Abstract
Objectives
To examine dose effects of Lactobacillus acidophilus NCFM (LA) ™ strain on rotavirus-specific antibody and B cell responses in gnotobiotic pigs vaccinated with an oral attenuated human rotavirus (AttHRV).
Methods
Pigs were inoculated with AttHRV vaccine in conjunction with high dose LA (14 doses, total 2.2×109 colony forming units [CFU]), intermediate dose LA (9 doses, total 3.2×106 CFU), low dose LA (5 doses, total 2.1×106 CFU) or without LA feeding. Protection against rotavirus shedding and diarrhea was assessed upon challenge with a virulent HRV. Rotavirus-specific IgA and IgG antibodies in serum and rotavirus-specific IgA and IgG antibody-secreting cells (ASC) and memory B cells in ileum, spleen and blood of the pigs were measured and compared among treatment groups.
Results
The intermediate dose LA (MidLA), but not high or low dose LA, significantly reduced rotavirus diarrhea (MidLA only group) and significantly improved the protection conferred by AttHRV vaccine (MidLA+AttHRV group). Associated with the increased protection, MidLA significantly enhanced rotavirus-specific antibody, ASC and memory B cell responses to AttHRV vaccine. High or low dose LA did not enhance virus-specific antibody and ASC responses, hence did not improve the vaccine efficacy.
Conclusions
These findings highlight the importance of dose selection and indicate that certain specific lactobacilli strains at the appropriate dose have the dual function of reducing rotavirus diarrhea and enhancing the immunogenicity and protective efficacy of rotavirus vaccines.
Keywords: Probiotics, Lactobacilli, Antibody-secreting cells, Rotavirus vaccine, Gnotobiotic pigs, Dose response
Introduction
Probiotics have been shown to reduce the severity of rotavirus diarrhea in a large number of clinical studies (1–5). An increasing number of clinical or experimental studies also showed that some selected probiotic lactobacilli strains have the potential as adjuvants to enhance the immunogenicity of viral vaccines (6). These probiotic adjuvants include Lactobacillus rhamnosus GG (LGG) for live-attenuated influenza virus (7) and rotavirus vaccines (8), L. acidophilus NCFM™ (LA) for rotavirus vaccine (9), L. paracasei CRL431 and LGG for live poliovirus vaccine (10), L. fermentum CECT5716, Bifidobacterium animalis lactis BB-12 and L. paracasei CRL431 for parenteral influenza vaccine (11, 12), and the mixture of B. longus and L. rhamnosus LPR for hepatitis B vaccine (13). Traditionally, an adjuvant is co-presented with a vaccine antigen to enhance specific immunity to that antigen and appropriate doses of the adjuvants are carefully determined through dose effect and toxicity studies to select the most effective doses without causing adverse effect (14, 15). However, probiotic adjuvants and vaccines do not need to be co-administered (6). The mechanisms of adjuvant effect of probiotics are to influence innate immune cells such as intestinal macrophages and dendritic cells in the gut associated lymphoid tissues (16, 17), which in turn results in enhanced antigen presentation and promotes preferential differentiation of mucosal lymphocytes towards the production of protective antibodies and effector αβ and γδ T cells (18–20). The strain specificity of the adjuvant effect of probiotics has been well-recognized (21–23); however, their dose effect remains an important and unaddressed issue.
Recent studies suggest that dose selection has an important impact on the immunomodulating functions of probiotics. High concentrations (≥ 1×106 colony forming unit [CFU]/ml) of a combination of LA and Bifidobacterium or B. infantis attenuates mitogen-induced overactive immune responses by inhibiting mitogen-induced cell proliferation and arresting the cell cycle at the G0/G1 stage in spleen and peripheral blood mononuclear cells. However, low concentrations (≤1×106 CFU/ml) promote a shift in the Th1/Th2 balance towards Th1-skewed immunity by enhancing IFN-γ and inhibiting IL-4 responses (24). A mixture of L. plantarum CEC 7315 and CEC 7316 at high dose (5×109 CFU/day) resulted in a significant increase in the percentage of activated T-suppressor cells, while at low dose (5×108 CFU/day) increased activated Th cells, B cells and APCs in the elderly (25). Dosing frequency may also have an important impact on the immunomodulating functions of probiotics. A study of gut mucosal immunostimulation by various lactic acid bacteria strains in mice showed that, among three dosing regimens (2, 5 or 7 consecutive days of feeding), L. acidophilus and L. plantarum were effective only in the 2-day feeding group, but not 5- or 7-day groups to significantly increase the numbers of total IgA secreting cells in the lamina propria of the small intestine (21).
