Abstract
Background
Human immunodeficiency virus (HIV)-infected patients are at increased risk for squamous cell carcinoma of the anal canal (SCCA) and the incidence of SCCA has increased in the era of highly active antiretroviral therapy. The outcome of SCCA in HIV-positive patients has not been evaluated in prospective trials and the published literature is limited to retrospective case series. The aim of this study is to describe the treatment, toxicity, and overall survival (OS) in patients with and without HIV infection.
Methods
We performed a retrospective chart review of all patients treated for invasive SCCA at Karmanos Cancer Institute, Wayne State University from 1991 to 2007 and collected data regarding HIV status, demographics, stage at diagnosis, treatment, response to treatment, toxicity, and survival.
Results
Forty-five patients with SCCA were identified, of whom 13 were HIV-positive and 32 were HIV-negative. HIV-positive patients were younger (median age, 45 vs. 57 years) and had a higher frequency of men (89% vs. 37%). Patients were balanced for presenting stage at diagnosis and rates of local recurrence were found to be similar between the 2 groups. HIV-positive patients were less likely to receive full dose chemoradiotherapy. Except for dermatitis, the incidence of grade 3 to 4 toxicities was similar in both groups. Median OS was 33.5 months for HIV-positive patients and 71.8 months for HIV-negative patients. Although limited by the small size of the study, the OS was not statistically significantly different by HIV status (P = 0.787).
Conclusion
Although the HIV-positive patients received lower dose chemoradiotherapy, no major difference in local control or overall survival was observed.
Squamous cell cancer of the anus (SCCA) is a rare disease with a projected incidence for the year 2009 of 5000 cases in the United States.1 Over the past 3 decades, the incidence of SCCA has increased by approximately 90% in men and 40% in women.2 The risk of SCCA is 120 times higher in human immunodeficiency virus (HIV)-infected patients.3, 4 With the advent of highly active antiretroviral therapy (HAART), the survival of patients with HIV infections has significantly increased. Unfortunately, the risk of developing SCCA, unlike other HIV-associated cancers has not been impacted by the introduction of HAART.4, 5 SCCA is also associated with human papillomavirus (HPV)-infection and may be preceded by high-grade anal intraepithelial neoplasia. Both high-grade anal intraepithelial neoplasia and anal HPV infection are highly prevalent in high risk groups such as HIV-infected patients. The problem of HPV-related cancers has also not declined among HIV-positive patients in the antiretroviral therapy era.6 Therefore, the incidence of SCCA in HIV-infected patients is expected to continue increasing.
Nigro et al established mitomycin, 5 fluorouracil (5-FU) concomitant with radiotherapy as the standard treatment for SCCA.7 Subsequent randomized trials confirmed that the 5-year colostomy-free survival and overall survival (OS) rates of patients with SCCA treated with chemoradiotherapy were 61% to 71%, and 71% to 78%, respectively. 8–10 The incidence of severe toxicities in HIV-negative patients associated with this regimen is relatively high and includes hematologic toxicity (18%–26%), dermatologic toxicity (47%–56%), and acute GI toxicity (33%–47%).8–10
The management of SCCA in the HIV-positive patients is complicated by the immune suppression from the HIV infection and the potential interaction between HAART and chemoradiotherapy. Several retrospective series of SCCA patients with HIV infection on HAART have been reported with conflicting results. Some series have reported comparable survival for HIV-positive and negative patients with SCCA.11, 12 Oehler-Janne et al reported comparable survival with a higher risk of local recurrence in HIV-infected patients.13 Other series have found a higher incidence of toxicities in the HIV-infected patients and worse outcome.14, 15 In the absence of prospective randomized trials, there is currently no consensus on the management of SCCA in HIV-infected patients. The aim of this study was to compare the treatment regimens, toxicity, and survival of HIV-positive patients with SCCA to HIV-negative patients treated at our institution between 1991 and 2007.
MATERIALS AND METHODS
The study proposal was approved by the Wayne State University Institutional Review Board (IRB), which ensures procedures that are Health Insurance Portability and Accountability Act (HIPAA) compliant and that maintain patient confidentiality.
