Abstract
We investigated the roles of specific subsets of donor APCs purified from bone marrow in donor T cell activation and graft-vs-leukemia (GvL) activity in murine models of hemopoietic stem cell transplantation. Lineage−CD11c+ APC precursors were separated from donor bone marrow based on expression of CD11b. Transplanting lineage−CD11c+CD11b− APC (CD11b− APC) in combination with c-kit+Sca-1+lineage− hemopoietic stem cells (HSC) and congenic donor T cells led to increased donor CD4+ and CD8+ T cell proliferation and higher donor T cell chimerism than with transplanting grafts containing HSC, T cells, and lineage−CD11c+CD11b+ APCs (CD11b+ APC), or grafts containing only HSC and T cells. Transplanting CD11b− APCs induced Th1/type 1 cytotoxic T lymphocyte donor T cell immune polarization and enhanced GvL activity of donor T cells without increased graft-vs-host disease in both MHC- and minor histocompatibility Ag-mismatched murine hemopoietic stem cell transplantation models, whereas CD11b+ APCs led to Th2/type 2 cytotoxic T lymphocyte donor T cell immune polarization. Donor CD11b− APCs were plasmacytoid dendritic cell progenitors (>90% CD317; PDCA-1+) and up-regulated CD80, CD86, and IL-12 during alloantigen presentation, whereas CD11b+ APCs expressed Gr-1 and up-regulated expression of programmed death ligands-1 and 2 after activation. These results are the first to show that manipulation of the content of donor APCs in allogeneic HSC grafts can regulate donor T cell immunity and enhance GvL without increasing graft-vs-host disease activity.
Keywords: Antigen presenting cells, Dendritic Cells, T-cells, Graft versus Host Disease, Cell Activity, Tumor Immunity
Introduction
Graft-vs-host disease (GvHD)3 and relapsed leukemia are the primary complications of hemopoietic stem cell transplantation (HSCT) in patients with leukemia. Both GvHD and graft-vs-leukemia (GvL) reactions require APCs to activate T cell effectors. Host APCs persist after high-dose chemotherapy (1), HSCT, and HSCT (2, 3) and initiate GvHD in mouse models of HSCT (4, 5, 6).
In contrast to the requirement for host-type APC in the initiation of GvHD, the role for donor APC on transplant outcomes is less clear (7). Experiments using MHC class II (MHC-II)-deficient radiation chimeras have demonstrated a requirement for host APCs on the initiation of CD4+ T cell-mediated GvHD (5) and a role for donor APC in increasing the severity of CD8+ T cell-dependent acute GvHD (5) and intestinal chronic GvHD (8). In allogeneic bone marrow (BM) transplant (BMT) from HLA-matched siblings, larger numbers of donor plasmacytoid dendritic cells (pDC) in allogeneic BM grafts were associated with more relapse and worse survival (9, 10). Additionally, higher numbers of donor dendritic cells (DC) in the blood of transplant recipients and higher serum IL-12 levels are associated with less relapse and less GvHD after allogeneic HSCT (11). Although these clinical studies suggest a role for donor APCs and DC in transplant outcomes, they do not formally evaluate the relative contribution of one donor cell subset over another. In this study, we tested the effects of transplanting phenotypically defined subsets of murine donor BM APCs on immune reconstitution following allogeneic HSCT.
Mouse APC and DC (the most potent type of APC) progenitors are contained within the population of CD11c+ cells that lack lineage (Lin) markers (12, 13, 14, 15). Specific DC subpopulations can be further defined by their expression of B220, CD11b, CD4, and CD8 (12). We have previously demonstrated that donor BM depleted of CD11b+ cells by MACS has an enhanced ability to polarize donor T cells to Th1 immunity and that tumor-bearing transplant recipients of allogeneic CD11b- depleted BM cells had prolonged survival compared with recipients of unmanipulated BM (16). These data suggested that phenotypically defined donor populations could modulate alloreactivity and GvL activity of donor T cells in allogeneic HSCT (17) and raise the interesting question of whether specific subsets of donor APCs could affect immune reconstitution in allogeneic HSCT.
To test this hypothesis, we used defined populations of donor APCs purified by FACS from donor BM to clarify their role in donor immunity following allogeneic HSCT. The expression of CD11b was used to divide Lin−CD11c+ BM APC into two distinct populations: a fairly homogeneous population of Lin− CD11c+ CD11b− APC precursors that are predominantly B220+CD11b−. and CD317+ (plasmacytoid DC Ag (PDCA)-1+) pDC precursors (18), and a more heterogeneous Lin−CD11c+CD11b+ population that includes myeloid DC progenitors as well as myeloid suppressor cells (16, 19, 20). We predicted that the murine CD11b− APC precursors would augment posttransplant cellular immune responses based on their ability to polarize cognate T cells toward Th1 immune responses (21, 22, 23, 24, 25) and that CD11b+ APC precursors would polarize T cells toward Th2 or mixed Th1/Th2 responses (23, 26). We herein demonstrate a striking ability of FACS-purified donor APC to regulate posttransplant immunity including the proliferation, cytokine synthesis, and antitumor cytotoxic activity of donor T cells.
Materials and Methods
Mice
B10.BR (B10, H-2Kk), PL/J (H-2Ks), BALB/c (B/c, H-2Kd), C3H.SW (H-2Kb), C57BL/6 (B6, H-2Kb) and congenic B6.SJL (H-2Kb, CD45.1, CD90.2) mice were purchased from The Jackson Laboratory. Congenic strains expressing CD90.1 and CD45.1 on a B6 (H-2Kb) background and CD90.1 and CD45.2 on a B10 (H-2Kk) background were bred at Emory University (Atlanta, GA). GFP-expressing B6 mice were a gift from Dr. Robert Taylor (Emory University).
Tumor cells
MMB3.19, a retrovirus-transformed myeloid leukemia line from B6 (27), was provided by Dr. R. Korngold (Jefferson Medical University, Philadelphia, PA). LBRM 33-5A4, a B10 T cell lymphoma cell line (28), was purchased from American Type Culture Collection (ATCC). Tumor cell lines were cultured according to ATCC recommendations, and per tests were free of lymphocytic choriomeningitis virus, mouse hepatitis virus, minute virus of mice, and mouse parvovirus by the University of Missouri Research Animal Diagnostic Laboratory (Columbia, MO).
Cell preparations
Donor mice were killed in a humane manner, and femurs, tibias, and spleens were removed aseptically. BM cells and splenocytes were harvested with sterile RPMI 1640 containing 1% heat-inactivated FCS. For purification of APC subsets, BM cells were incubated with anti-CD11c microbeads (Miltenyi Biotec) followed by anti-CD11c-allophycocyanin, CD11b-allophycocyanin-Cy7, and a mixture of PE-conjugated anti-CD3, IgM, CD19, DX5, and TER119 Abs (BD Biosciences). CD11c+ cells were then selected using LS magnetic columns (MACS; Miltenyi Biotec), and Lin−CD11c+CD11b+ and Lin−CD11c+CD11b− populations were sorted using the FACSAria (BD Biosciences). For hematopoietic stem cell (HSC) selection, BM cells were stained with biotinylated Abs to Lin markers CD11b, Gr-1, CD3, CD4, CD8, DX5, B220, I-Ab, and TER119 (BD Biosciences) and antibiotin microbeads (Miltenyi Biotech). Lin− cells were collected after MACS magnetic separation and stained with Abs to the stem cell factor receptor c-kit and to stem cell Ag (Sca)-1 (BD Biosciences); then Lin−c-kit+Sca-1+ HSCs were sorted by FACS. BM from one donor mouse provided sufficient sorted stem cells for three recipients and APC for five recipients. T cells were purified by incubating splenocytes with biotinylated anti-CD11b, B220, DX5, and TER119 Abs, followed by antibiotin microbeads, and negative MACS selection using an LS column. CD8 T cells were obtained by the addition of biotinylated anti-CD4 Ab to the T cell-negative selection mixture.
Characterization of BM APCs
MHC-II, CD40, CD80, CD86, ICOS ligand (ICOS-L), programmed death ligand-1 (PD-L1), and PD-L2 costimulatory molecule expression, and CD317, Ly-6C/G (Gr-1), F4/80, CD115, CD135, CD90, and NK1.1 lineage-related molecule expression on BM APCs were analyzed by flow cytometry using directly conjugated Abs as described by the manufacturer (BD Biosciences). For in vitro experiments, FACS-purified BM APCs (2 × 105/ml) were cultured with CD40L (1 μg/ml; PeproTech); with 1 × 106/ml irradiated (11 Gy) allogeneic splenocytes; with 1 × 106/ml syngeneic splenic T cells; with 1 × 106/ml irradiated allogeneic splenocytes plus 1 × 106/ml syngeneic splenic T cells; or with medium alone for 72 h in RPMI 1640 with 10% FCS. Flow cytometric analyses of costimulatory molecule expression were performed before and after the culture period. To analyze the maturation of BM APCs in vivo, mice were transplanted with 1 × 106 FACS-sorted APCs from GFP+ transgenic donors plus HSCs and donor T cells and then sacrificed 10 days posttransplant. Mononuclear cells were harvested from the BM and spleen and analyzed for Ag expression by flow cytometry, after electronically gating on GFP+ donor APC cells in list mode files of at least 1,000,000 events.
