Abstract
Background:
This study aims at investigating the possible effects of different daily doses of green tea (GT) intake for eight weeks on certain anthropometric, metabolic, and oxidative stress biomarkers of diabetic patients.
Materials and Methods:
This randomized clinical trial included 63 patients with type 2 diabetes (30 males and 33 females). After a two-week run-in period without green tea, they were randomly assigned into one of the three groups, with a different daily intake of green tea; four cups of green tea per day (n = 24), two cups of green tea per day (n = 25), and the control group (n = 14) with no green tea intake for two months. At baseline and after the intervention, blood tests, dietary, and anthropometric variables were assessed. The patients were instructed to maintain their usual dietary intake and normal physical activity.
Results:
Consumption of four cups of GT per day caused a significant decrease in body weight (73.2 to 71.9) (P < 0.001), body mass index (27.4 to 26.9) (P < 0.001), waist circumference (95.8 to 91.5) (P < 0.001), and systolic blood pressure (126.2 to 118.6) (P < 0.05) in this group. No significant change was seen in the other groups and between group comparisons. The metabolic and oxidative stress parameters did not show any significant differences within and between groups.
Conclusion:
Drinking four cups of green tea led to a significant reduction in weight and systolic blood pressure.
Keywords: Anthropometric indices, green tea, metabolic indices, Type 2 diabetes
INTRODUCTION
The occurrence of type 2 diabetes mellitus (T2DM) is increasing worldwide, particularly in developing countries, where it was previously much lower. It is estimated that by 2030, about 439 million adults will be diabetic.[1] In the United States, the prevalence of T2DM has doubled during the past 15 years. Also, diabetes is associated with an increased risk of cardiovascular diseases (CVD) accounting for approximately 80% of all diabetic deaths.[2] Abnormal lipid metabolism, glycation, and oxidative stress may play some role in the development of long-term diabetic complications.[3] Obesity also complicates the management of T2DM by increasing insulin resistance and blood glucose concentrations.[4] It is an independent risk factor for dyslipidemia, hypertension, and CVD.[5] Obesity at all ages increases diabetes prevalence and doubles it in Iranian women.[5] In T2DM patients, weight loss is significantly correlated with glycemic control. Losing more than 5% of their body weight significantly helps to keep the glycosylated hemoglobin lower.[6] The Third Report of the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) reaffirms that in the primary prevention of coronary heart disease, diet therapy is the initial recommended intervention.[7]
A rapidly growing area of using natural herbs to control metabolic problems has, therefore, attracted the researchers’ attention. Tea is the most popular beverage in the world after water. It is generally consumed in forms of green, oolong, and black tea, and all of them originate from the leaves of Camellia sinensis.[8] Among them green tea (GT) is an excellent source of phenolic antioxidants including catechins and epigallocatechin 3-gallate (EGCG).[9] A great deal of scientific interest has been focused on the beneficial health effects of green tea. Epidemiological surveys have provided evidence that heavy GT drinking populations like the Japanese have a lower risk of T2DM[10] as also lower mortality rates of coronary heart disease (CHD).[11] Green tea extract, rich in EGCG, has a beneficial effect on glucose control and weight management[12,13,14,15] and it has hypotensive effects[16] (1) GT catechins have been shown to stimulate thermogenesis,[17] hepatic β-oxidation,[18,19] and hypolipidemic activity by decreasing the intestinal absorption of lipids.[20] High intake of tea may reduce the risk of coronary disease because of the lowering of low-density lipoprotein (LDL) oxidation.[21] All of these effects may help diabetic patients to prevent long-term complications.
However, there have been few clinical trials of the effects of GT on diabetic patients. The aim of the present study is to evaluate the possible effects of different daily doses of GT intake for eight weeks on certain metabolic, oxidative stress, and anthropometric biomarkers in patients with T2DM.