Using the Detroit SEER Registry, we then identified all patients with invasive SCCA treated at the Karmanos Cancer Institute/Wayne State University between 1991–2007. We retrospectively reviewed the charts of these patients and collected information regarding: age, gender, race, stage of cancer, HIV status, the CD4 count at the time of diagnosis, and use of HAART.
Detailed information regarding the chemotherapy and radiotherapy administration was collected. Standard dose chemotherapy was defined as mitomycin C at 10mg/m2 and 5-FU at 750 to 1000 mg/m2/d for 4 to 5 days on weeks 1 and 5 of radiation. Patients receiving a lower dose of mitomycin C or 5-FU were considered to be treated with reduced dose chemotherapy. Standard radiotherapy dose was considered as 45 Gy or higher delivered to the primary tumor as well as the inguinal and pelvic nodes. Similarly, patients who received a lower dose of radiation therapy were considered as treated with reduced dose radiotherapy.
Toxicities were graded using the NCI Common Toxicity Criteria Version 2.0 manual and compared between HIV-positive and HIV-negative patients. Response duration (RD) was defined as the time from first documentation of response by digital rectal examination or anoscopy to date of relapse or date of last tumor assessment. OS was defined as the time from date of diagnosis to date of death or last contact date.
Statistical Methods
Baseline patient characteristics and toxicities were described with summary statistics. Standard Kaplan-Meier estimates of the censored RD and OS distributions were computed. Due to sometimes modest sample sizes (or numbers of events), survival statistics (eg, median, 1-year rate, etc.) were estimated more conservatively using linear interpolation among successive event times on the Kaplan-Meier curves.16 All point estimates were accompanied by 90% confidence limits whenever feasible. Censored RD and OS distributions were compared between HIV-positive and HIV-negative patients via the log-rank test.
RESULTS
Patient Characteristics
Table 1 summarizes the characteristics of 45 patients with invasive SCCA treated at Karmanos Cancer Institute between the years 1991–2007. Of these 45 patients, 13 were HIV-positive. Similarly to previously reported series, the SCCA patients with HIV infection at our institution tended to have a younger age, higher prevalence of males, and higher proportion of African Americans than the HIV-negative group. All 13 patients with HIV infection were on HAART. Their median CD4 count at the start of treatment was 232 (range, 125–460). There were no patients with severe HIV infection based on CD4 counts <100 cells/mm3. Both the HIV-positive and the HIV-negative group were balanced according to their stage at initial presentation.
TABLE 1.
Characteristics of 45 Patients With Invasive Anal Cancer Treated at Karmanos Cancer Institute Between the Years 1991–2007
| Characteristics | HIV Positive (N = 13) |
HIV Negative (N = 32) |
|---|---|---|
| Median age (yr) | 45 (range, 26–56) | 57 (range, 26–80) |
| Gender | ||
| Male | 11 (85%) | 12 (37%) |
| Female | 2 (15%) | 20 (63%) |
| Race | ||
| African-American | 10 (77%) | 20 (63%) |
| White | 3 (13%) | 10 (31%) |
| Other | 0 | 2 (6%) |
| CD4 count | ||
| 0–100 | 0 | — |
| 100–200 | 4 (31%) | — |
| 200–400 | 7 (54%) | — |
| >400 | 1 (8%) | — |
| Unknown | 1 (8%) | — |
| Stage (T, N, M) | ||
| I | 0 | 4 (13%) |
| II | 6 (46%) | 15 (47%) |
| III | 5 (38%) | 12 (38%) |
| IV | 1 (8%) | 0 |
| Unknown | 1 (8%) | 1 (3%) |
Percentages may not sum to 100 due to rounding.