Transplantation
Recipient B10, PL/J, or B6 mice were irradiated with two doses of 5.5 Gy separated by 3 h on day −2 (29). On day 0, recipient mice were transplanted with combinations of 3 × 103 HSCs, with varying numbers of APCs (5 × 104 or 1 × 106), and T cells (3 × 105 or 3 × 106) using B6 and C3H.SW donors in the B6→B6, B6→B10, B6→PL/J, and C3H.SW→B6 models. For tumor experiments, B10 mice received an i.v. dose of 1 × 105 cells of viable (Ficolled, from log-phase culture) LBRM (28) on day −1 (16, 30), whereas B6 recipients received an i.p. dose of 5 × 104 MMB3.19 cells on day −1 (27). Mice were weighed twice weekly and examined daily for signs of GvHD as described (31). Moribund animals losing >25% of initial body weight and mice surviving until the end of the experiment were euthanized, and tissues were processed for histopathological analysis of tumor-trophic sites including brain, lung, liver, spleen, and kidney. Flow cytometric chimerism analyses were performed on blood leukocytes on (mean ± SD) days 10, 30 ± 1, 60 ± 2, and 100 ± 5 posttransplant.
T cell proliferation, cytokine expression, and donor cell localization
The proliferation of donor T cells in recipient spleen was analyzed by CFSE dilution as described (32). Briefly, donor T cells were stained with CFSE before transplant and recipient spleens were removed 3 days later, and cell suspensions were prepared. Proliferation of donor T cells was determined by flow cytometric analysis of CFSE dilution profiles. In secondary MLR, responder T cells were recovered 15 days posttransplant, stained with CFSE before setting up the MLR culture, and analyzed 3 days later. Intracellular cytokine expression (IL-4, IL-10, IL-17, and IFN-γ; BD Biosciences) by CD4+ and CD8+ T cells was analyzed as described (30). In transplant experiments using GFP+ donors, the presence of GFP+ donor DCs in frozen sections of spleens, mesenteric lymph nodes, and ileum tissues was visualized by confocal microscopy (Zeiss LSM 510; Thornwood) using Zeiss LSM 5 Image Browser software with images captured using ×40 and ×100 objectives. Donor T cells were visualized using rat anti-mouse CD90.2 (BD Biosciences) and goat anti-rat IgG Alexa Fluor 555 (Invitrogen) with 4′,6′-diamidino-2-phenylindole to stain nuclei. The number of APCs per microliter of spleen tissue was determined by averaging the APCs found in 6-μm-thick sections of tissue (volumes calculated as area × thickness) from three mice per group in each of three separate transplant experiments.
Measurement of serum and secreted cytokine levels
Recipient mice were anesthetized by isofluorane inhalation, and peripheral blood was collected by tail vein bleeding into Microtainer brand serum separation tubes (BD Biosciences). After centrifugation, the serum was stored at −20°C, and cytokines were assayed in duplicate wells using OptEIA ELISA sets (IL-2, IL-10, IFN-γ, IL-12 p70, and TNF-α; BD Biosciences) and Ready-SET-Go ELISA kits (IL-4, IL-5, IL-23; eBioscience) and analyzed using a SpectraMax 340PC spectrophotometer (Molecular Devices).
CTL activity
Recipients of C3H.SW→B6 or B6→B10 transplants were euthanized on day 34 or 81, respectively, and donor T cells were isolated by MACS-negative selection of splenocytes using a mixture of Abs to CD11b, DX5, B220, and TER119 (BD Biosciences). To isolate spleen-derived donor T cells from B6→B10 transplant recipients, anti-CD45.2 (BD Biosciences) was added to the Ab cocktail to deplete host and HSC-derived T cells. In the C3H.SW→B6 transplants, >90% of splenic T cells were derived from the T cells in the graft on day 34 posttransplant. T cells (2 × 106/ml) were cultured with 2 × 106 cells/ml irradiated (30 Gy) allogeneic host-type splenocytes or tumor cells in 24-well plates in RPMI 1640 supplemented with 10% FCS and antibiotics. After 5 days, viable T cells were collected and assayed for cytotoxic activity against LBRM and MMB3.19 tumor cells by a flow cytometry assay using the CyToxiLux PLUS kit (OncoImmunin). Briefly, 2 × 106 effector cells were mixed with 2 × 105 surface-labeled target cells and incubated with caspase substrate for 30 min, washed twice with PBS, and the percentage of apoptotic target cells was calculated after flow cytometry analysis.
Statistical analyses
Analyses of data were performed using SPSS (version 17 Mac, SPSS Inc. Chicago, IL). Data are presented as mean ± SD. Survival differences between groups were calculated with the Kaplan-Meier log rank test in a pairwise manner. Differences in GvHD outcome between groups were compared using the Kruskal-Wallis test. Differences in the levels of donor T cells and other parametric tests were compared using the one-way ANOVA.
Results
Addition of BM CD11b− APCs to HSC grafts enhanced donor CD4 and CD8 T cell proliferation
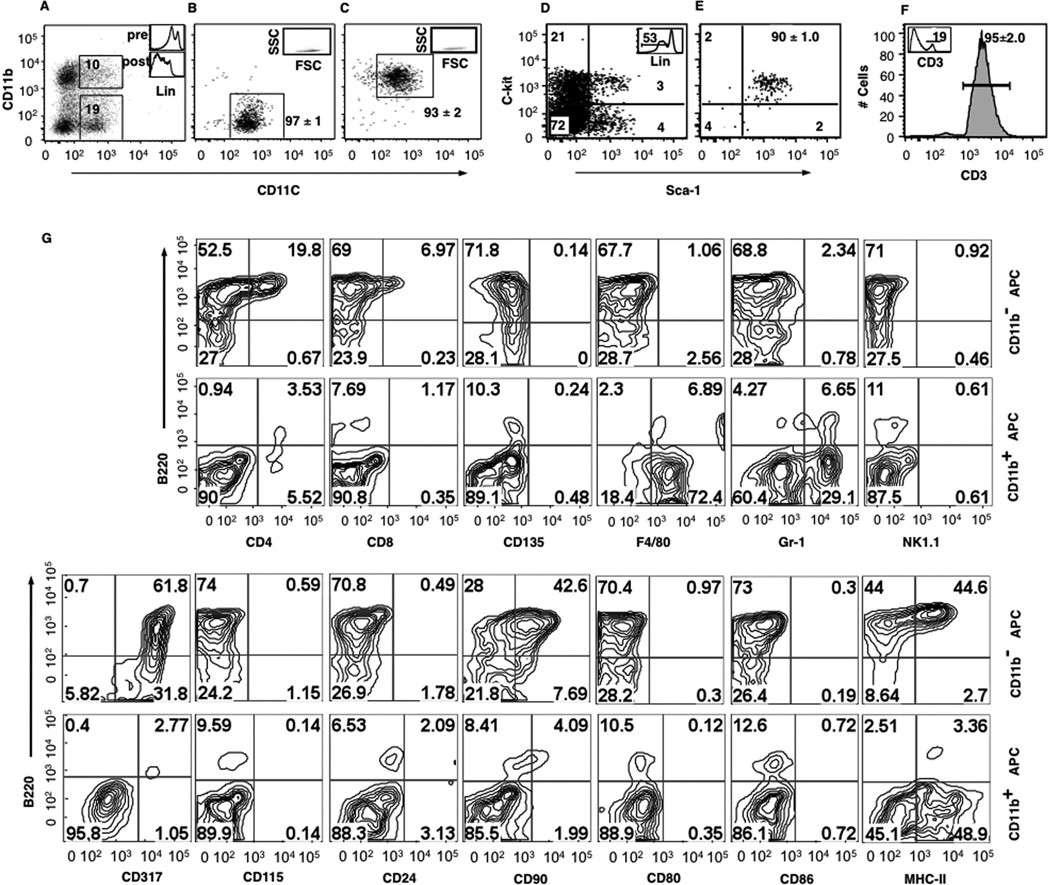
To test the hypothesis that the activation status of donor T cells can be modulated by the presence of donor APC in the graft, we used two MHC-mismatched allogeneic HSCT models (B6→B10 and B6→PL/J), as well as MHC-matched syngenic controls (B6→B6). Using high-speed FACS, we enriched APC from BM by selecting cells lacking lineage markers (TER119, CD3, DX5, CD19, and IgM) and expressing CD11c, and then sorted Lin−CD11c+CD11b− APCs and APC precursors (CD11b− APC) and Lin−CD11c+CD11b+ APCs and APC precursors (CD11b+ APCs) to average purities (±SD) of 97 ± 1% and 93 ±2%, respectively. Using separate congenic strains as donors of HSC and T cells, Lin−c-kit+Sca-1+ HSC were purified by FACS from donor BM and T cells were purified by MACS from splenocytes with average purities of 90 ± 1% and 95 ± 2%, respectively (Fig. 1, A–F). The CD11b− and CD11b+ APC subpopulations had the scatter properties of lymphocytes (Fig. 1, B and C). The majority of BM CD11b− APCs expressed B220 (72%), CD90 (51%), and CD317 (93%), had low levels of MHC-II, partial expression of CD4, and lacked expression of other markers including CD24, CD80, and CD86 as well as markers associated with lymphoid cell precursors and NK cell (CD135, NK1.1) or myeloid cell differentiation (F4/80, Gr-1, and CD115; Fig. 1G). In contrast, the majority of BM CD11b+ APCs lacked B220, CD4, CD8, CD24, CD80, CD86, MHC-II, and CD317 but expressed high levels of F4/80 and low levels Gr-1, consistent with recently described BM myeloid suppressor cells (Refs. 16, 19, and 20; Fig. 1G). Applying the same phenotypic analysis to Lin−CD11clow/+ cells in the spleen revealed three distinct populations: the same two populations of CD11clowCD11b+ APCs and CD11clowCD11b− APCs described above and a third population of Lin−CD11chigh cells that expressed high levels of CD4, CD80, CD86, and MHC-II which has been previously described as classical splenic DCs (Refs. 33 and 34 and supplemental Fig. 1).4
FIGURE 1.