MATERIALS AND METHODS
Subjects
The volunteers were recruited from the Gabric Diabetes Education Institute. Before inclusion, blood samples were drawn, to choose subjects with a fasting blood glucose (FBG) of 126 to 180 mg / dl. The other inclusion criteria were age (35 to 65 years), body mass index (BMI) (25 to 35 kg / m2), and willingness to participate in the study. Subjects with insulin therapy and history of stroke, cancer, or clinical diabetic nephropathy were excluded. Subjects who had to change their medications during the study were excluded. The study protocol was approved both ethically and scientifically by the Human Research Committee, Tehran University of Medical Sciences, and all the subjects had given their informed consent to participate. Finally, based on the mean difference of 1.2 nmol / l of Malondialdehyde (MDA) in the intervention group, a power of 80%, and a confidence interval of 95%, we calculated the sample size in accordance with our independent criteria (20 participants in each group, in addition to probable missing we tried to have 25 participants).
Study Design
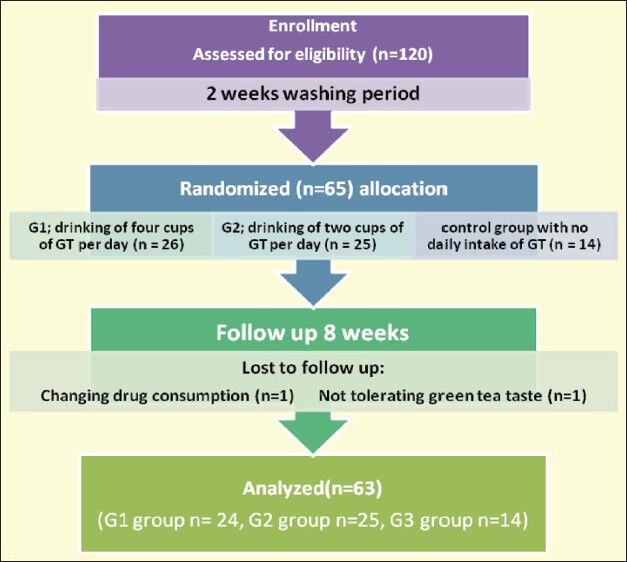
It was a randomized, parallel, clinical trial consisting of a two-week run-in period and an eight-week treatment period. After the run-in period, the participants were allocated into one of the three groups: G1, drinking four cups of GT per day (n = 26), G2, drinking two cups of GT per day (n = 25), and the control group with no daily intake of GT (n = 14). Randomization was done by entering the participants into the trial and randomly allocating them into three groups [Figure 1]. The participants in all groups were allowed to drink one cup of black tea a day, thus establishing a consistent baseline level. People in the G1 and G2 groups were instructed to prepare green tea using 2.5 g tea bags in 200 ml of boiled water for five minutes. All of volunteers had nutritional education, and supplemental food products or medications known to influence carbohydrate or lipid metabolism were prohibited. They were instructed to maintain their usual dietary intake and physical activity.
Figure 1.
Anthropometric Assays and Measurement of Circulatory Parameters
Body weight was measured with a digital balance (Beurer, Germany) to the nearest 0.1 kg, with light clothing, in a fasted state, and after voiding of the bladder. The height was measured using a wall-mounted stadiometer (Seca, Germany) to the nearest of 0.1 cm. The body mass index (BMI) was calculated using the equation weight (kg)/height2 (m). The waist circumference was measured by an elastic meter to the nearest of 0.1 cm. Blood pressure was measured with a regular mercury manometer and the mean of two consecutive measurements, with a 15-minute interval, was considered as the blood pressure of each subject. All anthropometric assessments were done on the first day and at the end of the intervention, in all three groups. Dietary records were taken by a 24-hour recall questionnaire for evaluating the probable changes in diets.