Therapy
Table 2 summarizes the treatment for the 45 patients with invasive SCCA. Chemotherapy was not given to 2 patients with HIV infection (1 due to end stage kidney disease and 1 lost to follow-up). The majority of the patients received mitomycin C and 5-FU (11 in the HIV and 28 in the non-HIV group). The 4 patients who did not receive mitomycin C and 5-FU were treated as follows: 2 with 5-FU, 1 with cisplatin and 5-FU, and 1 with oxaliplatin and 5-FU. Due to concerns regarding the tolerability of concurrent chemoradiotherapy, a reduced dose of chemotherapy was used in 6 (54%) HIV-positive patients and in 3 (12%) HIV-negative patients. These dose reductions were planned prior to the first cycle and not due to observed toxicities. The reduced doses of mitomycin C and 5-FU ranged from 5 to 7 mg/m2 and 600 to 750 mg/m2/d, respectively. Two patients did not receive radiation therapy. The administered radiation doses for the HIV-positive and HIV-negative patients were 45 to 59 Gy and 45 to 63 Gy, respectively. Radiation interruption was needed in 58% of HIV-positive and 42% of HIV-negative patients. Therefore, as a group HIV-positive patients received less chemotherapy but similar radiotherapy treatment as compared with the HIV-negative patients.
TABLE 2.
Treatment Received by 45 Patients With Invasive Anal Cancer Treated at Karmanos Cancer Institute Between the Years 1991–2007
| HIV Positive (N = 13) |
HIV Negative (N = 32) |
|
|---|---|---|
| Chemotherapy | ||
| MMC/FU | 11 (85%) | 28 (88%) |
| FU | 0 | 2 (6%) |
| Other | 0 | 2 (6%) |
| None | 2 (15%) | 0 |
| Chemotherapy MMC/FU | ||
| Standard dose | 5 (45%) | 25 (89%) |
| Reduced dose | 6 (54%) | 3 (11%) |
| Radiation Interruption | ||
| Yes | 7 (58%) | 13 (42%) |
| No | 5 (42%) | 16 (52%) |
| Unknown | 0 | 2 (6%) |
Counts of patients reflect exclusions due to occasional missing data.
Percentages may not sum to 100 due to rounding.
Toxicities
Table 3 summarizes the grade 3 and 4 observed toxicities in 36 patients treated with radiotherapy or chemoradiotherapy by HIV status. Toxicity data was not available on 9 patients in the HIV-negative group initially treated outside our institution. The most common toxicities included dermatitis, diarrhea, myelosuppression, dehydration, and nausea. Except for acute radiation dermatitis, the incidence of grade 3 and grade 4 toxicities was similar between patients with HIV-infection and patients without HIV infection.
TABLE 3.
The Frequency of Grade 3–4 Treatment-Related Toxicities in 36 Patients With Invasive Anal Cancer Expressed as the Worst Toxicity Per Patient*
| Type of Toxicity | HIV Positive (N = 13) |
HIV Negative (N = 23) |
|---|---|---|
| Neutropenia | ||
| Grade 3 | 2 (15%) | 3 (13%) |
| Thrombocytopenia | ||
| Grade 3 | 1 (8%) | 1 (4%) |
| Anemia | ||
| Grade 3 | 2 (15%) | 1 (4%) |
| Dermatitis | ||
| Grade 3 | 3 (23%) | 12 (52%) |
| Grade 4 | 0 | 1 (4%) |
| Mucositis | ||
| Grade 3 | 0 | 1 (4%) |
| Infection | ||
| Grade 3 | 1 (8%) | 2 (9%) |
| Dehydration | ||
| Grade 3 | 2 (15%) | 2 (9%) |
| Diarrhea | ||
| Grade 3 | 2 (15%) | 3 (13%) |
| Nausea | ||
| Grade 3 | 0 | 1 (4%) |
| Vomiting | ||
| Grade 3 | 0 | 1 (4%) |
| Encephalopathy | ||
| Grade 4 | 1 (8%) | 0 |
Toxicity data was not available on 9 patients in the HIV-negative group.
Relapse Site, Response Duration, and Survival
Table 4 and Figure 1 summarize the relapse status, RD, and OS for the 45 patients (13 HIV-positive and 32 HIV-negative) with invasive SCCA. In the HIV-positive group, the 3 (23%) patients had relapsed. All relapses were due to local recurrence. In the HIV-negative group, 10 (31%) patients relapsed with 8 being due to local recurrence and 2 due to distant metastasis.
TABLE 4.
