Abstract
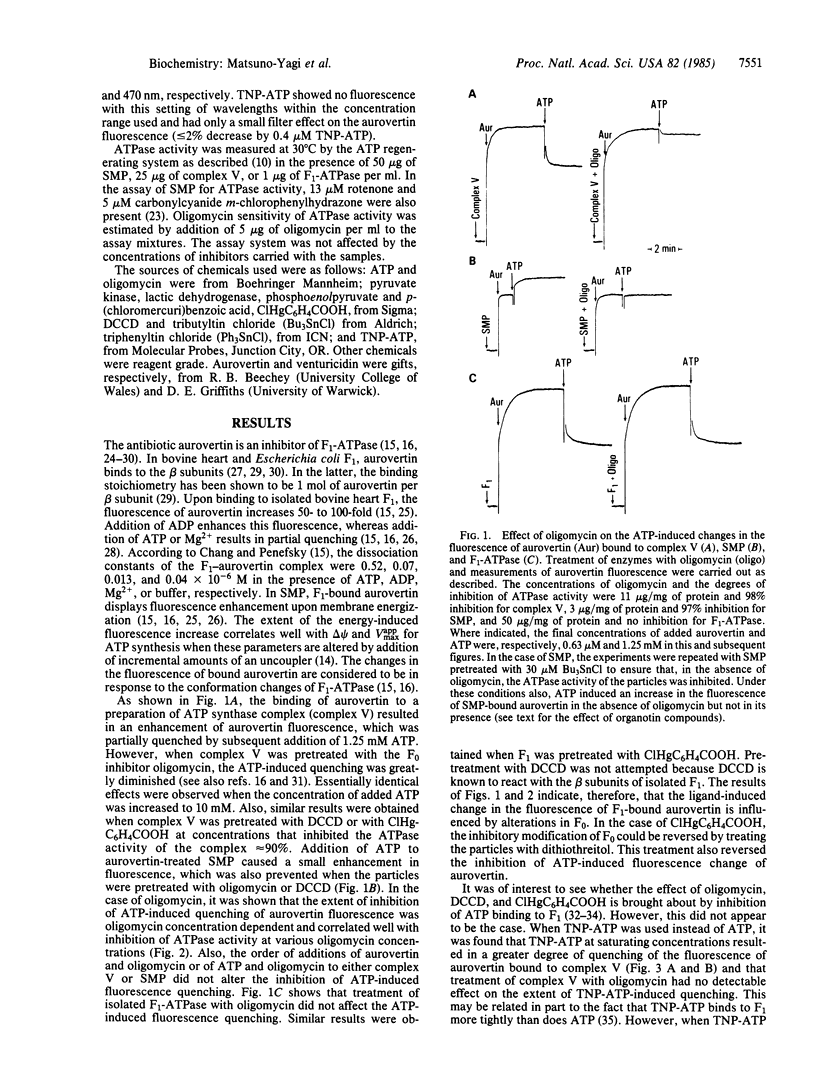
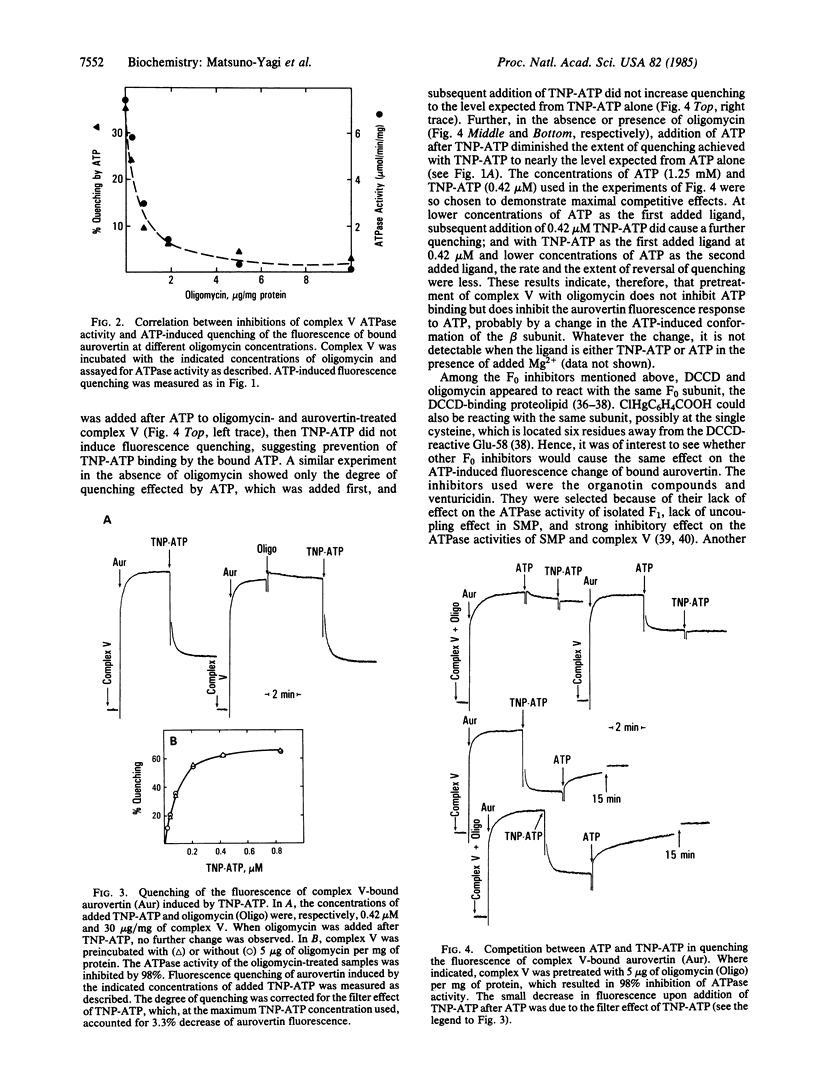
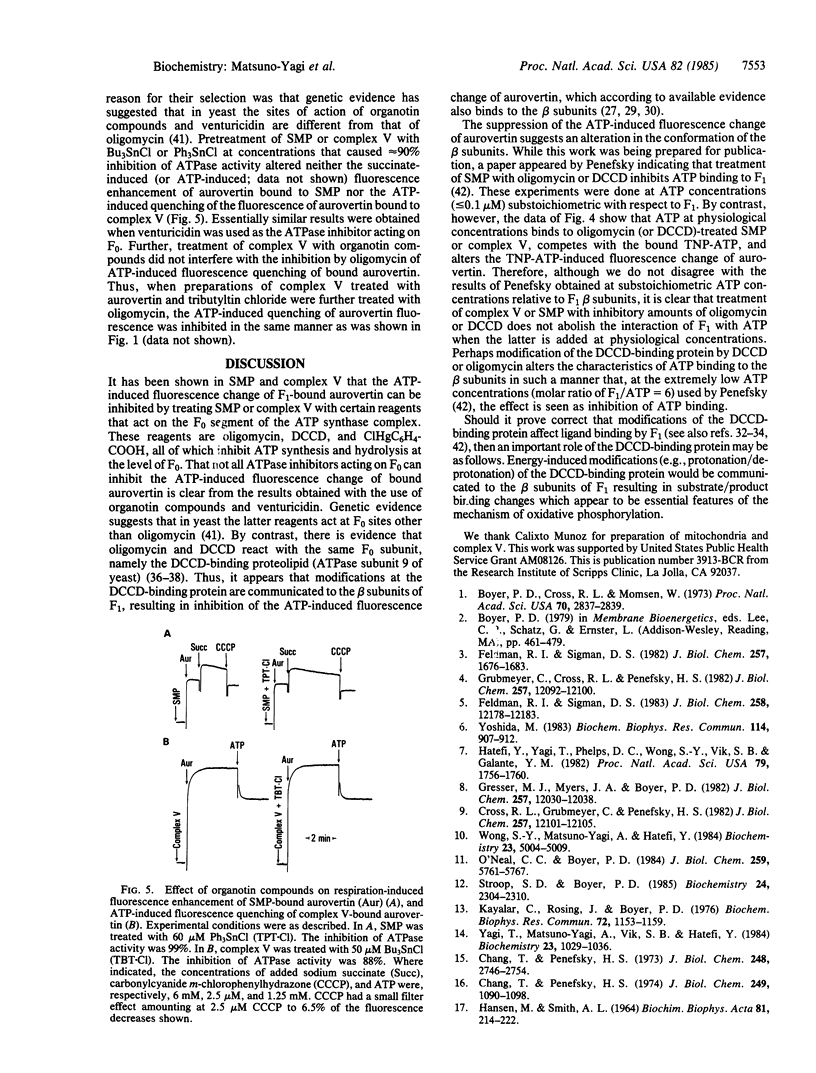
Aurovertin is a fluorescent antibiotic that binds to the catalytic beta subunits of the mitochondrial F1-ATPase and inhibits ATP synthesis and hydrolysis. ATP, ADP, and membrane energization in submitochondrial particles (SMP) alter the fluorescence of F1-bound aurovertin. These fluorescence changes are considered to be in response to the conformation changes of F1-ATPase. This paper shows that the ATP-induced fluorescence change of aurovertin bound to SMP or complex V (purified ATP synthase complex F0-F1) is inhibited when these preparations are pretreated with oligomycin or N,N'-dicyclohexylcarbodiimide (DCCD). This inhibition is not seen with isolated F1-ATPase. These and other results have suggested that modifications of the DCCD-binding protein in the membrane sector (F0) of the ATP synthase complex are communicated to F1, thereby altering the binding characteristics of ATP to the beta subunits. By analogy, it is proposed that modifications (e.g., protonation/deprotonation) of the DCCD-binding protein effected by protonic energy alter the conformation of F1 and bring about the substrate/product binding changes that appear to be essential features of the mechanism and regulation of oxidative phosphorylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandrowicz Z., Schuster S. M. Interactions of beef heart mitochondrial adenosine triphosphatase and aurovertin. Life Sci. 1979 Apr 9;24(15):1407–1417. doi: 10.1016/0024-3205(79)90012-2. [DOI] [PubMed] [Google Scholar]
- Bertina R. M., Schrier P. I., Slater E. C. The binding of aurovertin to mitochondria, and its effect on mitochondrial respiration. Biochim Biophys Acta. 1973 Jun 28;305(3):503–518. doi: 10.1016/0005-2728(73)90072-8. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Cross R. L., Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. M., Penefsky H. S. Energy-dependent enhancement of aurovertin fluorescence. An indicator of conformational changes in beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1090–1098. [PubMed] [Google Scholar]
- Chang T., Penefsky H. S. Aurovertin, a fluorescent probe of conformational change in beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1973 Apr 25;248(8):2746–2754. [PubMed] [Google Scholar]