Biochemical Assays
Fasting blood samples were taken before and after the intervention from all participants by venipuncture and then transferred to clean tubes either with or without an anticoagulant, like Ethylenediaminetetraacetic acid (EDTA), to recover plasma or serum, respectively. Blood glucose and lipid profile analyses were performed just on the day of sampling. Fasting blood glucose (FBG) was measured using the colorimetric method. Biochemical analysis of the serum total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, Apo A1, and Apo B100 was carried out on a Selectra E auto analyzer (Vita Laboratory, Netherlands) following standard procedures of the Pars Azmoon diagnostic kits (Iran). The LDL cholesterol was calculated using the Friedewald formula.[22] Plasma MDA concentration was measured using thiobarbituric acid reactive substances (TBARS) as described originally[23] with minor modifications.[24] Plasma total antioxidant capacity (TAC) was determined by the ferric reducing ability of plasma (FRAP) assay.[25]
Statistical Analysis
The analysis of consumed foods was carried out by using the Nutritionist III software, modified for Iranian foods, and statistical analysis was performed using the Statistical Package for Social Sciences (SPSS 16.0 for Windows, SPSS Inc. Headquarters, Chicago, USA). The values were expressed as means (standard deviation). The Kolmogorov-Smirnov test checked the normality of blood and anthropometric variables in every group. The results were expressed either in actual values or changes from week 0 to week 8. Baseline and treatment values were compared using the paired t-test in each group. For analyzing the changes in the dependent parameters of the three groups after the intervention, we used the one-way analysis of variance (ANOVA). A P-value less than 0.05 was considered to be significant.
RESULTS
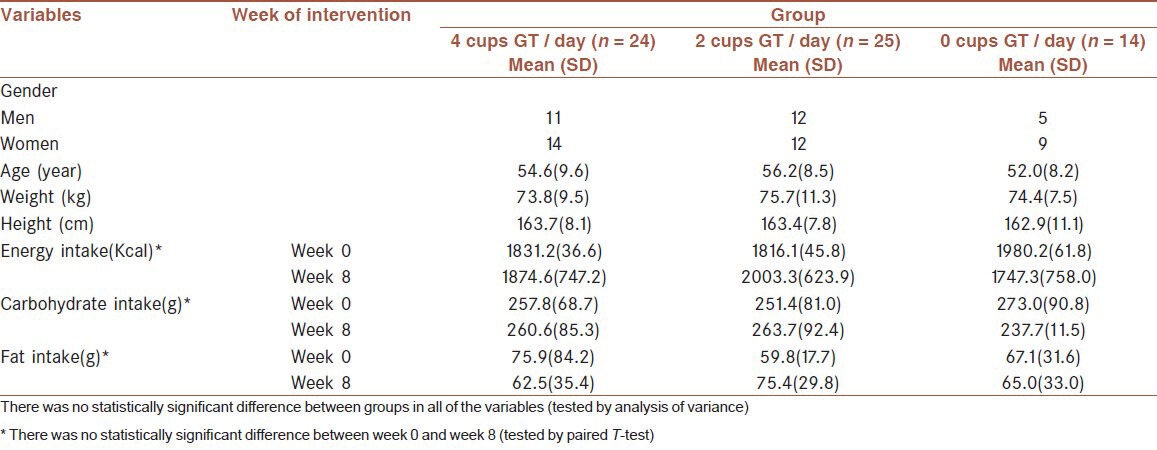
A total of 65 T2DM patients (30 men, 35 women) started the intervention, but two participants were excluded from the group having four cups of GT per day, because of their changing drug consumption and not tolerating the taste of green tea. The study was completed by 63 patients (30 men and 33 women). The mean and standard deviation (SD) of age and BMI of 63 patients were 54.6 (8.9) years and 27.9 (3.0) kg / m2, respectively. The normality of data in every group was confirmed. There was no significant difference in energy and nutrient intake both in the beginning and at the end of the intervention period between the three groups [Table 1].
Table 1.