Isolation of purified populations of donor APC subsets, HSCs, and donor T cells using FACS and MACS. A, Gating strategies for sorting CD11b− APC subsets and CD11b+ APC subsets from CD11c-enriched B6 BM. Insets, Histogram of lineage expression in BM before (top) and after (bottom) MACS enrichment of CD11c+ cells. B, Purity of sorted CD11b− APCs. Inset, Purified CD11c+CD11b− population back-gated in lymphocyte area in forward scatter (FSC) vs sideward scatter (SSC) flow cytometry plot. C, Purity of sorted CD11b+ APC. Insert: purified CD11c+CD11b+ population back-gated in lymphocyte area in a forward scatter vs sideward scatter flow cytometry plot. D, Gates (RUQ) for sorting Lin−Sca-1+c-kit+ HSCs from B6 BM. Inset, Histogram of lineage expression in BM after MACS enrichment of Lin− cells. E, Purity of sorted HSCs. F, Analysis of T cells purified by negative MACS selection. Insert shows the percentage of CD3+ T cells before immunomagnetic depletion. G, Phenotypes of CD11b− and CD11b+ APCs from BM. FACS plots are from a single representative experiment. Mean percentages (±SD) for each subset are shown from five replicate experiments.
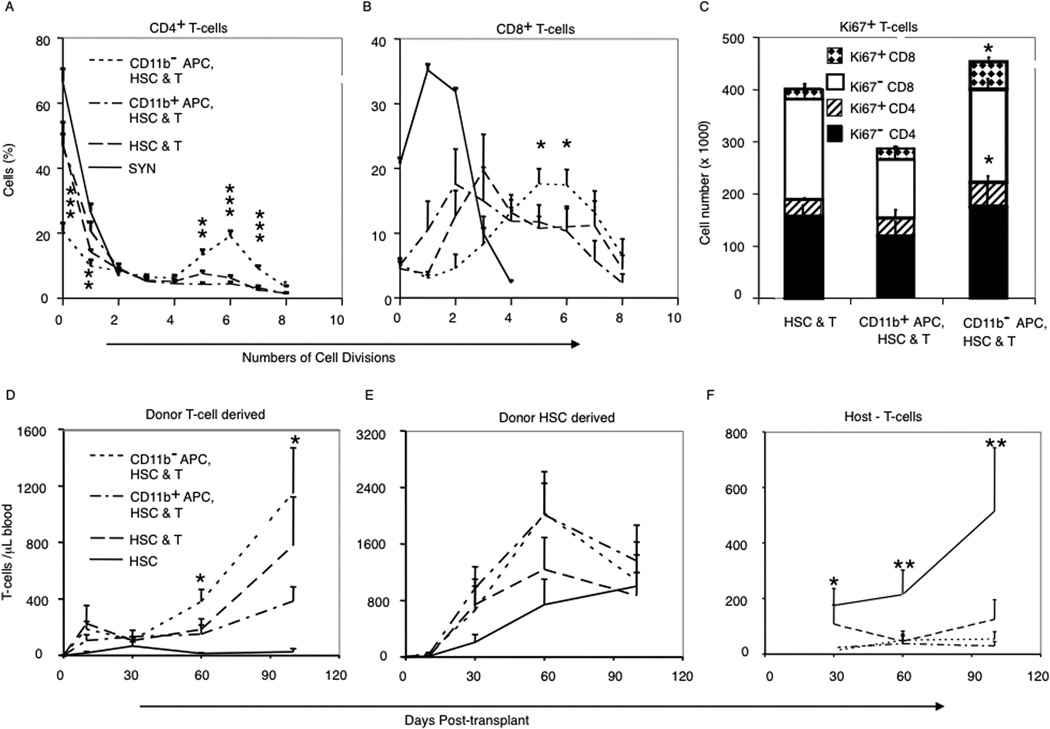
Next we measured the in vivo proliferation of CFSE-labeled donor T cells 3 days posttransplant in mice that received either CD11b− APCs or CD11b+ APCs. We transplanted 3 × 103 HSCs combined with 3 × 105 CFSE-labeled congenic CD90.1+ T cells and 5 × 104 FACS-sorted CD11b− APCs or CD11b+ APCs. The proliferation of donor T cells in syngeneic recipients were equivalent regardless of the presence or absence of donor APCs (Fig. 2, A and B; only the HSC plus T cells data are shown for clarity). In B6→B10 allogeneic recipients, there was greater initial proliferation of donor CD8+ T cells than of donor CD4+ T cells, and cotransplantation of CD11b− APCs led to higher proliferation rates of both donor CD4+ and CD8+ T cells compared with the proliferation of corresponding donor T cell subsets cotransplanted with CD11b+ APCs, or T cells transplanted into allogeneic mice that received no donor APCs (Fig. 2, A and B). After 3 days, CD4+ and CD8+ donor T cells recovered from recipients of CD11b− APCs expressed higher levels of CD25 and CD69 (data not shown) and Ki-67 (Fig. 2C) by FACS compared with T cells cotransplanted with HSCs alone or the combination of T cells, HSCs, and CD11b+ APCs.
FIGURE 2.
The addition of CD11b− donor APCs accelerated in vivo donor T cell proliferation in allogeneic BMT. A and B, Percentages of: donor CD4+ T cells (A) and CD8+ T cells (B) according to numbers of cell divisions (CFSE dilution). For B6→B6 syngeneic (Syn) transplants only data from HSCs plus T cells transplant; transplants adding CD11b+ or CD11b− APCs yielded similar results (data not shown). C, Numbers of total and Ki67+ donor CD4+ and CD8+ donor T cells at day 3 in the spleen of B6→B10 transplant recipients. Data are means ± SD from four replicate experiments with three mice per treatment group. D–F, T cell chimerism of blood T cells derived from mature T cells in the graft (D), donor HSC-derived T cells (E), and residual host T cells (F). Data are means (±SD) from four replicate experiments, five mice per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001 comparing T cell chimerism in groups receiving donor APCs with HSC and T cell groups.
The kinetics of donor T cell engraftment was analyzed by sampling blood on days 10, 30, 60, and 100 posttransplant and using congenic markers to distinguish T cells derived from donor HSCs (CD90.2+CD45.1+), donor BM APC subsets (CD90.1+CD45.2+), MACS-purified mature T cells in the graft (CD90.1+CD45.1+), and residual host T cells (CD90.2+CD45.2+). There was significantly greater expansion of donor T cells derived from the T cells in the graft among mice that received CD11b− APCs, compared with those that received CD11b+ APCs or grafts containing donor HSC and T cells without APC (Fig. 2D). In contrast, expansion of T cells derived from donor HSC was not significantly different among the groups (Fig. 2E). Persistence of host-type T cells was seen only among recipients of HSCs alone, consistent with mixed chimerism in the absence of donor T cells (Fig. 2F). Among recipients of CD11b− APCs, the graft-derived donor T cells were predominantly CD8+ (CD4:CD8 0.67:1) with an effector memory phenotype, compared with donor T cells from recipients of HSCs plus T cells (CD4:CD8 1.16:1), whereas the comparable donor T cells among recipients of CD11b+ APCs were predominantly CD4+ (CD4:CD8 1.28:1; p < 0.02) suggesting preferential activation of CD8+ donor T cells by CD11b− donor APCs.
Transplantation of purified CD11b− APCs promotes Th1 and cytotoxic T lymphocyte donor T cell (Tc) type 1 immune polarization of donor T cells
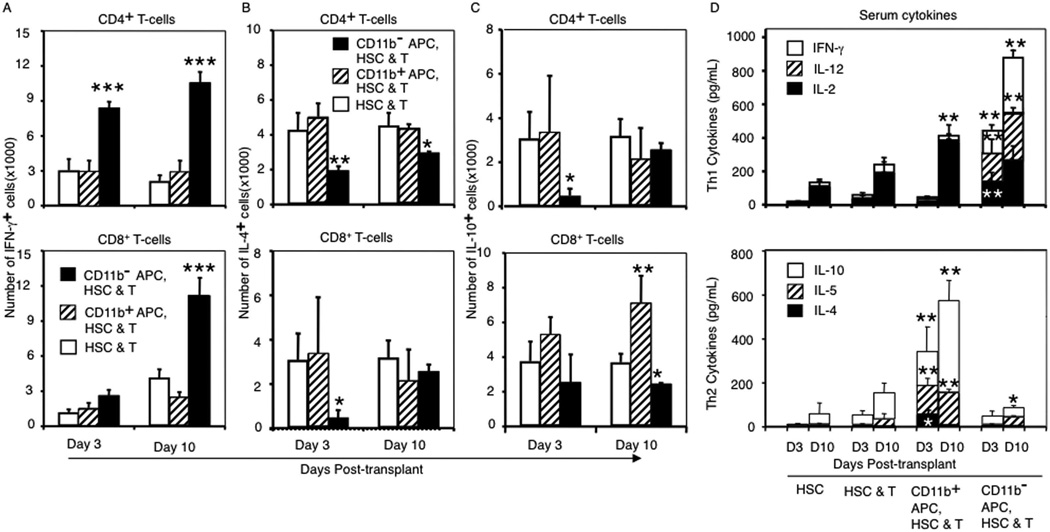
To characterize the effects of donor APC on the immune polarization of donor T cells, we measured intracellular synthesis of cytokines (IL-4, IL-10, IL-17, and IFN-γ) in donor (CD45.1+CD90.1+) CD4+ and CD8+ T cells on day 10 posttransplant and serum levels of IL-2, IL-4, IL-5, IL-10, IL-12, IL-23, IFN-γ and TNF-α. Recipients of CD11b− APC had higher frequencies and total numbers of Th1/Tc1 donor CD4+ and CD8+ T cells, lower numbers of Th2/Tc2 donor T cells, and significantly higher serum levels of Th1 cytokines compared with recipients of CD11b+ APC in which Th2/Tc2-polarized immune responses predominated (Fig. 3). There were no differences in frequencies of IL-17+ T cells comparing recipients of different donor APC preparations (data not shown).