In our previous study, low dose LA (5 feedings) significantly promoted IFN-γ producing T cell responses induced by an oral rotavirus vaccine and down-regulated regulatory T (Treg) cell responses, but high dose LA (14 feedings) increased the frequencies of Treg cells in most of the tissues of gnotobiotic (Gn) pigs when compared to the control groups (20). Whether different doses and dosing frequencies of the probiotic differentially modulated B cell and antibody responses to the vaccine was not reported previously. Because neither high dose nor low dose LA significantly reduced rotavirus diarrhea or increased the protection rate against rotavirus diarrhea conferred by the rotavirus vaccine (20), we investigated the effect of the intermediate dose LA (9 feedings) on rotavirus infection and diarrhea along with protection induced by the vaccine. We then compared the clinical protection rate and the immunomodulating effects of low, intermediate, and high dose LA as the vaccine adjuvant. Our eventual goal was to identify the minimal number and dose of LA feeding that is most effective as rotavirus vaccine adjuvant.
MATERIALS AND METHODS
Virus
The cell-culture adapted attenuated human rotavirus HRV (AttHRV) Wa strain (G1P1A[8]) derived from the 35th passage in MA104 cells was used as the vaccine at a dose of 5×107 fluorescent focus forming units (FFU) (26). The virulent human rotavirus HRV (VirHRV) Wa strain was passaged through Gn pigs and the pooled intestinal contents from the 27th passage were used for challenge of Gn pigs at a dose of ~105 FFU. The virus titer was determined by using cell culture immunofluorescence (CCIF) assay and was expressed as FFU/ml as described previously (27).
Probiotic adjuvant
Lactobacillus acidophilus NCFM (LA)™ were propagated in Lactobacilli MRS broth (Weber Scientific, USA) and the bacterial counts were titrated and expressed as CFU/ml as described previously (16). Prior to feeding, the bacteria were thawed and washed 2 times with 0.1% peptone water (BD Biosciences, Sparks, MD) by centrifuging at 2000rpm/min for 10 min at 4°C and were diluted to the specified CFU/ml.
Treatment groups and inoculation of Gn pigs
Gnotobiotic pigs were derived by hysterectomy from near-term sows (Landrace and Large White crossbred) and maintained in germ-free isolator units (28). Pigs were fed commercial ultra-high temperature-treated sterile milk. Pigs (both males and females) were randomly assigned to different treatment groups as follows: (1) high dose LA plus AttHRV (HiLA+AttHRV), (2) intermediate dose LA plus AttHRV (MidLA+AttHRV), (3) low dose LA plus AttHRV (LoLA+AttHRV), (4) AttHRV only (AttHRV), (5) high dose LA only (HiLA), (6) intermediate dose LA only (MidLA), (7) low dose LA only (LoLA), and (8) mock control (Mock). The detailed LA dosing regimen, AttHRV inoculation and VirHRV challenge are shown in Table 1. The incremental dose increases of the LA feeding regimen was determined empirically to avoid potentially causing diarrhea by high dose probiotic bacteria during the first few days of life since Gn pigs lack protection conferred by maternal antibodies. Pigs in LA-fed groups were orally dosed with LA using a syringe as previously described (9) at the specified CFU suspended in 3 ml of 0.1% peptone water starting from post-partum day (PPD) 3. Non-LA fed pigs were given only 3 ml of 0.1% peptone water. At PPD 5 (post-AttHRV inoculation day [PID] 0), pigs in AttHRV inoculated groups were orally inoculated with 5×107 FFU of AttHRV in 5 ml of diluent (minimum essential medium) and re-inoculated with the same dose at PID 10. Pigs in non-AttHRV inoculated groups were given an equal volume of diluent. At PID 28 (post-VirHRV challenge day [PCD] 0), subsets of pigs from all groups were orally challenged with 105 FFU of VirHRV. Pigs were given 8 ml of 100 mM sodium bicarbonate 20 min before virus inoculation to reduce gastric acidity. Pigs were euthanized at PID 28 or PCD 7. Ileum, spleen, and peripheral blood samples were collected at euthanasia for isolation of mononuclear cells (MNCs) as previously described (29). All animal experimental procedures were conducted in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committee of Virginia Polytechnic Institute and State University.
Table 1.
Probiotic doses and feeding regimens, vaccine inoculations and challenge
| Age (PPD)a | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 26 | 33 | 34 | 44 | Total # of feedings | Accumulative dosage |
| PIDb | −5 | −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 21 | 28 | 29 | 35 | ||
| PCDc | −17 | −16 | −15 | −14 | −13 | −12 | −11 | −10 | −9 | −8 | −7 | −6 | −5 | −4 | −3 | −2 | −1 | 0 | 1 | 7 | |||
| High dose (CFUd) | 103 | 103 | 104 | 104 | 105 | 105 | 106 | 106 | 107 | 107 | 108 | 108 | 109 | 109 | 14 | 2.22 × 109 | |||||||
| Intermediate dose (CFU) | 103 | 103 | 104 | 104 | 105 | 105 | 106 | 106 | 106 | 9 | 3.22 × 106 | ||||||||||||
| Low dose (CFU) | 103 | 104 | 105 | 106 | 106 | 5 | 2.11 × 106 | ||||||||||||||||
| AttHRV inoculation (FFUe ) | 5×107 | 5×107 | |||||||||||||||||||||
| VirHRV challenge (FFU ) | 1×105 |
PPD, post-partum day;
PID, post-inoculation day;
PCD, post-challenge day;
CFU, colony forming unit;
FFU, fluorescent focus forming units.
