10.3748/wjg.v11.i38.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzaei N, Poursina F, Faghri J, Talebi M, Khataminezhad MR, Hasanzadeh A, et al. Prevalence of resistance to Helicobacter pylori strains to selected antibiotics in Isfahan, Iran. J J Microbiol. 2013;6:e6342. [Google Scholar]
- 21.Naser D. Genetic diversity and drug resistance of Helicobacter pylori strains in Isfahan, Iran. Iran J Basic Med Sci. 2008;11:174–82. [Google Scholar]
- 22.Kargar M, Baghernejad M, Doosti A, Ghorbani S. Clarithromycin resistance and 23S rRNA mutations in Helicobacter pylori isolates in Iran. Afr J Microbiol Res. 2011;5:853–6. [Google Scholar]
- 23.Ahmad N, Zakaria WR, Abdullah SA, Mohamed R. Characterization of clarithromycin resistance in Malaysian isolates of Helicobacter pylori. World J Gastroenterol. 2009;15:3161–5. doi: 10.3748/wjg.15.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling TK, Leung WK, Lee CC, Ng EK, Yung MY, Chung MY, et al. The antimicrobial susceptibility of Helicobacter pylori in Hong Kong (1997-2001) Helicobacter. 2002;7:327–8. doi: 10.1046/j.1523-5378.2002.00101_1.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates form Korea. J Antimicrob Chemother. 2001;47:459–61. doi: 10.1093/jac/47.4.459. [DOI] [PubMed] [Google Scholar]
- 26.Andrzejewska E, Szkaradkiewicz A, Karpinski T. Antimicrobial resistance of Helicobacter pylori clinical strains in the last 10 years. Pol J Microbiol. 2009;58:301–5. [PubMed] [Google Scholar]
- 27.Gerrits MM, van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: Molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6:699–709. doi: 10.1016/S1473-3099(06)70627-2. [DOI] [PubMed] [Google Scholar]
- 28.Siavoshi F, Saniee P, Latifi-Navid S, Massarrat S, Sheykholeslami A. Increase in resistance rates of H. pylori isolates to metronidazole and tetracycline-comparison of three 3-year studies. Arch Iran Med. 2010;13:177–87. [PubMed] [Google Scholar]
- 29.Rafeey M, Ghotaslou R, Nikvash S, Hafez AA. Primary resistance in Helicobacter pylori isolated in children from Iran. J Infect Chemother. 2007;13:291–5. doi: 10.1007/s10156-007-0543-6. [DOI] [PubMed] [Google Scholar]
- 30.Megraud F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut. 2004;53:1374–84. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendonca S, Ecclissato C, Sartori MS, Godoy AP, Guerzoni RA, Degger M, et al. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxicillin, tetracycline, and furazolidone in Brazil. Helicobacter. 2000;5:79–83. doi: 10.1046/j.1523-5378.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 32.Kohanteb J, Bazargani A, Saberi- Firoozi M, Mobasser A. Antimicrobial susceptibility testing of Helicobacter pylori to selected agents by agar dilution method in Shiraz-Iran. Indian J Med Microbiol. 2007;25:374–7. doi: 10.4103/0255-0857.37342. [DOI] [PubMed] [Google Scholar]
- 33.Farshad S, Alborzi A, Japoni A, Ranjbar R, Hosseini Asl K, Badiee P, et al. Antimicrobial susceptibility of Helicobacter pylori strains isolated from patients in Shiraz, Southern Iran. World J Gastroenterol. 2010;16:5746–51. doi: 10.3748/wjg.v16.i45.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimoyama T, Fukuda S, Mikami T, Fukushi M, Munakata A. Efficacy of metronidazole for the treatment of clarithromycin-resistant Helicobacter pylori infection in a Japanese population. J Gastroenterol. 2004;39:927–30. doi: 10.1007/s00535-004-1424-8. [DOI] [PubMed] [Google Scholar]
- 35.Dzierzanowska-Fangrat K, Rozynek E, Celińska-Cedro D, Jarosz M, Pawłowska J, Szadkowski A, et al. Antimicrobial resistance of Helicobacter pylori in Poland: A multicentre study. Int J Antimicrob Agents. 2005;26:230–4. doi: 10.1016/j.ijantimicag.2005.06.015. [DOI] [PubMed] [Google Scholar]