Relapse Site, Response Duration, and Overall Survival of 45 Patients With Invasive Anal Cancer Treated at Karmanos Cancer Institute Between the Years 1991–2007
| HIV Positive (N = 13) |
HIV Negative (N = 32) |
|
|---|---|---|
| Relapse | ||
| Local | 3 (23%) | 8 (25%) |
| Distant | 0 | 2 (6%) |
| No relapse | 10 (77%) | 22 (69%) |
| (N = 9) | (N = 16) | |
| Response duration (90% CI) | ||
| Median (mo) | Not reached | Not reached |
| 12-mo rate | 72% (55%–100%) | 73% (52%–95%) |
| (N = 13) | (N = 32) | |
| Survival (90% CI) | ||
| Median (mo) | 33.5 (0.0–*) | 71.8 (16.6–109.3) |
| 12-mo rate | 74% (55%–94%) | 89% (80%–98%) |
| 24-mo rate | 58% (34%–82%) | 77% (65%–90%) |
| 36-mo rate | 48% (11%–85%) | 62% (46%–77%) |
| 48-mo rate | 38% (1%–75%) | 58% (43%–74%) |
This confidence limit is not available, ie, not estimable due to censoring patterns in the data.
CI indicates confidence interval.
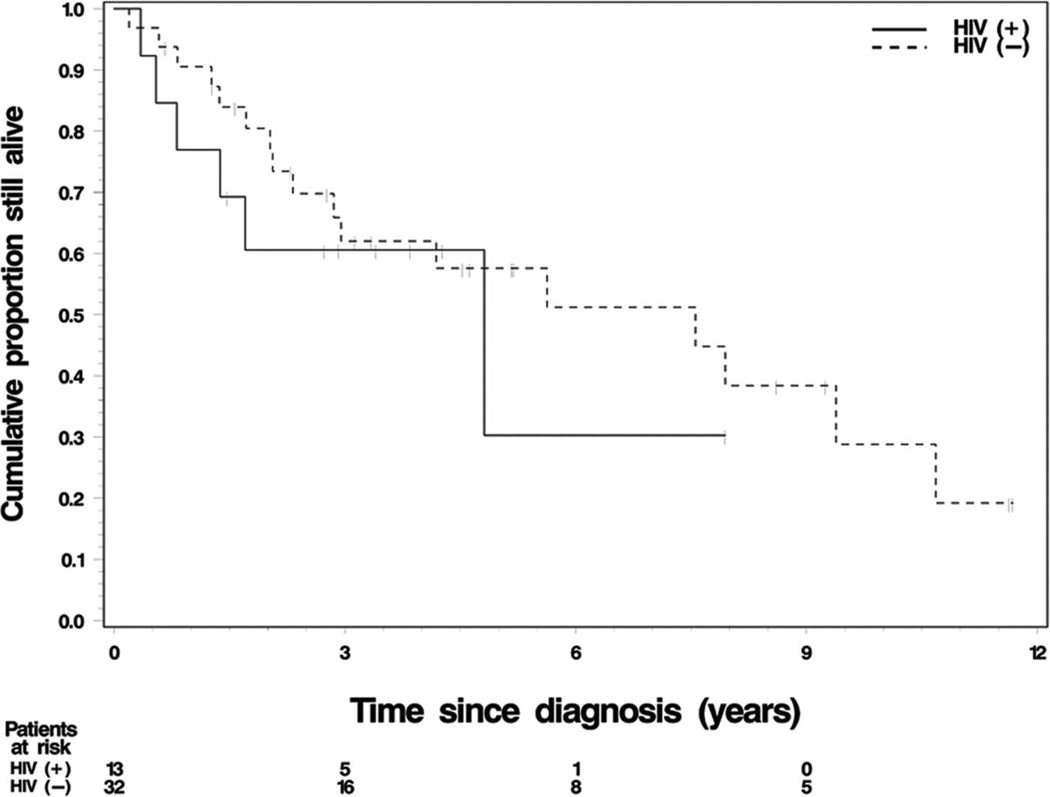
FIGURE 1.
The Kaplan-Meier estimate of overall survival by HIV status in the 45 patients with invasive anal cancer treated at Karmanos Cancer Institute between the years 1991–2007. The median survival for the HIV-positive and HIV-negative patients was 33.5 and 71.8 months, respectively.