Characteristics of sixty-five volunteers who participated in the study
Anthropometric Data: There were no significant differences in any of the initial anthropometric parameters between the three groups; 46.4% of the men and 65.7% of the women were overweight, and 28.6% men and 28.6% women were obese (BMI > 30). Therefore, most of the women and more than two-thirds of the men were overweight or obese. The BMI decreased in both the G1 and G2 groups, although only in G1 it was statistically significant (P < 0.001). At the end of the intervention period, the waist circumference also showed a significant decrease in both the G1 and G2 groups (P = 0.001) compared to the baseline measures. However, the magnitude of the decrease in the G1 group was greater than in the G2 group, but the between-group difference was not significant [Table 2].
Table 2.
Body and blood characteristics of subjects of three groups, before and after the intervention
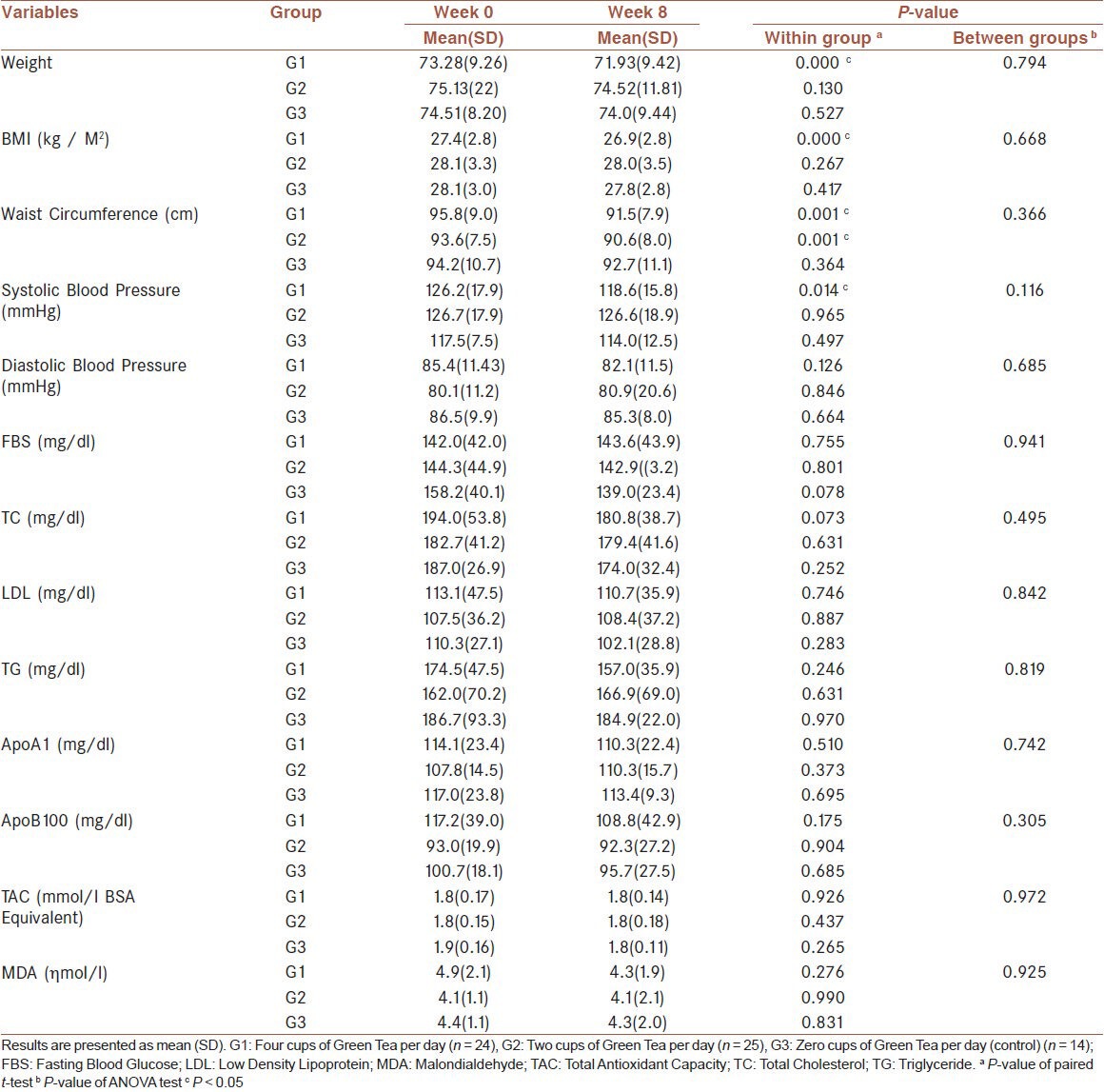
Blood Pressure: Systolic blood pressure decreased in all three groups, but only the change in the G1 group (26.2 to 118.6 mmHg) was significant (P < 0.01). No significant changes in diastolic blood pressure were observed in the three groups [Table 2].