FIGURE 3.
Transplanting CD11b− donor APCs lead to increased IFN-γ production by donor T cells. A–C, Mean numbers (±SD) of cytokine producing CD4 (top) and CD8 (bottom) T cells were determined per spleen. A, IFN-γ+ donor T cells; B, IL-4+ donor T cells; C, IL-10+ donor T cells. D, Th1 (top) and Th2 (bottom) cytokine concentrations determined in serum of recipients 3 and 10 days posttransplant. Data are from three experiments with a total of nine mice at each time point per group. p values are based on comparisons of cytokine levels from mice that received donor APCs with those that received HSCs and T cells. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To test whether B6→B10 T cell transfer resulted in activation of donor T cells specifically responding directly to host APCs (H2k, host restricted), we assessed the proliferative capacity of B6 donor T cells harvested 15 days posttransplant from B10 recipients in one-way MLR cultures. Donor T cells were harvested from HSCT recipients, stained with CFSE, and cultured for 3 days with irradiated syngeneic B6 splenocytes, recipient-type B10 splenocytes, or third-party BALB/c splenocytes. For all groups, donor T cells had minimal proliferation when cultured with syngeneic B6 or third-party BALB/c irradiated splenocytes (data not shown). In MLRs with recipient-type B10 irradiated splenocytes, donor CD4 and CD8 T cells from recipients of CD11b− APCs proliferated more than T cells from recipients of HSCs and T cells or recipients of HSCs, T cells, and CD11b+ APCs (supplemental Fig. 2A and B). Donor T cells from recipients of CD11b− APCs were Th1/Tc1 polarized compared with Th2/Tc2-polarization of T cells from recipients of CD11b+ APCs (supplemental Fig. 2C and D).
Donor APC and donor T cells colocalize in peripheral lymphoid tissues
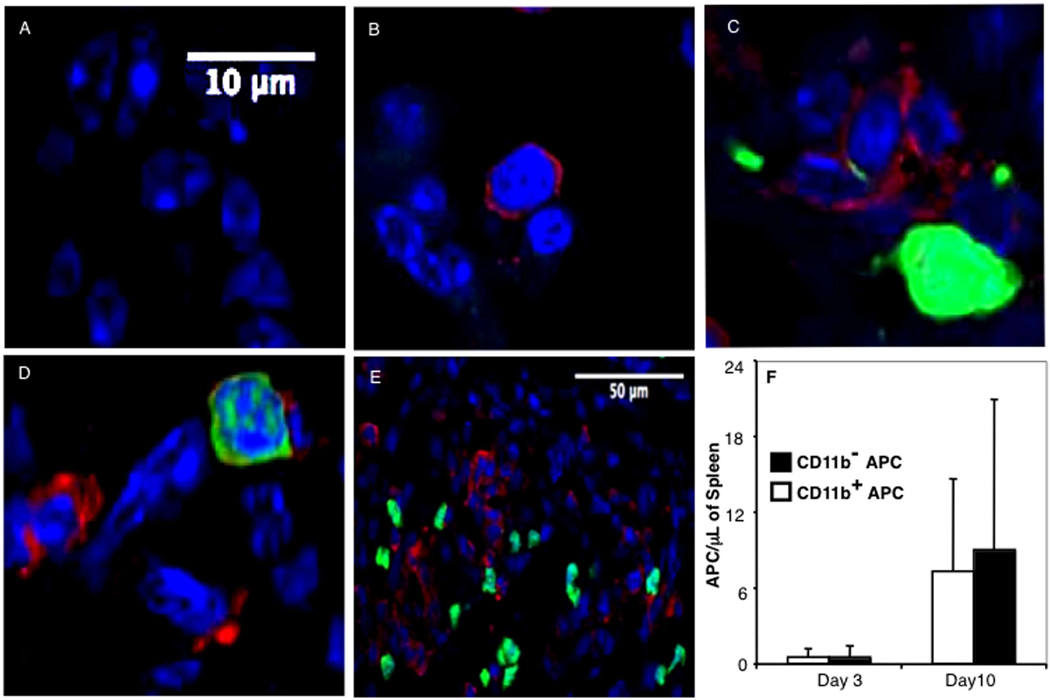
FACS-purified CD11b+ APC or CD11b− APCs from GFP-transgenic B6 donor mice were transplanted along with non-GFP B6 CD90.1+ HSCs and CD90.2+ T cells into lethally irradiated CD90.1+ B10 recipients. No GFP signal was observed in spleens of control recipients of HSCs or HSCs and T cells (Fig. 4, A and B). Equal numbers of GFP+ donor CD11b+, and CD11b− APCs with visible dendrites were seen in close physical proximity to donor T cells (and unstained host mononuclear cells) in sections of recipient spleen on days +3 and +10 posttransplant (Fig. 4, C–E), and the numbers of both APC subsets increased in the recipient spleen from day +3 to day +10 posttransplant over this time (Fig. 4F), consistent with in situ proliferation and/or continued migration of donor GFP+ APCs from other sites (35). GFP+CD11b− APCs and CD11b+ APCs were also observed in proximity to donor T cells in mesenteric lymph nodes and Peyer’s patches of transplant recipients (data not shown). These observations suggest equivalent abilities of both donor APC subsets to home to secondary lymphoid organs and argue that increased proliferation and Th1 polarization of donor T cells cotransplanted with donor CD11b− APCs is not due to differences in microanatomic localization.
FIGURE 4.
CD11b+ and CD11b− donor APCs home equivalently to lymphoid organs after transplantation. CD90.1+ B10 mice were transplanted with 3 × 103 B6 CD90.1+ HSC, 3 × 105 CD90.2+ T cells, and 5 × 104 GFP+ APC subsets. Spleen sections were prepared from mice sacrificed on days 3 and 10 posttransplant and were examined by confocal microscopy for donor APC (green) and donor T cells (red). Day 10 spleen sections were photographed using ×100 (A–D) and ×40 (E) objectives. A, Mice transplanted with HSCs alone. B, Mice transplanted with HSCs T cells. C, Mice transplanted with HSCs, T cells, and GFP+CD11b+ APCs. D–E, Mice transplanted with HSCs, T cells, and GFP+CD11b− APCs. F, The average number of APCs per microliter of splenic tissue was determined by microscopic analysis of histological sections. The experiment was repeated four times with three mice per group. Three slides per organ were screened, and five high-powered fields per slide were examined.