Clinical signs, rotavirus shedding and LA counts
After VirHRV challenge, pigs were examined daily from PCD 0 to PCD 7 for clinical signs, including number with diarrhea, duration of diarrhea, and fecal consistency (diarrhea scores) as previously described (26). Fecal swabs were collected daily for detection of virus shedding. Rotavirus infection in Gn pigs was confirmed by fecal virus shedding using enzyme-linked immunosorbent assay (ELISA) and CCIF assay as previously described (27, 30). Fecal swabs were collected at PID 5, 10, 21 and 28 for enumeration of LA shedding. LA enumeration was processed as previously described (9, 16, 29). From PPD 2, fecal swabs were also collected weekly, diluted, plated on regular blood agar plates and in NIH thioglycollate broth, and cultured at 37°C for 24–72 h to check for the sterility.
Assessment of rotavirus-specific IgA and IgG antibody responses in serum and antibody-secreting cell (ASC) and memory B cell responses in the intestinal and systemic lymphoid tissues
Rotavirus-specific serum IgA and IgG antibody titers in AttHRV and/or VirHRV inoculated pigs were measured using indirect isotype-specific antibody ELISAs as previously described (31, 32).
The MNCs from ileum, spleen, and peripheral blood were isolated and subjected to ELISPOT assays for detection of ASC (26) and memory B cells (33) as we previously described. The ELISPOT plates were scanned with a CTL-ImmunoSpot® S5 Core Analyzer (CTL Analyzers LLC, OH). Numbers of rotavirus-specific ASC or memory B cells were determined by counting blue spots in the wells and were reported as the numbers per 5×105 of MNCs.
Statistical analysis
The LA counts were compared among different time points using one-way analysis of variance (ANOVA-general linear model [GLM]). Mean duration of virus shedding and diarrhea and mean cumulative fecal consistence scores among the treatment groups were compared using ANOVA-GLM, followed by Duncan’s multiple range test. Proportions of virus shedding and diarrhea among treatment groups were compared using Fisher’s exact test. Virus titers, antibody titers, LA counts and numbers of ASC among treatment groups were compared using Kruskal-Wallis rank sum test. Statistical significance was assessed at p<0.05. All statistical analysis was performed using SAS program 9.2 (SAS Institute, INC, USA).
RESULTS
Fecal LA counts
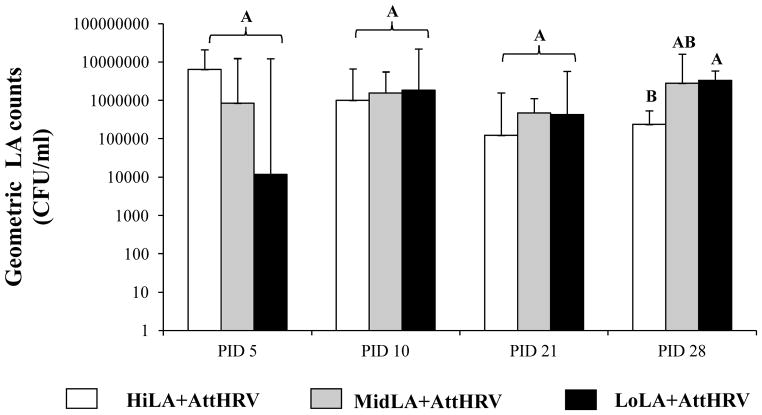
LA colonization in HiLA+AttHRV, MidLA+AttHRV and LoLA+AttHRV pigs was confirmed by bacterial enumeration in rectal swab samples collected on PID 5, 10, 21, and 28 (10, 15, 26 and 33 days of age) respectively (Fig. 1). The LA counts in the MidLA+AttHRV pigs did not differ significantly compared to HiLA+AttHRV or LoLA+AttHRV pigs at any time. The LA counts in the HiLA+AttHRV pigs were significantly lower than the LoLA+AttHRV pigs at PID 28.
Fig. 1. LA counts in fecal samples of Gn pigs vaccinated with AttHRV and fed different doses of LA.
The LA counting was performed by plating the fecal swab samples on MSR selective medium and was recorded as CFU/ml. The counts were presented as geometric mean counts for each treatment group. Error bars indicate the standard error of the mean. Different capital letters (A, B) indicate significant difference among different groups (Kruskal Wallis Test, p<0.05, n=7–9), while shared letters indicate no significant difference. The data from HiLA+AttHRV and LoLA+AttHRV groups was published in our previous study (20)
The non-LA fed pigs remained bacteria free. The bacterial cultures from LA fed pigs resembled the original LA inoculum and were confirmed by biochemical staining, indicating no extraneous bacterial contamination during the experiment.