Plasma Variables: Table 2 shows laboratory findings before and after the intervention. No significant difference was seen in the initial values of the biochemical parameters between the three groups. No significant difference was found in the serum FBG levels or lipid profiles, including, serum total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol, throughout the intervention. Apo A1 and Apo B100 did not show a significant difference before or after the intervention, between the three groups.
Meanwhile, there was no significant difference in the serum TAC and MDA levels within the groups at baseline or after two months of intervention. However, in the G1 group, there was a decreasing trend in the serum MDA level, but it was not significant.
DISCUSSION
The effects of green tea consumption on the anthropometric and biochemical parameters of patients with type 2 diabetes were investigated in an interventional trial, without changing the usual lifestyle of the participants. In the nutritional assessments, there were no significant differences among the three groups in the intake of energy, fat, carbohydrate, and other dietary factors. In each group the usual dietary and exercise habits were maintained throughout the trial. Compliance of GT and adherence to the intervention was good because of their enthusiasm for herbal medication.
The results suggest that drinking four cups of green tea per day decreased body weight, waist circumference, and the systolic blood pressure of these patients [Table 2]. Our results support the findings of several observational studies that indicate an association between green tea consumption and weight control[12,26,27,28] and waist circumference reduction.[29] It is consistent with animal experiments that demonstrate the weight reduction effect of GT.[18] The plasma total cholesterol decreased in all groups, but to a greater extent in GT drinkers, although it was not significant.
Previous studies show that EGCG, the most effective ingredient of GT, could increase thermogenesis and 24-hour energy expenditure by about 4%.[30] It increases fat oxidation[31] and prolongs sympathetic stimulation of thermogenesis by the inhibition of catechol O-methyltransferase activity.[19,32,33] Some previous studies examined the effect of EGCG on weight control. Chantre et al. used the EGCG extract in moderately obese patients and after three months their body weight decreased by 4.6% and waist circumference decreased by 4.4 percent.[32] As our patients were overweight too, in the four cups group of GT drinkers, the weight decreased by 1.8% and waist circumference decreased by 4.4%. Nagao et al. used GT extract drinks for three months in healthy Japanese men with a slightly lower energy diet.[34] The subjects’ weight, waist circumference, and body fat mass decreased by 1.5, 2.0, and 3.7%, in comparison to the placebo group. In different studies the amount of tea consumed, its variety, and the physiological status of the subjects have great effects on the result.[35] We used brewed GT leaves (not extract) so the amount of ingested polyphenols was lower, and hence, we predicted less significant results.