- Cross R. L., Grubmeyer C., Penefsky H. S. Mechanism of ATP hydrolysis by beef heart mitochondrial ATPase. Rate enhancements resulting from cooperative interactions between multiple catalytic sites. J Biol Chem. 1982 Oct 25;257(20):12101–12105. [PubMed] [Google Scholar]
- Emanuel E. L., Carver M. A., Solani G. C., Griffiths D. E. Differential inhibition of F0F1-ATPase-catalysed reactions in bovine-heart submitochondrial particles by organotin compounds. Biochim Biophys Acta. 1984 Jul 27;766(1):209–214. doi: 10.1016/0005-2728(84)90233-0. [DOI] [PubMed] [Google Scholar]
- Enns R. K., Criddle R. S. Affinity labeling of yeast mitochondrial adenosine triphosphatase by reduction with [3H]borohydride. Arch Biochem Biophys. 1977 Aug;182(2):587–600. doi: 10.1016/0003-9861(77)90540-9. [DOI] [PubMed] [Google Scholar]
- Feldman R. I., Sigman D. S. The synthesis of ATP by the membrane-bound ATP synthase complex from medium 32Pi under completely uncoupled conditions. J Biol Chem. 1983 Oct 25;258(20):12178–12183. [PubMed] [Google Scholar]
- Feldman R. I., Sigman D. S. The synthesis of enzyme-bound ATP by soluble chloroplast coupling factor 1. J Biol Chem. 1982 Feb 25;257(4):1676–1683. [PubMed] [Google Scholar]
- Fillingame R. H., Peters L. K., White L. K., Mosher M. E., Paule C. R. Mutations altering aspartyl-61 of the omega subunit (uncE protein) of Escherichia coli H+ -ATPase differ in effect on coupled ATP hydrolysis. J Bacteriol. 1984 Jun;158(3):1078–1083. doi: 10.1128/jb.158.3.1078-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser M. J., Myers J. A., Boyer P. D. Catalytic site cooperativity of beef heart mitochondrial F1 adenosine triphosphatase. Correlations of initial velocity, bound intermediate, and oxygen exchange measurements with an alternating three-site model. J Biol Chem. 1982 Oct 25;257(20):12030–12038. [PubMed] [Google Scholar]
- Grubmeyer C., Cross R. L., Penefsky H. S. Mechanism of ATP hydrolysis by beef heart mitochondrial ATPase. Rate constants for elementary steps in catalysis at a single site. J Biol Chem. 1982 Oct 25;257(20):12092–12100. [PubMed] [Google Scholar]
- Grubmeyer C., Penefsky H. S. The presence of two hydrolytic sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1981 Apr 25;256(8):3718–3727. [PubMed] [Google Scholar]