The response duration was calculated for 25 of the 45 patients (9 HIV-positive patients and 16 HIV-negative patients). The 12-month relapse-free rate was 72% (90% confidence interval [CI], 41%–100%) for the HIV-positive group and 73% (90% CI, 52%–95%) for the HIV-negative patients. Median OS for the HIV-positive patients was 33.5 months, with a 24-month rate of 58% (90% CI, 34%–82%). Median OS for HIV-negative patients was 71.8 months, with a 24-month rate of 77% (90% CI, 65%–90%). The causes of death for the patients in the HIV-positive group were: anal cancer related (3 patients), 5-FU related neurotoxicity (1 patient), HIV related (1 patient), and unknown (1 patient). Therefore, HIV-positive patients had a shorter RD and OS than HIV-negative patients, but the differences were not statistically significant (P = 0.89 for RD; P = 0.49 for OS).
DISCUSSION
In this study, we evaluated our institutional experience with SCCA in HIV-positive and negative patients. The patient characteristics demonstrate that SCCA in our HIV-positive patients occurs at a younger age (median 47 vs. 57 years) and more frequently in men (89% in HIV-positive vs. 37% in HIV-negative). Our results are similar to a recent report from a multicenter study where the mean age and percentage of men in HIV-positive and negative patients were 48 and 62 years and 93% and 25%, respectively.13 Chaio et al reported on 1184 patients with SCCA treated in the Veterans Administration Health System. Similarly, the median ages of those HIV-positive and negative patients were 49 and 63 years, respectively.11 These demographic differences in SCCA in HIV-positive patients carry 2 important implications. First, a review of the SEER registry indicates a disproportionate and rising incidence in SCCA in young African American men.2 This demographic change in SCCA may be in part explained by a rising incidence of SCCA in HIV infected patients in the HAART era. Second, gender is an established prognostic factor in SCCA with women having a better outcome with treatment as demonstrated in randomized trials9 and in registry based studies.2 The high incidence of men with SCCA in the HIV-infected patients may be a poor prognostic factor for that group of patients independent of the HIV infection.
In our study, HIV-infected patients were less likely to receive full dose of chemotherapy (45% in HIV-positive vs. 88% in HIV-negative). This difference in treatment was mainly due to concerns by the treating physicians regarding the increased toxicity in the HIV-positive patients and the resulting dose reductions were planned prior to the actual administration of chemotherapy. The toxicity profile observed in our patients was similar in the 2 groups except for acute radiation dermatitis, which was more common in the HIV-negative group. This may be explained by the lower dose of chemotherapy used in the HIV-positive patients. No significant difference in outcome of therapy (either RD or OS) between the HIV-positive and negative patients was observed. Both the HIV-positive and negative groups were balanced by their stage at initial presentation. The major site of treatment failure for both groups was local recurrence and the risk of local recurrence was also nearly identical for the 2 groups suggesting that the outcome of therapy is similar in the HIV-positive and negative patients. Similarly, the survival at 2 years for the HIV-negative patients was within the expected rates reported in randomized trials8–10 and was similar to that of the HIV-negative patients. Study limitations include the modest sample sizes, especially the number of HIV-positive patients, just 13, and the small number of deaths among that subgroup, only 6. Although we did not detect a statistically significant difference in OS duration by HIV status, the retrospective power for that was only 51%, approximately. The lower dose of chemotherapy used in our patient series did not seem to negatively impact the disease control in the HIV-positive patients but did improve the toxicity profile. The ability to deliver full dose radiotherapy to the HIV-positive patients may have resulted in improvement in local control.