High systolic blood pressure is an important parameter of the metabolic syndrome, established by the National Cholesterol Education Program for Americans.[36] Results from both cohort studies and randomized trials suggest that antioxidant-rich foods like green tea (source of flavan-3-ols) may lower the risk of type 2 diabetes and cardiovascular risk factors like blood pressure.[37] The hypotensive effect of GT has been studied. Nagao et al. showed that drinking GT containing 583 mg catechins in obese Japanese women for three months decreases SBP, especially in hypertensive ones.[26] Yang et al. have shown that consumption of GT for a long time has a good effect on the BP of old women and clearly decreases BP in hypertension.[38] We found the same hypotensive results. On account of the nutritional education before the intervention, a decreasing change was seen in all three groups, but it was significant only in the group drinking four cups of GT, which showed that besides nutritional education, using herbal medications like GT helped. As in the animal studies GT polyphenols decreased BP by improving the endothelial function and inhibiting left ventricular hypertrophy.[39]
Cohort studies showed many beneficial effects of drinking GT, in diabetic patients.[14] Also in streptozotocin-induced diabetic rats, EGCG affected glucose tolerance, insulin sensitivity, and insulin secretion,[40,41,42] but in most of the human clinical studies,[43,44,45] like ours, GT did not have any effect on blood glucose. The blood lipid lowering mechanism of green tea, especially in hypercholesterolemic animal models included, reducing micellar solubility and intestinal absorption of cholesterol and reducing hepatic cholesterol concentration.[46] Using GT water extract in streptozotocin-diabetic rats decreased blood lipids, especially cholesterol, and this decreasing effect was dose-dependent.[47,48] In human studies, using a daily capsule containing theaflavin-enriched GT extract (375 mg) with a low-fat diet in mild-to-moderate hypercholesterolemic adults decreased their total cholesterol, LDL-C, HDL-C, and triglyceride levels,[49] but in other newer studies using even one year of GT extract capsules without a diet showed no significant change in the lipid profile of diabetic patients.[29] In our study, after two months of drinking green tea, although we did not see a significant effect on the lipid profile, all the parameters; TC, TG, LDL, Apo A1, and Apo B100 decreased in the GT group that drank four cups of GT, which showed the there could be a clear decrease in lipids, especially in total cholesterol and LDL, with a longer interventional period or higher doses. Furthermore, there is also evidence which shows that EGCG can accumulate in the liver microsomes, and therefore, a long-term effect of GT in Chinese and Japanese is predictable.[50]
Catechins are hypothesized to help protect against oxidative damages by contributing, along with the antioxidant vitamins (i.e., vitamins C and E) and enzymes (i.e., superoxide dismutase and catalase), to the total antioxidant defense system.[51] Consumption of vitamin C and omega 3 fatty acids decrease malondialdehyde, a marker of oxidative stress in diabetic patients.[52] Malondialdehyde also decreases after green tea intake.[53] Measurements of MDA and TAC have been used in the present study as the patients’ oxidative and antioxidative statuses. GT consumption did not have any effect on the oxidative status of our diabetic patients, but in some of the previous studies drinking one liter of GT in four weeks[54] and consuming encapsulated green tea extract[55] decreased the MDA levels in healthy subjects. Therefore, in diabetic patients, we may need higher doses to see clear effects.
In conclusion, this study indicates that drinking four cups of green tea per day, may help weight control, and may help to prevent hypertension in diabetic patients. However, more clinical trials may be required to evaluate the effects of longer consumption of green tea in diabetic patients.