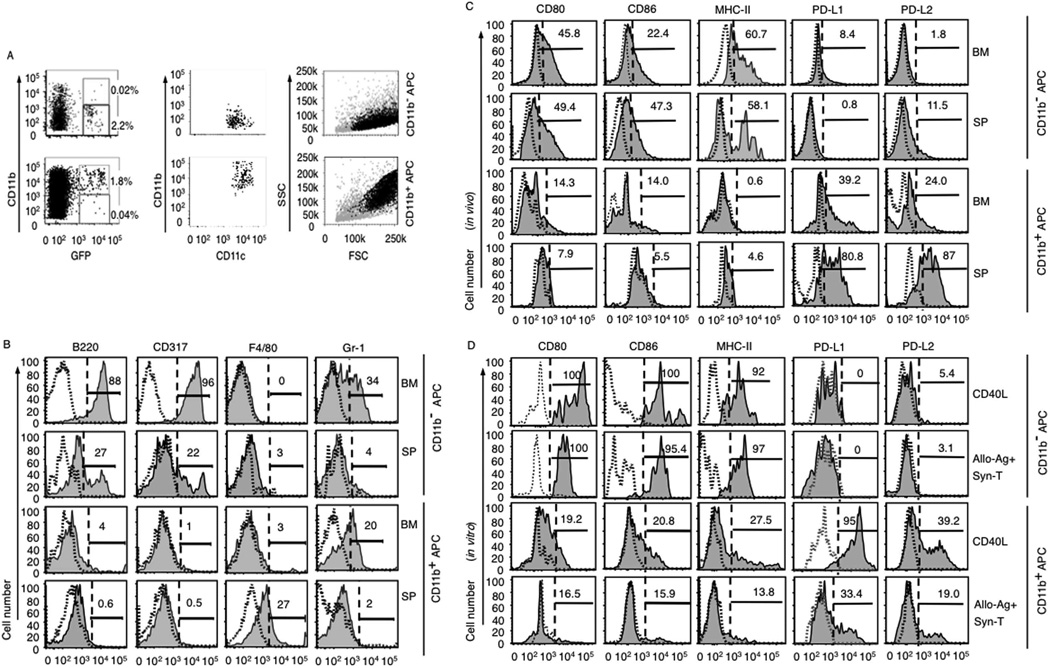
Differential development of APC precursors and expression of costimulatory molecules on CD11b+ APCs vs CD11b− APCs
To explore the impact of CD11b− and CD11b+ APC precursor development and differentiation on donor immune polarization, we tracked expression of lineage markers on GFP+ donor cells in recipient BM and spleen 10 days after transplantation of GFP+CD11c+CD11b− or CD11b+ APCs. The frequencies of GFP+CD11c+CD11b− and CD11b+ APCs were similar in BM (2.2 ± 0.2 and 1.8 ± 0.3%, respectively; Fig. 5A) and spleen (data not shown). Based on scatter plots, GFP+CD11b+ APCs had higher side scatter, consistent with myelomonocytic differentiation compared with GFP+CD11b− APC (Fig. 5A). Analysis of donor GFP+CD11b− APCs in recipient BM showed similar lineage expression patterns compared with cells taken directly from BM, whereas donor GFP+CD11b− APCs in recipient spleen showed moderately decreased frequencies of cells expressing B220, Gr-1, and CD317. In contrast, the frequency of GFP+CD11b+ APC-expressing F4/80 and Gr-1 were slightly increased in the spleen compared with GFP+CD11b− cells recovered from BM (Figs. 1G and 5B). Expression of CD3 and NK1.1 on GFP+ donor cells isolated from recipients of either GFP+CD11b− APCs or GFP+CD11b+ APCs was absent (data not shown). GFP+CD11b− APCs recovered from the spleens and BM of allogeneic transplant recipients had higher levels of MHC-II, CD80, and CD86, as well as lower levels of PD-L1 and PD-L2, compared with the GFP+ progeny of CD11b+ APCs (Fig. 5C). Low levels of expression of CD40 were seen only on CD11b+ APCs, and ICOS-L was largely absent on both CD11b− and CD11b+ APCs (data not shown). There were higher levels of costimulatory and coinhibitory molecule expression on GFP+ donor APCs recovered from recipient spleen vs recipient BM, indicating that factors in the tissue microenvironment may also have a significant role in the maturation of transplanted donor APCs. In parallel, FACS-purified CD11b+ and CD11b− APCs were analyzed for MHC-II, CD80, CD86, PD-L1, and PD-L2 expression before and after stimulation with CD40L, irradiated allogeneic splenocytes, and the combination of syngeneic T cells and allogeneic Ags. In vitro stimulation with CD40L (36, 37) or alloantigen with syngeneic T cells led to marked up-regulation of MHC-II, CD80, and CD86 expression on CD11b− APC, and up-regulation of PD-L1 and PD-L2 on CD11b+ APC (Fig. 5D). The level of expression of activation and maturation markers on donor APC in vivo were lower than the levels of the same markers expressed on APC populations cultured in vitro with CD40L (Fig. 5, C and D).
FIGURE 5.
Costimulatory molecule expression is increased following activation of CD11b− APCs. A, Initial gating on donor-derived GFP+ cells in recipient BM and spleen, and subsequent identification of GFP+CD11c+CD11b+ APC populations and GFP+CD11c+CD11b− APC populations. B–D, Isotype controls are shown as dotted line histograms. B, Differentiation of CD11b− APC precursors and CD11b+ APC precursors tracked by expression of lineage markers B220, F4/80, Gr-1, and CD317 on GFP+ donor cells 10 days posttransplant. C, Expression of costimulatory molecules on APC subsets from B6 GFP+ BM 10 days following transplantation in B10 recipients. Recipient BM and splenocytes (SP) were analyzed by flow cytometry with electronic gates on GFP+ cells and concatenation of list mode files of at least 1,000,000 events from each of 3 mice per treatment group. Results are from one of five independent experiments with similar results. D, Costimulatory molecule expression on BM CD11b− and CD11b+ APC subpopulations after stimulation with either CD40L or syngeneic T cells with allogeneic Ag. Data represent analysis of 6 × 105 cultured APCs and are representative of three replicate experiments, each with thee parallel wells per experimental condition. FSC, Forward scatter.
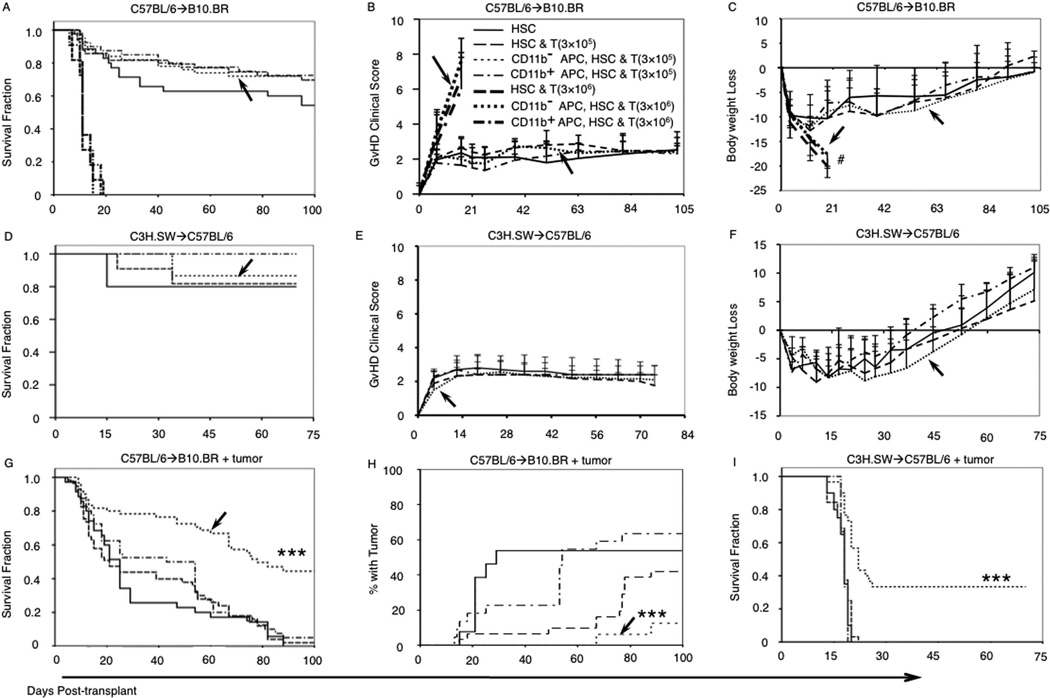
Donor CD11b− APCs augmented GvL activity of donor T cells without increasing GvHD
We next tested the effect of varying numbers of donor T cells transplanted with CD11b+ APCs or CD11b− APCs on GvHD in the MHC-mismatched (B6→B10) murine allogeneic HSCT model. As expected, recipients of a larger dose of donor T cells (3 × 106) developed lethal GvHD compared with minimal GvHD among recipients of a lower donor T cell dose (3 × 105; Fig. 6, A and B). Clinical GvHD scores and body weight loss for the recipients of 3 × 105 T cells plus CD11b− APCs were low and not significantly different from other treatment groups with the same T cell dose and different donor APCs (Fig. 6, B and C); mean clinical GvHD scores for recipients of 3 × 106 T cells were much higher but did not vary significantly among groups with different donor APCs (Fig. 6B). In the MHC-matched, MiHA-mismatched model (C3H.SW→B6), there was no difference in survival, GvHD clinical sores, and body weight loss between recipients of different grafts in the absence of tumor (Fig. 6, D–F). To test whether donor CD11b− APC could augment the GvL activity of low-dose T cells, we transplanted 3 × 105 donor T cells and 3000 HSC with 5 × 104 amounts of either population of donor BM APCs, using two distinct allogeneic HSCT models. Host-type tumor cells were injected one day before HSCT and following total body irradiation. In the MHC-mismatched B6→B10.BR model, recipients of CD11b− APCs had 45% durable survival (Fig. 6G) compared with <5% survival among tumor-bearing recipients of HSCs alone; HSCs plus T cells; or HSCs, T cells, and CD11b+ APCs (p < 0.001). Necropsy of moribund B10.BR recipient mice that were euthanized showed a lower incidence of detectable leukemia among recipients of CD11b− APCs compared with other groups (Fig. 6H). In the C3H.SW→B6 MiHA-mismatched model, recipients of CD11b− APCs had 35% long-term survival compared with uniform early mortality among other treatment groups (Fig. 6I). The GvL activities in the C3H.SW→B6 model observed using mixed CD4+ and CD8+ donor T cells or purified CD8 T cells were identical (data not shown), indicating that our transplant model is comparable with others that use only CD8 T cells (4, 17).
FIGURE 6.