Our results are in agreement with the largest reported series in HIV-infected patients with SCCA. That study included 175 HIV-positive patients and 1009 HIV-negative patients.11 Their 2-year survival rates for HIV-positive and negative patients were 77% and 75%, respectively. Since this was a registry study limited information was available regarding the details of chemoradiotherapy regimens used and the observed toxicities. Oehler-Janne et al reported results on 40 HIV-positive and 81 HIV-negative patients with SCCA treated at multiple institutions.13 Similar to our series, patients with HIV infection were less likely to receive full dose chemotherapy which was administered to 55% of the HIV-positive population and 72% of the HIV-negative group. Although the radiotherapy doses administered in the HIV-positive and negative patients were similar, the duration was significantly longer in the HIV-positive population resulting in a lower corrected dose. In contrast to our series, grade 3 to grade 4 toxicities were more common in the HIV-infected patients (50% in HIV-positive versus 31% in HIV-negative patients). The 5-year survival rates for HIV-positive and negative patients were 61% and 65%, respectively. However, the risk of local recurrence was higher in the HIV-positive patients (62% vs. 13%) which may be related to the lower radiation dose used. Several smaller series have reported comparable tolerability, local control, and overall survival in HIV-positive patients with SCCA as compared with HIV-negative patients.17–19
In conclusion, in the era of HAART, the outcome with respect to local recurrence and overall survival of HIV-infected patients with SCCA appears to be similar to that of HIV-negative patients. A reduced dose of chemotherapy in the HIV-positive patients may yield adequate disease control with lower incidence of toxicity. Future trials evaluating lower dose of chemotherapy or intensity-modulated radiotherapy in HIV patients are needed to optimize therapy in this group of patients.
Acknowledgments
Supported in part by Cancer Center Support Grant CA-22453 from the National Cancer Institute.
Footnotes
All authors have no financial and personal relationships with other people or organizations that would inappropriately influence (bias) this work.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE, Li Y, McDonald A, et al. Rates of non-AIDS-defining cancers in people with HIV infection before and after AIDS diagnosis. Aids. 2002;16:1155–1161. doi: 10.1097/00002030-200205240-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bower M, Powles T, Newsom-Davis T, et al. HIV-associated anal cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr. 2004;37:1563–1565. doi: 10.1097/00126334-200412150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 6.Palefsky J. Human papillomavirus and anal neoplasia. Curr HIV/AIDS Rep. 2008;5:78–85. doi: 10.1007/s11904-008-0013-5. [DOI] [PubMed] [Google Scholar]
- 7.Nigro ND, Vaitkevicius VK, Considine B., Jr Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–356. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 8.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 9.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 10.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 11.Chiao EY, Giordano TP, Richardson P, et al. Human immunodeficiency virus-associated squamous cell cancer of the anus: epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol. 2008;26:474–479. doi: 10.1200/JCO.2007.14.2810. [DOI] [PubMed] [Google Scholar]
- 12.Holland JM, Swift PS. Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology. 1994;193:251–254. doi: 10.1148/radiology.193.1.8090901. [DOI] [PubMed] [Google Scholar]
- 13.Oehler-Janne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26:2550–2557. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 14.Vatra B, Sobhani I, Aparicio T, et al. Anal canal squamous-cell carcinomas in HIV positive patients: clinical features, treatments and prognosis [Article in French] Gastroenterol Clin Biol. 2002;26:150–156. [PubMed] [Google Scholar]
- 15.Place RJ, Gregorcyk SG, Huber PJ, et al. Outcome analysis of HIV-positive patients with anal squamous cell carcinoma. Dis Colon Rectum. 2001;44:506–512. doi: 10.1007/BF02234322. [DOI] [PubMed] [Google Scholar]
- 16.Lee E, Wang JW. Statistical Methods for Survival Data Analysis. 3rd ed. Hoboken, NJ: Wiley & Sons, Inc; 2003. pp. 76–91. [Google Scholar]
- 17.Blazy A, Hennequin C, Gornet JM, et al. Anal carcinomas in HIV-positive patients: high-dose chemoradiotherapy is feasible in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2005;48:1176–1181. doi: 10.1007/s10350-004-0910-7. [DOI] [PubMed] [Google Scholar]
- 18.Seo Y, Kinsella MT, Reynolds HL, et al. Outcomes of chemoradiotherapy with 5-fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys. 2009;75:143–149. doi: 10.1016/j.ijrobp.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 19.Allen-Mersh T, Hanna-Morris A, Goldstone S. ASCO Gastrointestinal Cancer Symposium. San Francisco, CA: 2004. Is chemoradiaiton the treatment of choice for anal squamous cell carcinoma developing in HIV-positive patients with access to HAART. [Google Scholar]