Intermediate dose LA was most effective in enhancing protection against rotavirus diarrhea
Clinical signs (diarrhea) and fecal virus shedding in the HiLA+AttHRV, MidLA+AttHRV, LoLA+AttHRV, AttHRV-only, HiLA only, MidLA only, LoLA only and mock control groups after VirHRV challenge are summarized in Table 2. Compared to the AttHRV-only pigs, the MidLA+AttHRV pigs had significantly shorter mean duration of diarrhea (by 2.1 days), and slightly lower incidence of diarrhea (57% vs 67%) and fecal virus shedding, and a lower mean cumulative score of diarrhea (Table 2-I). On the other hand, neither low nor high dose LA improved the protection conferred by the AttHRV vaccine on clinical signs (Table 2-I). The percentages of fecal virus shedding in the three LA fed and AttHRV vaccinated groups were all lower than the AttHRV only group, but they did not differ significantly (Table 2-I).
Table 2.
Clinical signs and rotavirus fecal shedding in Gn pigs postchallenge△
| Treatments | n | Clinical signs
|
Fecal virus shedding (by CCIF and/or ELISA)
|
||||
|---|---|---|---|---|---|---|---|
| % with diarrhea ▼ ▮ | Mean duration days #, ▼▼ | Mean cumulative score #, ▼▼ | % shedding virus ▼ | Mean duration days #, ▼▼ | Geometric mean peak titer (FFU/ml) #, ◆, ▼▼ | ||
| (I) Vaccinated groups | |||||||
|
| |||||||
| HiLA+AttHRV | 13 | 92A | 4.0 (0.7^)A | 12.5 (1.4)A | 31A, * | 1.2 (0.5)A, * | <200A, * |
| MidLA+AttHRV | 7 | 57A | 1.0 (0.4)B | 8.4 (0.5)B | 43A, * | 1.1 (0.6)A, * | <200A, * |
| LoLA+AttHRV | 8 | 88A | 2.1 (0.7)AB, * | 8.4 (1.3)AB, * | 36A, * | 0.4 (0.2)A, * | <200A, * |
| AttHRV only | 12 | 67A | 3.1 (0.7)AB, * | 9.8 (1.4)AB, * | 50A, * | 0.7 (0.2)A, * | <200A, * |
|
| |||||||
| (II) Control groups | |||||||
|
| |||||||
| HiLA only | 8 | 100a | 5.1 (0.6)a | 14.3 (1.0)a | 100a | 4.0 (0.8)a | 9.96 × 102 a |
| MidLA only | 5 | 75a | 1.6 (0.7)b | 8.4 (1.0)b | 100a | 6.0 (0.4)a | 4.86 × 103 a |
| LoLA only | 3 | 100a | 5.3 (0.7)a | 15.3 (0.6)a | 100a | 7.0 (0.0)a | 2.61 × 103 a |
| Mock control | 9 | 100a | 5.6 (0.3)a | 14.4(1.0)a | 100a | 4.7 (0.7)a | 4.56 × 103 a |
The data from HiLA+AttHRV, LoLA+AttHRV, AttHRV only and Mock control groups was partially published previously (20).
Pigs with daily fecal scores of ≥2 were considered diarrheic. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid.
Mean value calculation included all the pigs in each group.
FFU, fluorescent focus forming units. Geometric mean peak titers were calculated among pigs that shed virus.
Standard error of the mean.
Proportions in the same column with different superscript captain letters (A, B ) among vaccinated groups (I) or lower case letters (a, b) among control groups (II) indicate significant difference (Fisher’s exact test, p≤0.05), while shared letters indicate no significant difference.
Means in the same column with different superscript captain letters (A, B ) among vaccinated groups (I) or lower case letters (a, b) among control groups (II) indicate significant difference (ANOVA-GLM followed by Duncan’s multiple range test, p≤0.05), while shared letters indicate no significant difference.
Indicates the values in the vaccinated group differ significantly from the corresponding control group.
Interestingly, among the three LA-only control groups, the MidLA group had reduced percentage of diarrhea (from 100% to 75%). The MidLA-only pigs had significantly shorter mean duration of diarrhea (1.6 vs. 5.1–5.6 days) and significantly lower mean cumulative score (8.4 vs. 14.3–15.3 days) compared to HiLA-only, LoLA-only and mock control pigs (Table 2-II). The clinical signs among HiLA-only, LoLA-only and mock control pigs and the fecal virus shedding among the four control groups did not differ (Table 2-II).
Comparing between AttHRV vaccinated and the corresponding non-vaccinated control groups, the percentage of fecal virus shedding was significantly reduced in all the vaccinated pigs from 100% to 31–50%; and the mean duration and mean peak titer of fecal virus shedding were significantly reduced (Table 2-I and 2-II). For clinical signs, vaccinated groups had overall reduced percentages, mean duration and cumulative scores of diarrhea compared to the corresponding controls (Table 2-I and 2-II). Among them, the LoLA+AttHRV and AttHRV pigs had significantly reduced mean durations of diarrhea (2.1 vs 5.3 and 3.1 vs 5.6) and mean cumulative scores (8.4 vs 15.3 and 9.8 vs 14.4) compared to the LoLA only pigs and Mock, respectively (Table 2-I and 2-II). The MidLA+AttHRV pigs had a 24% percent reduction in the percent of diarrhea (57% vs 75%) and a slightly shorter mean duration of diarrhea (1.0 vs 1.6) than the MidLA-only pigs (Table 2-I and 2-II).