Addition of CD11b− APCs to an allogeneic graft of HSC and T cells increased leukemia-free survival without increasing GvHD. A, Survival of mice that received various doses (0, 3 × 105, or 3 × 106) of donor splenic T cells and CD11b− or CD11b+ APCs in B6→B10 transplant pair with no leukemia cells (see legend in B). B, Mean GvHD clinical scores in B6→B10 recipients with no leukemia. C, Body weight loss in B6→B10 recipients with no leukemia. D–I, HSCs and 3 × 105 T cell dose groups only. D, Survival of mice in C3H→B6 transplants with no leukemia cells. E, Mean GvHD clinical scores in C3H→B6 recipients with no leukemia cells. F, Body weight loss in C3H→B6 recipients with no leukemia cells. G, Survival of B10 mice that received B6 transplants with 1 × 105 viable LBRM leukemia cells in 5 separate experiments with 10 mice per experimental group. H, The fraction of mice from G with pathological evidence for tumor at necropsy. I, Survival of B6 mice that received C3H.SW transplants with 5 × 104 viable MMB3.19 leukemia cells, in 2 experiments, with 10 mice per experimental group. Arrows,i transplant groups that received CD11b− APC. #, Mice euthanized due to body weight loss were ≥25%. p values (***, p < 0.001) represent log-rank comparison of survival of recipients of CD11b− APCs with recipients of HSCs and T cells.
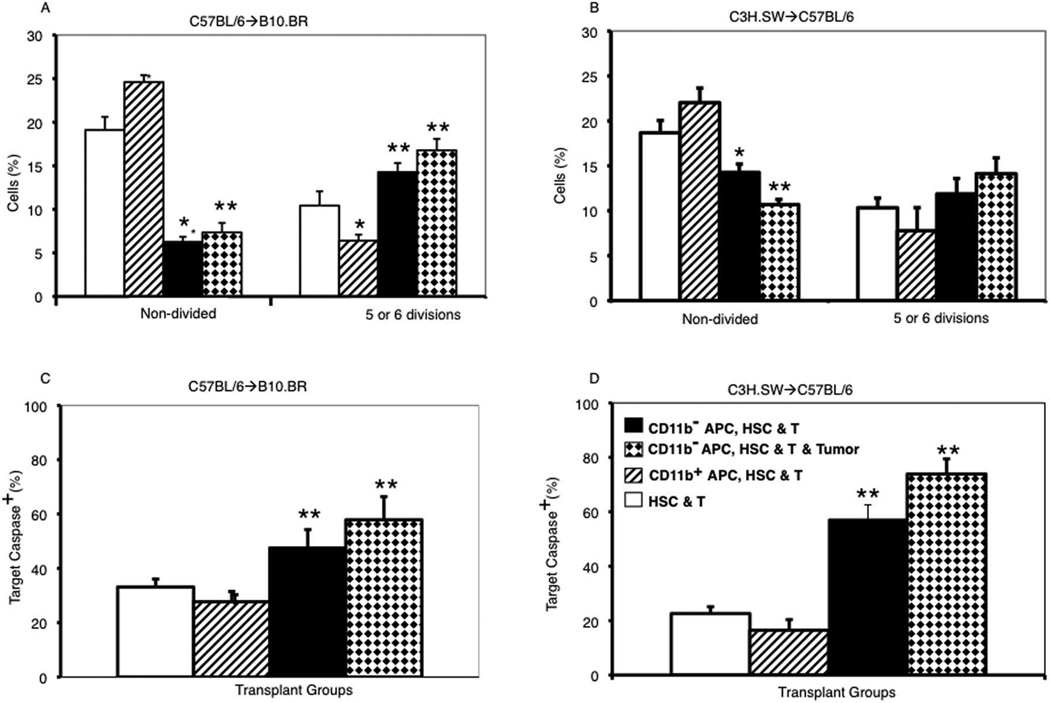
Donor CD11b− APCs enhanced T cell proliferation and CTL activity ex vivo
To further address mechanisms whereby CD11b− APC enhanced the GvL activity of low-dose donor T cells, we assessed the cytotoxic activity of donor T cells recovered from recipients of CD11b− APCs compared with recipients of other graft combinations. Well-appearing recipients of MHC-mismatched and MiHA-mismatched transplants were euthanized on days 81 and 34 posttransplant, respectively, and donor T cells derived from the mature donor T cells in the original transplant graft were selected by MACS using the CD45.1 congenic marker. Donor T cells recovered from recipients of CD11b− APCs had increased proliferation in a one-way MLR following culture with irradiated host-type allogeneic splenocytes compared with donor T cells recovered from recipients of CD11b+ APCs or T cells from recipients without donor APCs (Fig. 7, A and B). In addition, antileukemia cytotoxic activity was significantly greater among T cells recovered from recipients of CD11b− APCs than among T cells from other treatment groups (Fig. 7, C and D).
FIGURE 7.
Transplanting donor CD11b− APC increased long-term alloreactivity, Th1 polarization and antitumor cytotoxicity of donor T cells. Donor T cells were recovered from the spleens of B6→B10 or C3H.SW→B6 recipients on days 81 or 34 posttransplant, respectively, and CFSE-labeled as described in Materials and Methods. A and B, Mean percentages (±SD) of nondivided T cells and T cells that had undergone five to six cell divisions after 3 days of culture with irradiated host-type splenocytes are shown (three mice from each transplant group). C and D, Killing of allogeneic leukemia cells by donor T cells. Mean percentages (±SD) of caspase+ LBRM targets (C) or MMB3.19 targets (D) following a 3-h incubation with T cells at an E:T ratio of 10:1 are shown (three to five mice from each transplant group). *, p < 0.05; **, p < 0.01, comparing T cells from recipients of HSCs and T cells plus CD11b− APCs with T cells from recipients of HSCs and T cells without DC.
IFN-γ does not directly kill leukemia cells
To rule out the possibility that the observed GvL effect of CD11b− APCs was due to direct antitumor effects of IFN-γ, we cocultured LBRM or MMB3.19 cells (2 × 10/ml) with various concentrations of IFN-γ. In vitro exposure of leukemia cells to IFN-γ, at doses of 10–300 pg/ml, similar to concentrations observed in vivo, demonstrated neither cytotoxicity nor long-term growth-inhibitory effects on either leukemia cell line over 5 days of culture (data not shown).
Discussion
Our findings indicate that distinct subsets of donor APC are associated with quite different immunological effects posttransplant. Using MHC-mismatched and MiHA-mismatched HSCT model systems, leukemia-bearing recipients of CD11b− APC had substantially enhanced survival compared with recipients of HSCs alone, HSCs plus T cells, or HSCs plus CD11b+ APCs. Significantly, the addition of CD11b− APCs to grafts containing a low number of donor T cells did not significantly affect clinical GvHD mortality compared with mice transplanted with HSC and T cells (without donor APC). These findings represent, to our knowledge, the first clear demonstration that a purified subset of donor APCs can increase GvL activity without a concomitant increase in GvHD when added to grafts containing purified HSCs and T cells.
Previous studies have shown that donor T cells and DCs isolated from donors pretreated with hemopoietic cytokines or cytokine receptor agonists led to decreased GvL (38) and increased acute and chronic GvHD (38, 39). In contrast to using donor DCs mobilized by cytokines (23, 40), or DCs derived from cultured BM (41) or spleens (17), or bulk BM cells as donor APCs (4), we chose to use unstimulated populations of phenotypically defined APC subsets isolated by FACS from the BM in an attempt to recapitulate our previous clinical observations that more BM pDCs were associated with reduced GvL activity after allogeneic HSCT (42). The BM CD11b− APC used in this study were nearly all pDC progenitors as evidenced by their expression of B220 and PDCA-1 (43, 44). In contrast, the CD11b+ APCs are heterogeneous and contained cells with the phenotypes of myelomonocytic precursors (16, 19, 20).
Although the current findings support clinical observations that donor DCs have a role in transplant outcomes, there is no direct correspondence between the immunological functions of murine vs human pDC. Our previous report on human BMT indicated that larger numbers of donor pDC suppressed GvL (42), whereas the current study, using murine BMT models, indicates that donor CD11b− APCs (the majority of which are pDC) augment the GvL activity of donor T cells. Although both human and murine pDCs are important in innate immunity and synthesize large amounts of IFN-α in response to viral infection or to the binding of CpG sequences to TLR9 (24, 45), their effects on T cell immune polarization are quite different. Mature murine BM-derived pDCs (but not blood-derived pDCs) produce significant amounts of IL-12 and polarize T cells toward Th1 immune responses in vitro (21, 22, 23, 24). In contrast, CD11c− human pDCs do not make significant amounts of IL-12, and they generate Th2 immunity in cognate T cells responding to direct or indirect Ag presentation (21, 46). In humans, the CD11c+ myeloid DCs make IL-12 and polarize responding T cells toward Th1 immune responses (46, 47). Thus, from the standpoint of IL-12 production and Th1 polarization of donor T cells, we believe that the function of the murine CD11b− APCs is analogous to the CD11c+ human myeloid DC subset.