ACKNOWLEDGEMENTS
The study was supported by a grant from the office of the Vice Chancellor for Research, Iran University of Medical Sciences. We are grateful to the Gabric Diabetes Education Institute and Golestan Company for equipping green tea.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet RA. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–9. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 3.Kuyvenhoven JP, Meinders AE. Oxidative stress and diabetes mellitus pathogenesis of long-term complications. Eur J Intern Med. 1999;10:9–19. [Google Scholar]
- 4.Maggio CA, Pi-Sunyer FX. The prevention and treatment of obesity: Application to type 2 diabetes. Diabetes Care. 1997;20:1744–66. doi: 10.2337/diacare.20.11.1744. [DOI] [PubMed] [Google Scholar]
- 5.Golozar A, Khademi H, Kamangar F. Diabetes mellitus and its correlates in an Iranian adult population. PLoS One. 2011;6:e26725. doi: 10.1371/journal.pone.0026725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Buse JB, Davidson MB. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 8.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA. 2006;296:1255–65. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 9.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 10.Iso HC, Date K, Wakai M, Fukui A, Tamakoshi E. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554–62. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 11.Unno T, Tago M, Suzuki Y, Nozawa A, Sagesaka YM, Kakuda T, et al. Effect of tea catechins on postprandial plasma lipid responses in human subjects. Br J Nutr. 2005;93:543–7. doi: 10.1079/bjn20041379. [DOI] [PubMed] [Google Scholar]
- 12.Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome. Phytochemistry. 2009;70:11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Nahas R, Moher M. Complementary and alternative medicine for the treatment of type 2 diabetes. Can Fam Physician. 2009;55:591–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: A literature review. Chin Med. 2010;5:1–9. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortsäter H, Grankvist N, Wolfram S, Kuehn N, Sjöholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr Metab. 2012;14:9–11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgson JM, Devine A, Puddey IB, Chan SY, Beilin LJ, Prince RL. Tea intake is inversely related to blood pressure in older women. J Nutr. 2003;133:2883–6. doi: 10.1093/jn/133.9.2883. [DOI] [PubMed] [Google Scholar]
- 17.Bracco D, Ferrarra JM, Arnaud MJ, Jequier E, Schutz Y. Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am J Physiol. 1995;269:E671–8. doi: 10.1152/ajpendo.1995.269.4.E671. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Kim C, Kim Y. Green tea -epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-Induced obese mice. Ann Nutr Metab. 2009;54:151–7. doi: 10.1159/000214834. [DOI] [PubMed] [Google Scholar]
- 19.Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70:1040–5. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 20.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–83. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinson JA, Dabbagh YA. Effect of green and black tea supplementation on lipids, lipid oxidation and fibrinogen in the hamster: Mechanisms for the epidemiological benefits of tea drinking. FEBS Lett. 1998;433:44–6. doi: 10.1016/s0014-5793(98)00880-1. [DOI] [PubMed] [Google Scholar]
- 22.McNamara JR, Cohn JS, Wilson PW, Schaefer EJ. Calculated values for low-density lipoprotein cholesterol in the assessment of lipid abnormalities and coronary disease risk. Clin Chem. 1990;36:36–42. [PubMed] [Google Scholar]
- 23.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 24.Neyestani TR, Shariatzadeh N, Gharavi A, Kalayi A, Khalaji N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patiens with type 2 diabetes mellitus: A possible role in the prevention of long-term complications. J Endocrinol Invest. 2007;30:833–8. doi: 10.1007/BF03349224. [DOI] [PubMed] [Google Scholar]
- 25.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 26.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity. 2007;15:1473–83. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 27.Rudelle S, Ferruzzi MG, Cristiani I, Moulin J, Macé K, Acheson KJ, et al. Effect of a thermogenic beverage on 24-hour energy metabolism in humans. Obesity. 2007;15:349–55. doi: 10.1038/oby.2007.529. [DOI] [PubMed] [Google Scholar]
- 28.Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50:188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- 29.Hsu CH, Liao YL, Lin SC, Tsai TH, Huang CJ, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev. 2011;16:157–63. [PubMed] [Google Scholar]
- 30.Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes. 2005;29:615–23. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 31.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int Obes Relat Metab Disord. 2002;26:1459–64. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- 32.Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine. 2002;9:3–8. doi: 10.1078/0944-7113-00078. [DOI] [PubMed] [Google Scholar]