Intermediate dose LA significantly enhanced rotavirus-specific serum IgA and IgG antibody responses to AttHRV vaccine pre- and postchallenge
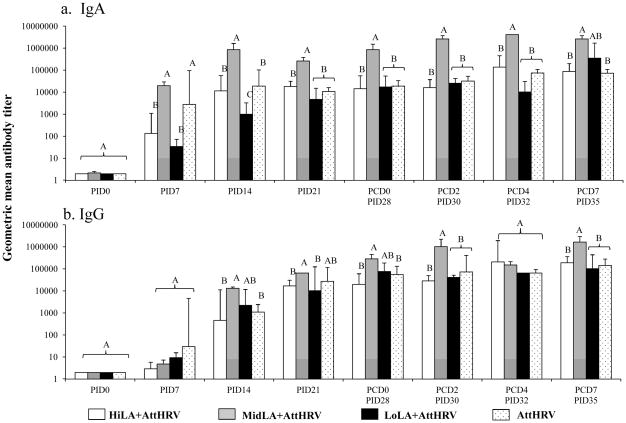
Rotavirus-specific serum IgA and IgG antibody titers were compared among AttHRV vaccinated pigs fed high, intermediate, low dose LA and without LA (AttHRV only) on PID 0, 14, 21, 28 (PCD 0), 30 (PCD 2), 32 (PCD 4) and 35 (PCD 7) (Fig. 2). From PID 14 to PCD 7, MidLA+AttHRV pigs had significantly higher IgA titers than the HiLA+AttHRV, LoLA+AttHRV and AttHRV-only pigs (Fig. 2a). In contrast, LoLA+AttHRV pigs had significantly lower IgA titers than the AttHRV-only pigs on PID 7 and 14. There were no significant differences in IgA titers among the HiLA+AttHRV, LoLA+AttHRV and AttHRV-only pigs from PID 21 to PCD 7.
Fig. 2. Rotavirus-specific serum IgA and IgG antibody responses in Gn pigs vaccinated with AttHRV, with or without high, intermediate or low dose LA feeding.
Rotavirus-specific serum IgA (a) and IgG (b) antibody titers were measured by an indirect isotype-specific antibody ELISA and presented as geometric mean titers for each treatment group (n=3–27). Samples negative at a dilution of 1:4 were assigned a titer of 1:2 for the calculation of geometric mean antibody titers. See Fig. 1 legend for description of error bars and statistical analysis.
From PID 14 to PCD 7, MidLA+AttHRV pigs had higher or significantly higher IgG titers than the HiLA+AttHRV, LoLA+AttHRV and AttHRV pigs excepting from PCD4 (PID 32) (Fig. 2b). There were no significant differences in the IgG titers among the HiLA+AttHRV, LoLA+AttHRV and AttHRV-only pigs.
Intermediate dose LA significantly enhanced rotavirus-specific IgA and IgG ASC responses to AttHRV vaccine pre- and postchallenge
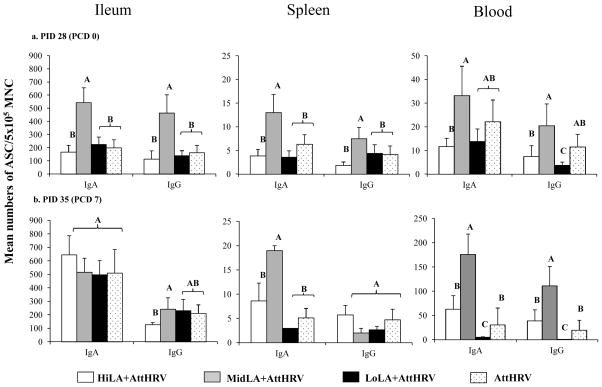
Rotavirus-specific IgA and IgG ASC in ileum, spleen, and blood are compared among the four AttHRV-vaccinated pig groups on PID 28 (PCD 0) and PID35 (PCD 7) (Fig. 3). At PID 28, numbers of IgA and IgG ASC in ileum, spleen and blood of the MidLA+AttHRV pigs were higher or significantly higher than the HiLA+AttHRV, LoLA+AttHRV and AttHRV-only pigs (Fig. 3a). There were no significant differences in numbers of the IgA and IgG ASC in ileum and spleen and the IgA ASC in blood among the HiLA+AttHRV, LoLA+AttHRV and AttHRV-only pigs. At PCD 7, numbers of IgA ASC in spleen and blood and IgG ASC in blood of MiLA+AttHRV pigs were significantly higher than the HiLA+AttHRV, LoLA+AttHRV and AttHRV-only pigs (Fig. 3b). Notably, the LoLA+AttHRV pigs had significantly lower numbers of IgG ASC at PID 28 and IgA and IgG ASC at PCD 7 in blood than the other three groups.