To delineate the mechanisms by which the CD11b− subset of donor APCs enhanced GvL activity compared with CD11b+ APCs, we evaluated the homing and proliferation of APCs to secondary lymphoid organs on transplant recipients and equivalent numbers of both APC subsets were seen in the spleens and lymph nodes during the first 10 days posttransplant. Both types of APC subsets freshly isolated from BM lacked activation and maturation markers MHC-II, CD80, CD86, and ICOS-L, indicating an immature level of differentiation that was not affected by FACS isolation or exposure to medium containing FBS (48, 49, 50). Donor CD11b− APCs that homed to the BM retained an immature phenotype, whereas donor CD11b− APCs that homed to and proliferated in the spleen up-regulated the same pattern of activation and maturation markers after in vitro exposure to CD40L, or following exposure to alloantigen and homologous T cells in one-way MLR, consistent with pDC differentiation. The levels of activation and maturation marker expression on the donor APCs recovered from allogeneic transplant recipients were lower compared with the expression of those markers in vitro, suggesting that GvHD may impair the maturation of pDC (51). The lack of increased GvHD seen among recipients of CD11b− APCs are consistent with the conclusions of Banovic et al. (51) that bone marrow pDCs are immunomodulatory and may decrease GvHD. The Th1 polarization of donor T cells seen in our study was not described by Banovic, possibly due to differences in the model systems and the absence of more potent APCs (donor conventional DCs) and other accessory cells in grafts composed of purified cell subsets that were used in our studies.
Transplanting CD11b− APCs resulted in a global effect of early Th1 polarization with higher serum levels of IL-12, IFN-γ, IL-2, and Th1-polarized donor T cells. The increased numbers of donor T cells with direct antitumor cytotoxicity seen with CD11b− APCs in the MHC-mismatched model are consistent with cross-presentation (52) of alloantigen by CD11b− APCs to donor T cells, leading to activation and subsequent activation and Th1 polarization of additional donor T cells with direct cytotoxic activity against host hemopoietic cells (including leukemia targets). Furthermore, IFN-γ did not have direct tumoricidal activity, indicating that the observed increase in GvL activity of donor T cells cotransplanted with CD11b− APCs was due to their enhanced antitumor cytotoxicity. We have also shown, using IFN-γ knockout mice as T cell donors in the B6→B10 transplant model, that IFN-γ synthesis by donor T cells is critical to the survival of tumor-bearing recipients of CD11b− APCs (Y. Lu et al., manuscript in preparation), consistent with models in which IFN-γ production enhances the GvL activity of donor T cells while limiting GvHD mortality (7, 53, 54, 55).
These results are in contrast with reports by Shlomchik and others in which persistent host APCs are integral to the initiation of GvHD, and donor APCs have only a weak effect on GvHD and no effect on GvL (4, 5, 17). These models used much higher doses of T cells that caused lethal GvHD. Other differences between our results and previous reports include the use of FACS-purified populations of donor BM APC in our study, whereas other studies used unfractionated BM or splenic donor APCs that may have obscured the effect of donor APC subpopulations on donor T cell immune function. A recent report by Koyama et al. (25) also show that host-type pDCs harvested from Flt2/Flk3 ligand-treated mice primed Th1 immune responses in donor T cells and induced lethal GvHD when transplanted into lethally irradiated allogeneic recipients. The higher level of GvHD observed in this study likely results from the use of larger numbers of donor T cells (2 × 106) and adoptive transfer of recipient-type Flt2/Flk3 ligand-activated pDC rather than resting BM donor pDCs in our study. The radiation doses used in the study by Koyama et al. (56) ablate endogenous host-type pDCs, raising the question of the physiological relevance of host pDCs in the initiation of GvHD following myeloablative conditioning. Nevertheless, these results support our findings that donor pDCs can initiate Th1 immune responses in responding T cells. A recent report by Markey et al. (57) explored the role for different donor DC populations in activating donor T cells in allogeneic BMT. In this study, transgenic donor T cells were administered on day 10 posttransplant, and different subpopulations of donor DCs deleted through the administration of toxins or mAbs. The results indicate that classical CD11c+ DCs were most efficient in stimulating donor T cell proliferation by indirect presentation of donor alloantigens and that the role of donor pDC was minimal. Although these two model systems are quite different, both results support the importance of donor APCs in activating donor T cells. Ongoing experiments will test whether the salutorious antitumor effect of donor pDC in the experiments presented herein are due either to 1) an ability of pDCs to present alloantigen indirectly to donor T cells (in contrast to the data presented by Markey et al.) or 2) the ability of donor pDCs to modulate T cell function in response to host APCs through local production of cytokines such as IL-12 or IFN-α. Additional reasons for the improved survival seen among recipients of pDCs in our study may be that donor pDCs more efficiently activate donor T cells administered with the stem cell graft vs donor T cells administered at later time points posttransplant; that donor pDCs may facilitate donor HSC engraftment (33, 58); or that donor pDCs may induce IFN-γ production by donor T cells and thereby limit the development of GvHD (7, 53, 54, 55).
In contrast to the Th1 polarization seen with donor pDCs, transplanting CD11b+ APCs yielded donor T cells with Th2 polarization and higher serum IL-4, IL-5, and IL-10. Whereas the CD11b− APC are nearly all pDC precursors, the CD11b+ APCs are more heterogeneous, and express a pattern of markers that are expressed on precursors for monocytes as well as myeloid suppressor cells. The expression of MHC-II and CD80CD86 on GFP+CD11b+ APCs recovered from transplant recipients was minimal, indicating limited differentiation to DCs. In contrast, the progeny of CD11b+ APC expressed high levels of immunosuppressing costimulatory molecules PD-L1 and PD-L2 (36, 59) and increased levels of Gr-1 and F4/80 expression, consistent with differentiation of some CD11b+ APCs into myeloid suppressor cells (16, 19, 20) or immunosuppressive monocytes (60). The expression of PD-L1 and PD-L2 on CD11b+ APCs is consistent with previous reports showing that CD11b+ donor splenocytes and CD11b+ donor BM cells suppress host-vs-graft alloreactivity in myeloablative and nonmyeloablative transplants, respectively (16, 32), and that murine CD11b+CD8α− DCs result in Th2 or mixed responses (23, 26).
Some of the limitations of this study are that we did not attempt to isolate the subpopulations of pDCs with varying abilities to polarize Th1 and Th2 immunity depending on the level of the Rag1 promoter (61, 62), nor could we vary the level of alloantigen presented by donor DCs, an additional factor that has been shown to affect the ability of DC subsets to direct Th1 vs Th2 T cell polarization (63). The population of CD11b+ APC was clearly heterogeneous, and we cannot be certain which subpopulation is responsible for the observed Th2 polarization of donor T cells. Nevertheless, our data support a model in which different APC subsets result in distinctly different types of T cell polarization (14).
The current findings from murine HSCT models may have potential for clinical translation, as selective depletion of human pDCs from mononuclear cells isolated from blood, using a mAb to BDCA2 and immunomagnetic columns, resulted in marked enhancement of the ability of the remaining cells to activate homologous T cells to alloantigen via cross presentation (46). Taken together, these data suggest that selective manipulation of the content of donor APCs from BM grafts could be a novel method to enhance the GvL activity of allogeneic HSC transplantation.
In summary, using two distinct HSCT model systems in which donors and recipients are either MHC or MiHA mismatched, we observed highly significant Th1 polarization of donor T cells and improvements in the survival of tumor-bearing recipients of FACS-purified CD11b− pDCs compared with other transplant groups. These observations indicate a role for donor APC in regulating GvL activities.
Supplementary Material
Acknowledgments
We thank Drs. James C. Zimring and Robert Mittler for their careful reading of the manuscript and helpful comments.
National Institute of Health grants R01 CA-74364-03 to EK Waller and NHLBI P01Hl086773 to CD Hillyer and EK Waller supported this study. An Amy Strelzer Manasevit fellowship sponsored by the National Marrow Donor Program and the SuperGen Corporation and a research fund from When Everyone Survives Foundation, Inc. supported J-M. Li.
Footnotes
Disclosures
E.K.W. has nonlicensed patents in China and Australia that describe the potential clinical utility of manipulating the DC content of hemopoietic progenitor cell allografts. The other authors declare no competing financial interests.
This work was supported by National Institute of Health Grants R01 CA-74364-03 (to E.K.W.) and NHLBI P01Hl086773 (to C.D.H. and E.K.W.) and an Amy Strelzer Manasevit fellowship sponsored by the National Marrow Donor Program and the SuperGen Corporation and a research fund from When Everyone Survives Foundation, Inc. (J.-M.L.).
Abbreviations used in this paper: GvHD, graft-vs-host disease; HSCT, hemopoietic stem cell transplantation; MHC-II, MHC class II; BM, bone marrow; DC, dendritic cell; GvL, graft-vs-leukemia; B6, C57BL/6; B10, B10.BR; B/c, BALB/c; Lin, lineage; MiHA, minor histocompatibility Ag; Syn, syngenic; pDC, plasmacytoid DC precursor; PD-L, programmed death ligand; PD-L2, programmed death ligand-2; Tc, cytotoxic T lymphocyte donor T cell; PDCA, plasmacytoid DC Ag; Tc, cytotoxic T lymphocyte donor T cell; HSC, hematopoietic stem cell.
The online version of this article contains supplemental material.
