Abstract
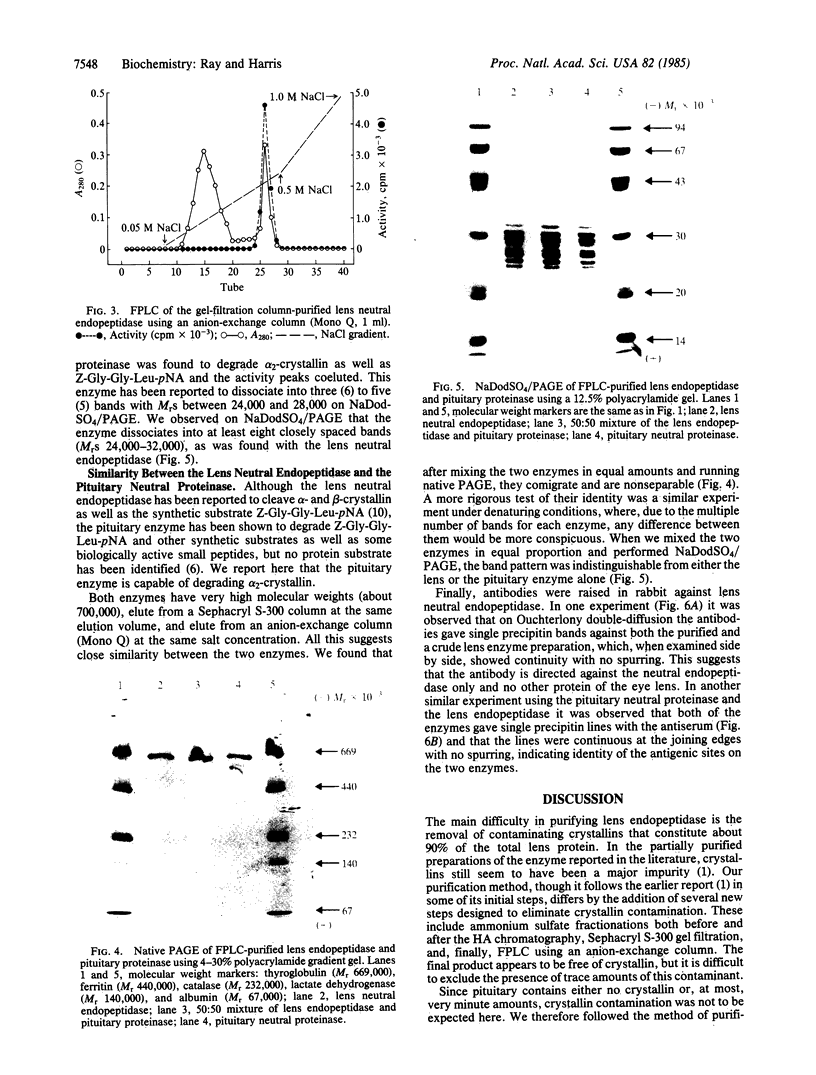
A neutral endopeptidase (EC 3.4.24.5) that degrades alpha- and beta-crystallins occurs in mammalian lens. A procedure for purification of this enzyme from bovine lens is described. The enzyme appears to have a high molecular weight (Mr approximately equal to 700,000) and under denaturing conditions dissociates into at least eight polypeptide subunits with Mrs ranging from 24,000 to 32,000. A neutral proteinase in bovine pituitary has been reported previously to have similar structural characteristics. We have found that this enzyme purified from bovine pituitary is indistinguishable in molecular weight and in subunit composition from bovine lens endopeptidase. In addition, antiserum raised in rabbit against the purified lens enzyme crossreacts with bovine pituitary enzyme. When examined side by side in Ouchterlony double-diffusion tests, the two enzymes give a continuous precipitin line with no spurring. It is concluded that lens neutral endopeptidase and pituitary neutral proteinase are structurally closely similar, if not identical. This is a surprising result because it had been thought previously that the lens endopeptidase was unique to lens, where its crystallin substrates comprise a large proportion of the total tissue protein. In other tissues, crystallin is either absent or occurs, at most, in trace amounts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blow A. M., Heyningen R. V., Barrett A. J. Metal-dependent proteinase of the lens. Assay, purification and properties of the bovine enzyme. Biochem J. 1975 Mar;145(3):591–599. doi: 10.1042/bj1450591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., van Heyningen R. Neutral proteinase activity in the human lens. Exp Eye Res. 1976 Mar;22(3):251–257. doi: 10.1016/0014-4835(76)90052-x. [DOI] [PubMed] [Google Scholar]
- Wagner B. J., Fu S. C., Margolis J. W., Fleshman K. R. A synthetic endopeptidase substrate hydrolyzed by the bovine lens neutral proteinase preparation. Exp Eye Res. 1984 May;38(5):477–483. doi: 10.1016/0014-4835(84)90125-8. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme. J Neurochem. 1980 Nov;35(5):1172–1182. doi: 10.1111/j.1471-4159.1980.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983 Mar;40(3):842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]
- van Heyningen R. Experimental studies on cataract. Invest Ophthalmol Vis Sci. 1976 Sep;15(9):685–697. [PubMed] [Google Scholar]