Fig. 3. Rotavirus-specific IgA and IgG ASC responses in Gn pigs vaccinated with AttHRV, with or without high, intermediate or low dose LA feeding.
Rotavirus-specific IgA and IgG ASC in the MNC isolated from ileum, spleen, and blood of AttHRV-vaccinated pigs at challenge (a) and postchallenge (b) were enumerated by using an ELISPOT assay and were reported as the mean numbers of virus-specific IgA and IgG ASC per 5×105 MNC (n=3–14). See Fig. 1 legend for description of error bars and statistical analysis.
Intermediate dose LA promoted the development of memory B cell responses induced by the AttHRV vaccine and resided in spleen
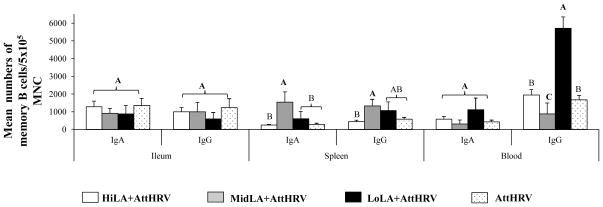
Rotavirus-specific IgA and IgG memory B cell in ileum, spleen, and blood are compared among the four AttHRV-vaccinated pig groups on PID 28 (PCD 0) (Fig. 4). The numbers of rotavirus-specific IgA and IgG memory B cells in spleen of the MidLA+AttHRV pigs were higher or significantly higher than the HiLA+AttHRV, LoLA+AttHRV and AttHRV-only pigs. In blood, the LoLA+AttHRV pigs had significantly higher numbers of IgG memory B cells than the other three groups while the MidLA+AttHRV pigs had significantly lower numbers of IgG memory B cells than the other groups. The numbers of IgA and IgG memory B cells in ileum and IgA memory B cells in blood did not differ significantly among the four groups.
Fig. 4. Rotavirus-specific IgA and IgG memory B cell responses in Gn pigs vaccinated with AttHRV with or without high, intermediate or low dose LA feeding on PID 28.
The MNC isolated on PID28 (PCD 0) were stimulated with semipurified AttHRV antigen for 96 hours. The rotavirus-specific IgA and IgG memory B cells was enumerated by using ELISPOT assay and were reported as the mean numbers of virus-specific IgA and IgG memory B cells per 5×105 MNC (n=3–10). See Fig. 1 legend for description of error bars and statistical analysis.
Discussion
In this study, we demonstrated for the first time that probiotic LA NCFM™ strain at the intermediate dose has dual functions. Similar to previous findings for LGG (2, 8), MidLA exerted not only adjuvant effect for the AttHRV vaccine, but also directly reduced rotavirus diarrhea. Associated with the improvement in the protection against the rotavirus diarrhea, MidLA strongly enhanced rotavirus-specific serum IgA and IgG antibody responses and intestinal IgA and IgG ASC responses at challenge compared to the AttHRV-only pigs. In contrast, high or low dose LA did not reduce the incidence of rotavirus diarrhea in vaccinated or non-vaccinated pigs and did not enhance the antibody and ASC responses to the AttHRV vaccine. The underlying mechanism for the direct protection of MidLA against rotavirus diarrhea is likely related to its direct or indirect effect on the intestinal epithelial barrier function as has been shown for LGG (34, 35). LGG is another probiotic strain which has been reported to reduce rotavirus diarrhea (2) and to enhance the immunogenicity of an oral rotavirus vaccine in separate clinical studies (8).
Previous studies in Gn pigs demonstrated that protective immunity against rotavirus diarrhea is positively correlated with the magnitude of rotavirus-specific serum and intestinal IgA antibody, intestinal IgA ASC, and IFN-γ producing T cell responses at challenge (26, 31, 36, 37). Our current study further highlighted the association of rotavirus-specific serum antibody and intestinal ASC responses with rotavirus protective immunity. Although low dose LA significantly promoted IFN-γ producing CD4+ and CD8+ T cell responses and suppressed regulatory T cell responses and their TGF-β and IL-10 productions (29); it did not enhance ASC, memory B cell and antibody responses to rotavirus vaccine in the LoLA+AttHRV pigs, hence the protection rate against rotavirus diarrhea was not increased. High dose LA increased the frequencies of Treg cells in most of the tissues of the HiLA+AttHRV pigs at PID 28 compared to the control groups (29). The increased Treg cell responses suppressed effector T cell activation, including Th1 and Th2 effector T cells, leading to weakened protective immunity (38). Indeed, we observed prolonged virus shedding and increased severity of diarrhea in the HiLA+AttHRV pigs upon challenge with VirHRV.
It is worth noting that, compared with the AttHRV only pigs; the MidLA+AttHRV pigs had significantly increased rotavirus-specific IgA and IgG memory B cell responses in spleen at PID 28, which were associated with the increased protection against the rotavirus diarrhea. Previous studies showed that after AttHRV inoculation, IgA and IgG memory B cells mainly reside in spleen whereas after VirHRV infection, memory B cells mainly reside in intestinal lymphoid tissues (33). Our data indicated that LA adjuvant at the intermediate dose significantly promoted the development and homing of memory B cells induced by the AttHRV. The increased numbers of splenic memory B cells are likely the main source of the increased serum antibody titers in the MidLA+AttHRV pigs at challenge and postchallenge (33).