- Hatefi Y., Yagi T., Phelps D. C., Wong S. Y., Vik S. B., Galante Y. M. Substrate binding affinity changes in mitochondrial energy-linked reactions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1756–1760. doi: 10.1073/pnas.79.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Schairer H. U., Friedl P., Sebald W. An Asp-Asn substitution in the proteolipid subunit of the ATP-synthase from Escherichia coli leads to a non-functional proton channel. FEBS Lett. 1982 Aug 16;145(1):21–29. doi: 10.1016/0014-5793(82)81198-8. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Sebald W. The proton conducting F0-part of bacterial ATP synthases. Biochim Biophys Acta. 1984 Apr 9;768(1):1–27. doi: 10.1016/0304-4173(84)90005-3. [DOI] [PubMed] [Google Scholar]
- Issartel J. P., Klein G., Satre M., Vignais P. V. Aurovertin binding sites on beef heart mitochondrial F1-ATPase. Study with [14C]aurovertin D of the binding stoichiometry and of the interaction between aurovertin and the natural ATPase inhibitor for binding to F1. Biochemistry. 1983 Jul 5;22(14):3492–3497. doi: 10.1021/bi00283a028. [DOI] [PubMed] [Google Scholar]
- Issartel J. P., Vignais P. V. Evidence for a nucleotide binding site on the isolated beta subunit from Escherichia coli F1-ATPase. Interaction between nucleotide and aurovertin D binding sites. Biochemistry. 1984 Dec 18;23(26):6591–6595. doi: 10.1021/bi00321a048. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Rosing J., Boyer P. D. 2,4-Dinitrophenol causes a marked increase in the apparent Km of Pi and of ADP for oxidative phosphorylation. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1153–1159. doi: 10.1016/s0006-291x(76)80252-5. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuno-Yagi A., Hatefi Y. Inhibitory chemical modifications of F1-ATPase: effects on the kinetics of adenosine 5'-triphosphate synthesis and hydrolysis in reconstituted systems. Biochemistry. 1984 Jul 17;23(15):3508–3514. doi: 10.1021/bi00310a019. [DOI] [PubMed] [Google Scholar]
- O'Neal C. C., Boyer P. D. Assessment of the rate of bound substrate interconversion and of ATP acceleration of product release during catalysis by mitochondrial adenosine triphosphatase. J Biol Chem. 1984 May 10;259(9):5761–5767. [PubMed] [Google Scholar]