Supplementary Material http://www.jimmunol.org/content/suppl/2009/11/23/jimmunol.0900155.DC1.html
References
- 1.Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, Najfeld V, Phelps RG, Grosskreutz C, Scigliano E, Frenette PS, Merad M. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J. Exp. Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durakovic N, Bezak KB, Skarica M, Radojcic V, Fuchs EJ, Murphy GF, Luznik L. Host-derived Langerhans cells persist after MHC-matched allografting independent of donor T cells and critically influence the alloresponses mediated by donor lymphocyte infusions. J. Immunol. 2006;177:4414–4425. doi: 10.4049/jimmunol.177.7.4414. [DOI] [PubMed] [Google Scholar]
- 4.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 5.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 6.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat. Med. 2004;10:510–517. doi: 10.1038/nm1038. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 9.Waller EK, Rosenthal H, Jones TW, Peel J, Lonial S, Langston A, Redei I, Jurickova I, Boyer MW. Larger numbers of CD4bright dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood. 2001;97:2948–2956. doi: 10.1182/blood.v97.10.2948. [DOI] [PubMed] [Google Scholar]
- 10.Dean R, Masci P, Pohlman B, Andresen S, Serafino S, Sobecks R, Kuczkowski E, Curtis J, Maciejewski J, Rybicki L, et al. Dendritic cells in autologous hematopoietic stem cell transplantation for diffuse large B-cell lymphoma: graft content and post transplant recovery predict survival. Bone Marrow Transplant. 2005;36:1049–1052. doi: 10.1038/sj.bmt.1705183. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 11.Reddy V, Iturraspe JA, Tzolas AC, Meier-Kriesche HU, Schold J, Wingard JR. Low dendritic cell count after allogeneic hematopoietic stem cell transplantation predicts relapse, death, and acute graft-versus-host disease. Blood. 2004;103:4330–4335. doi: 10.1182/blood-2003-09-3325. [DOI] [PubMed] [Google Scholar]
- 12.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 13.Hume DA. Macrophages as APC and the dendritic cell myth. J. Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 14.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002;2:151–161. doi: 10.1038/nri746. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JM, Waller EK. Donor antigen-presenting cells regulate T-cell expansion and antitumor activity after allogeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 2004;10:540–551. doi: 10.1016/j.bbmt.2004.05.007. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 17.Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat. Med. 2004;10:987–992. doi: 10.1038/nm1089. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 18.O'Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J. Exp. Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg TK, Kraal G. A function for the macrophage F4/80 molecule in tolerance induction. Trends Immunol. 2005;26:506–509. doi: 10.1016/j.it.2005.07.008. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 20.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 21.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 22.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Keeffe M, Hochrein H, Vremec D, Scott B, Hertzog P, Tatarczuch L, Shortman K. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. 2003;101:1453–1459. doi: 10.1182/blood-2002-03-0974. [DOI] [PubMed] [Google Scholar]
- 25.Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, Niiro H, Harada M, Tanimoto M, Akashi K, Teshima T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8±+ and CD8±− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korngold R, Leighton C, Manser T. Graft-versus-myeloid leukemia responses following syngeneic and allogeneic bone marrow transplantation. Transplantation. 1994;58:278–287. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [PubMed] [Google Scholar]
- 28.Gillis S, Mizel SB. T-cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc. Natl. Acad. Sci. USA. 1981;78:1133–1137. doi: 10.1073/pnas.78.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waller EK, Ship AM, Mittelstaedt S, Murray TW, Carter R, Kakhniashvili I, Lonial S, Holden JT, Boyer MW. Irradiated donor leukocytes promote engraftment of allogeneic bone marrow in major histocompatibility complex mismatched recipients without causing graft-versus-host disease. Blood. 1999;94:3222–3233. [PubMed] [Google Scholar]
- 30.Giver CR, Montes RO, Mittelstaedt S, Li JM, Jaye DL, Lonial S, Boyer MW, Waller EK. Ex vivo fludarabine exposure inhibits graft-versus-host activity of allogeneic T cells while preserving graft-versus-leukemia effects. Biol. Blood Marrow Transplant. 2003;9:616–632. doi: 10.1016/s1083-8791(03)00229-5. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 31.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte, Jr J, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation. I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 32.Li JM, Gorechlad J, Larsen CP, Waller EK. Apoptotic donor leukocytes limit mixed-chimerism induced by CD40-CD154 blockade in allogeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 2006;12:1239–1249. doi: 10.1016/j.bbmt.2006.08.038. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 33.Fugier-Vivier IJ, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, Chilton PM, Ildstad ST. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J. Exp. Med. 2005;201:373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: crosscorrelation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 35.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J. Immunol. 2004;173:2632–2640. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]
- 38.Banovic T, MacDonald KP, Morris ES, Rowe V, Kuns R, Don A, Kelly J, Ledbetter S, Clouston AD, Hill GR. TGF-β in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106:2206–2214. doi: 10.1182/blood-2005-01-0062. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald KP, Rowe V, Filippich C, Thomas R, Clouston AD, Welply JK, Hart DN, Ferrara JL, Hill GR. Donor pretreatment with progenipoietin-1 is superior to granulocyte colony-stimulating factor in preventing graft-versus-host disease after allogeneic stem cell transplantation. Blood. 2003;101:2033–2042. doi: 10.1182/blood-2002-05-1529. [DOI] [PubMed] [Google Scholar]
- 40.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell. Immunol. 1996;170:111–119. doi: 10.1006/cimm.1996.0140. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 42.Waller EK, Rosenthal H, Sagar L. DC2 effect on survival following allogeneic bone marrow transplantation. Oncology. 2002;16:19–26. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [PubMed] [Google Scholar]
- 43.Li JM, Waller EK. The yin and yang of adaptive immunity in allogeneic hematopoietic cell transplantation: donor antigen-presenting cells can either augment or inhibit donor T cell alloreactivity. Adv. Exp. Med. Biol. 2007;590:69–87. doi: 10.1007/978-0-387-34814-8_5. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 44.Kohara H, Omatsu Y, Sugiyama T, Noda M, Fujii N, Nagasawa T. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12-CXCR4 chemokine signaling. Blood. 2007;110:4153–4160. doi: 10.1182/blood-2007-04-084210. [DOI] [PubMed] [Google Scholar]
- 45.Martin P, Del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez LA, Parrillas V, Ardavin C. Characterization of a new subpopulation of mouse CD8±+B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 46.Lonial S, Torre C, David E, Harris W, Arellano M, Waller EK. Regulation of alloimmune responses by dendritic cell subsets. Exp. Hematol. 2008;36:1309–1317. doi: 10.1016/j.exphem.2008.04.021. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 47.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 48.Haase C, Ejrnaes M, Juedes AE, Wolfe T, Markholst H, von Herrath MG. Immunomodulatory dendritic cells require autologous serum to circumvent nonspecific immunosuppressive activity in vivo. Blood. 2005;106:4225–4233. doi: 10.1182/blood-2005-03-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 2004;114:280–290. doi: 10.1172/JCI21583. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 2006;7:663–671. doi: 10.1038/ni1340. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 51.Banovic T, Markey KA, Kuns RD, Olver SD, Raffelt NC, Don AL, Degli-Esposti MA, Engwerda CR, MacDonald KP, Hill GR. Graft-versus-host disease prevents the maturation of plasmacytoid dendritic cells. J. Immunol. 2009;182:912–920. doi: 10.4049/jimmunol.182.2.912. [DOI] [PubMed] [Google Scholar]
- 52.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang YG, Qi J, Wang MG, Sykes M. Donor-derived interferon γ separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8 T cells. Blood. 2002;99:4207–4215. doi: 10.1182/blood.v99.11.4207. [DOI] [PubMed] [Google Scholar]
- 54.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat. Med. 2005;11:1244–1249. doi: 10.1038/nm1309. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PubMed] [Google Scholar]
- 55.Asavaroengchai W, Wang H, Wang S, Wang L, Bronson R, Sykes M, Yang YG. An essential role for IFN-γ in regulation of alloreactive CD8 T cells following allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2007;13:46–55. doi: 10.1016/j.bbmt.2006.09.014. http://sfxhostedexlibrisgroupcom/emu/sfxgif. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markey KA, MacDonald KP, Hill GR. Recipient plasmacytoid DCs are not required to prime allogeneic T-cell responses after BMT. Blood. 2009;113:6038–6039. doi: 10.1182/blood-2009-03-212944. [DOI] [PubMed] [Google Scholar]
- 57.Markey KA, Banovic T, Kuns RD, Olver SD, Don AL, Raffelt NC, Wilson YA, Raggatt LJ, Pettit AR, Bromberg JS, Hill GR, MacDonald KP. Conventional dendritic cells are the critical donor APC presenting alloantigen after experimental bone marrow transplantation. Blood. 2009;113:5644–5649. doi: 10.1182/blood-2008-12-191833. [DOI] [PubMed] [Google Scholar]
- 58.Colson YL, Christopher K, Glickman J, Taylor KN, Wright R, Perkins DL. Absence of clinical GVHD and the in vivo induction of regulatory T cells after transplantation of facilitating cells. Blood. 2004;104:3829–3835. doi: 10.1182/blood-2004-01-0393. [DOI] [PubMed] [Google Scholar]
- 59.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 60.Mielcarek M, Graf L, Johnson G, Torok-Storb B. Production of interleukin-10 by granulocyte colony-stimulating factor-mobilized blood products: a mechanism for monocyte-mediated suppression of T-cell proliferation. Blood. 1998;92:215–222. [PubMed] [Google Scholar]
- 61.Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, Busslinger M, Kincade PW. Derivation of two categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamogawa-Schifter Y, Ohkawa J, Namiki S, Arai N, Arai K, Liu Y. Ly49Q defines 2 pDC subsets in mice. Blood. 2005;105:2787–2792. doi: 10.1182/blood-2004-09-3388. [DOI] [PubMed] [Google Scholar]
- 63.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O'Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J. Exp. Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.