The neonatal Gn pig model represents a unique model to study the immune responses induced by HRV vaccines and the protective efficacy upon VirHRV challenge and the effects of immune modulation by probiotics. Gnotobiotic pigs are born devoid of maternal antibodies, but are immunocompetent, allowing assessment of true primary immune responses (36). Extraneous microorganisms and enteropathogens are absent (i.e. wild type rotavirus, E. coli), thus immune responses to a single pathogen, a vaccine, a probiotic bacterial strain or a well-defined gut microflora can be assessed. This study clearly demonstrated that different doses of a particular probiotic strain can exert qualitatively different effects in terms of protective benefit and immunomodulation. Probiotics can be less effective or ineffective (the low dose LA) or even detrimental (the high dose LA) if not used at the optimal dosage, highlighting the importance of dose-effect studies for all probiotic uses. It is possible that the dose effects exist for all probiotic strains and the dose range that can be considered low, intermediate or high dose differs for each probiotic strain in different host populations, depending on species, age, composition of intestinal microbiota, immune status, diets, etc. The initial dosing regimen (low dose) was selected based on an early study showing that LGG (5×1010 CFU/dose) given to infants for 5 days twice daily around the time of an oral live rotavirus vaccine administration enhanced the rotavirus IgA seroconversion rate (8). In our previous studies (9, 20), we started with testing LA at 5 doses (once daily every other day) but at a markedly lower CFU/dose based on the consideration that unlike human infants, Gn pigs do not have indigenous gut microbiota to compete with LA for colonization.
It is interesting that LA counts in the MidLA+AttHRV pigs did not differ significantly from HiLA+AttHRV or LoLA+AttHRV pigs. Similarly, in our previous study Lactobacillus rhamnosus GG (LGG) counts in fecal samples from the LGG-fed Gn pigs remained within the range of 107–108 CFU/ml and did not exhibit a gradual increase with the progressively augmented intake of the LGG dose from 103 to 1012 CFU (39). Additionally, a study using doses of 108, 109, 1010 or 1011 CFU of Bifidobacterium animalis or Lactobacillus paracasei daily in healthy adults reported no recovery of L. paracasei for any of the doses but the recovery of B. animalis exhibited a dose-dependent manner (40). In contrast, another study using 108 CFU of L. paracasei DN-114001 daily in healthy adults found that the fecal recovery increased 1000-fold after 10 days in all adults (41). It is still debatable that adherence and colonization of the gastrointestinal tract is essential for probiotics to exert biological activity (42). Nonetheless, based on our results, the effectiveness of the probiotic LA strain as a vaccine adjuvant does not depend on the colonization and growth capability of the LA in the gut. More studies are required to understand the underlying mechanisms.
Consistent with our findings of the differential modulating effect of different dosing regimen of LA on adaptive humoral and T cell immune responses, a study of gut mucosal immunostimulation by various lactic acid bacteria strains in mice showed that among three dosing regimens (2, 5 or 7 consecutive days of feeding), L. acidophilus and L. plantarum were effective only in the 2-day feeding group, but not 5- or 7-day groups to significantly increase the numbers of total IgA secreting cells in the lamina propria of the small intestine (21). In another recent study, a low and high dose pretreatment of L. rhamnosus ATCC 7469 (1010 and 1012 CFU/day, respectively) was used to elucidate dose effects of L. rhamnosus on the gut microbiota and mucosal immune responses in a pig model of F4+ETEC challenge (43). Piglets pretreated with high dose L. rhamnosus had stronger downregulation of F4+ ETEC-induced innate immune responses (jejunal TLR4, IL-8 and ileal porcine β-defensins 2 mRNA expressions) compared to those pretreated with low dose. Piglets pretreated with high dose L. rhamnosus failed to upregulate TLR2, TLR9, NOD1 and TNF-α mRNA expression. Consequently, pretreatment with the low dose L. rhamnosus is more effective at ameliorating F4+ ETEC-induced diarrhea than with the high dose. Collectively, the previous reports and our present studies indicate that both dose (CFU/day) and dosing regimen (numbers of feeding) of probiotics have significant effects on the immune modulatory functions of probiotics. High dose or high number of dosing may have a negative impact on the immunostimulatory effect of probiotics and reduce or abolish their effectiveness.