- 33.Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: Interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000;24:252–8. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- 34.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005;81:122–9. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- 35.Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome-a randomized placebo-controlled trial. J Soc Gynecol Investig. 2006;13:63–8. doi: 10.1016/j.jsgi.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Bethesda, MD: National Institutes of Health; 2001. National Institutes of Health. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) [NIH Publication 01-3670] [Google Scholar]
- 37.van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: Review of recent findings. Curr Opin Lipidol. 2013;24:25–33. doi: 10.1097/MOL.0b013e32835bcdff. [DOI] [PubMed] [Google Scholar]
- 38.Yang YC, Lu FH, Wu JS, Wu CH, Chang CJ. The protective effect of habitual tea consumption on hypertension. Arch Intern Med. 2004;164:1534–40. doi: 10.1001/archinte.164.14.1534. [DOI] [PubMed] [Google Scholar]
- 39.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, et al. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I / R injury in SHR. Am J Physiol Endocrinol Metab. 2007;292:E1378–87. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 40.Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002;83:109–16. doi: 10.1016/s0378-8741(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, et al. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem. 2006;281:10214–21. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- 42.Yan J, Zhao Y, Suo S, Liu Y, Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic Biol Med. 2012;52:1648–57. doi: 10.1016/j.freeradbiomed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Ryan EA, Imes S, Wallace C, Jones S. Herbal tea in the treatment of diabetes mellitus. Clin Invest Med. 2000;23:311–7. [PubMed] [Google Scholar]
- 44.Ryu OH, Lee J, Lee KW, Kim HY, Seo JA, Kim SG, et al. Effects of green tea consumption on inflammation, insulin resistance and pulse wave velocity in type 2 diabetes patients. Diabetes Res Clin Pract. 2006;71:356–8. doi: 10.1016/j.diabres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Josic J, Olsson AT, Wickeberg J, Lindstedt S, Hlebowicz J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: A randomized controlled trial. Nutr J. 2010;63:1–8. doi: 10.1186/1475-2891-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003;14:326–32. doi: 10.1016/s0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 47.Anandh Babu PV, Sabitha KE, Shyamaladevi CS. Green tea extract impedes dyslipidaemia and develo pment of cardiac dysfunction in streptozotocin-diabetic rats. Clin Exp Pharmacol Physiol. 2006;33:1184–9. doi: 10.1111/j.1440-1681.2006.04509.x. [DOI] [PubMed] [Google Scholar]
- 48.Haidari F, Shahi MM, Zarei M, Rafiei H, Omidian K. Effect of green tea extract on body weight, serum glucose and lipid profile in streptozotocin-induced diabetic rats. A dose response study. Saudi Med J. 2012;33:128–33. [PubMed] [Google Scholar]
- 49.Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH, Chen H, et al. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: A randomized controlled trial. Arch Intern Med. 2003;163:1448–53. doi: 10.1001/archinte.163.12.1448. [DOI] [PubMed] [Google Scholar]
- 50.Antonello M, Montemurro D, Bolognesi M. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am J Hypertens. 2007;20:1321–8. doi: 10.1016/j.amjhyper.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7:135–44. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- 52.Jazayery A, Jalali M, Keshavarz SA, Shakouri Mahmoudabadi MM, Eshraghian MR, Saboor Yaraghi AA, et al. Inflammatory biomarkers, antioxidant enzyme activities, and oxidative stress in Iranian male patients with type 2 diabetes mellitus: Effects of eicosapentaenoic acid and vitamin C supplementation. J Res Med Sci. 2012;17:S38–41. [Google Scholar]
- 53.Yokozawa T, Nakagawa T, Kitani K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J Agric Food Chem. 2002;50:3549–52. doi: 10.1021/jf020029h. [DOI] [PubMed] [Google Scholar]
- 54.Coimbra S, Castro E, Rocha-Pereira P, Rebelo I, Rocha S, Santos-Silva A. The effect of green tea in oxidative stress. Clin Nutr. 2006;25:790–6. doi: 10.1016/j.clnu.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Freese R, Basu S, Hietanen E, Nair J, Nakachi K, Bartsch H, et al. Green tea extract decreases plasma malondialdehyde concentration but does not affect other indicators of oxidative stress, nitric oxide production, or hemostatic factors during a high-linoleic acid diet in healthy females. Eur J Nutr. 1999;38:149–57. doi: 10.1007/s003940050056. [DOI] [PubMed] [Google Scholar]