- Penefsky H. S. Mechanism of inhibition of mitochondrial adenosine triphosphatase by dicyclohexylcarbodiimide and oligomycin: relationship to ATP synthesis. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1589–1593. doi: 10.1073/pnas.82.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. Studies on the mitochondrial oligomycin-insensitivt ATPase. I. An improved method of purification and the behavior of the enzyme in solutions of various depolymerizing agents. Arch Biochem Biophys. 1970 Sep;140(1):257–266. doi: 10.1016/0003-9861(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Stiggall D. L., Galante Y. M., Hatefi Y. Preparation and properties of an ATP-Pi exchange complex (complex V) from bovine heart mitochondria. J Biol Chem. 1978 Feb 10;253(3):956–964. [PubMed] [Google Scholar]
- Stiggall D. L., Galante Y. M., Hatefi Y. Preparation and properties of complex V. Methods Enzymol. 1979;55:308-15, 819-21. doi: 10.1016/0076-6879(79)55036-8. [DOI] [PubMed] [Google Scholar]
- Verschoor G. J., van der Sluis P. R., Slater E. C. The binding of aurovertin to isolated beta subunit of F1 (mitochondrial ATPase). Stoicheiometry of beta subunit in F1. Biochim Biophys Acta. 1977 Nov 17;462(2):438–449. doi: 10.1016/0005-2728(77)90141-4. [DOI] [PubMed] [Google Scholar]
- Wong S. Y., Matsuno-Yagi A., Hatefi Y. Kinetics of ATP hydrolysis by F1-ATPase and the effects of anion activation, removal of tightly bound nucleotides, and partial inhibition of the ATPase by covalent modification. Biochemistry. 1984 Oct 9;23(21):5004–5009. doi: 10.1021/bi00316a027. [DOI] [PubMed] [Google Scholar]
- Yagi T., Matsuno-Yagi A., Vik S. B., Hatefi Y. Modulation of the kinetics and the steady-state level of intermediates of mitochondrial coupled reactions by inhibitors and uncouplers. Biochemistry. 1984 Feb 28;23(5):1029–1036. doi: 10.1021/bi00300a035. [DOI] [PubMed] [Google Scholar]
- Yoshida M. The synthesis of enzyme-bound ATP by the F1-ATPase from the thermophilic bacterium PS3 in 50% dimethylsulfoxide. Biochem Biophys Res Commun. 1983 Aug 12;114(3):907–912. doi: 10.1016/0006-291x(83)90646-0. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., van Dam K. Binding of aurovertin to phosphorylating submitochondrial particles. Biochim Biophys Acta. 1974 May 22;347(2):253–263. doi: 10.1016/0005-2728(74)90049-8. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., van Dam K., Slater E. C. Interaction of aurovertin with submitochondrial particles, deficient in ATPase inhibitor. Biochim Biophys Acta. 1974 May 22;347(2):224–239. doi: 10.1016/0005-2728(74)90047-4. [DOI] [PubMed] [Google Scholar]