To date, five randomized, placebo-controlled clinical trials evaluating the effectiveness of probiotics in stimulating mucosal vaccine-specific humoral immune responses have been reported. The range of probiotic doses is between 1 to 4×1010 CFU/day, the range of dosing is between one to five weeks, and the group size is between 9 to 25 (7, 8, 10, 44, 45). Six probiotic strains were shown to be effective (L. rhamnosus GG, L. paracasei CRL431, L. acidophilus La-14, Bifidobacterium lactis Bl-04 and BI-07) by one or more studies. Four strains (L. acidophilus NCFM, L. plantarum Lp-115, L. paracasei Lpc-37, L. salivarius Ls-33) were reported to be ineffective by one pilot study with a small group size of only 9 volunteers aged between 18–62 years old (44). There are many variables which can impact the efficacy of probiotic adjuvants. The main reason for the ineffectiveness of LA and other three strains in the clinical study may be due to strain specific effects, but it is also likely due to the non-optimal dose and dosing regimen for these four strains. Optimal dose and dosing regimen needs to be determined for each probiotic strain in clinical trials. There will probably be no one standardized dose and dosing regimen for uses of all probiotic adjuvants in humans. While the optimal dose and dosing regimen identified in the Gn pigs cannot be directly extrapolated to humans, it can serve as a reference for future studies on using LA as rotavirus vaccine adjuvant in human infants.
The limitation of this study is that the precise mechanisms for the observed direct reduction of diarrhea and adjuvant effect of the MidLA are not clearly defined. Thus, it is difficult to predict how the results in Gn pigs will compare to those in conventional pigs or humans which have indigenous gut microflora. The interactions between LA and gut microbiota can influence the functions of LA through competition for space or nutrients, production of antimicrobial agents, or immunomodulation, thus different doses and dosing regimens may be needed to achieve the dual functions in conventional hosts. Further studies are under way in our laboratory to address these questions using neonatal human gut microbiota colonized Gn pigs to more closely model human infants. The findings in Gn pigs are pertinent and can provide guidance to further studies in humanized pigs or human clinical trials.
Acknowledgments
We thank Marlice Vonck, Pete Jobst, Andrea Pulliam, Kimberly Allen, and Shannon Viers for animal care at Virginia-Maryland Regional College of Veterinary Medicine, Virginia Polytechnic Institute and State University. We thank Dr. Linda J. Saif from The Ohio State University for providing virulent and attenuated human rotavirus.
Funds: This work was supported by a grant (R01AT004789) from the National Center of Complementary and Alternative Medicine, National Institutes of Health, Bethesda, MD.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Guarino A, Canani RB, Spagnuolo MI, et al. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25(5):516–9. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Isolauri E, Juntunen M, Rautanen T, et al. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88(1):90–7. [PubMed] [Google Scholar]
- 3.Teran CG, Teran-Escalera CN, Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. Int J Infect Dis. 2009;13(4):518–23. doi: 10.1016/j.ijid.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeldt V, Michaelsen KF, Jakobsen M, et al. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr Infect Dis J. 2002;21(5):411–6. doi: 10.1097/00006454-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30(1):54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Licciardi PV, Tang ML. Vaccine adjuvant properties of probiotic bacteria. Discov Med. 2011;12(67):525–33. [PubMed] [Google Scholar]
- 7.Davidson LE, Fiorino AM, Snydman DR, et al. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011;65(4):501–7. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isolauri E, Joensuu J, Suomalainen H, et al. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13(3):310–2. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Azevedo MS, Wen K, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26(29–30):3655–61. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vrese M, Rautenberg P, Laue C, et al. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005;44(7):406–13. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- 11.Olivares M, Diaz-Ropero MP, Sierra S, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23(3):254–60. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Rizzardini G, Eskesen D, Calder PC, et al. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2012;107(6):876–84. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 13.Soh SE, Ong DQ, Gerez I, et al. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine. 2010;28(14):2577–9. doi: 10.1016/j.vaccine.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Baldrick P, Richardson D, Elliott G, et al. Safety evaluation of monophosphoryl lipid A (MPL): an immunostimulatory adjuvant. Regul Toxicol Pharmacol. 2002;35(3):398–413. doi: 10.1006/rtph.2002.1541. [DOI] [PubMed] [Google Scholar]
- 15.Vicari AP, Schmalbach T, Lekstrom-Himes J, et al. Safety, pharmacokinetics and immune effects in normal volunteers of CPG 10101 (ACTILON), an investigational synthetic toll-like receptor 9 agonist. Antivir Ther. 2007;12(5):741–51. [PubMed] [Google Scholar]
- 16.Zhang W, Wen K, Azevedo MS, et al. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008;121(3–4):222–31. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen K, Azevedo MS, Gonzalez A, et al. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Vet Immunol Immunopathol. 2009;127(3–4):304–15. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Azevedo MS, Gonzalez AM, et al. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008;122(1–2):175–81. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen K, Li G, Zhang W, et al. Development of gammadelta T cell subset responses in gnotobiotic pigs infected with human rotaviruses and colonized with probiotic lactobacilli. Vet Immunol Immunopathol. 2011;141(3–4):267–75. doi: 10.1016/j.vetimm.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen K, Li G, Bui T, et al. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine. 2012;30(6):1198–207. doi: 10.1016/j.vaccine.2011.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitini E, Alvarez S, Medina M, et al. Gut mucosal immunostimulation by lactic acid bacteria. Biocell. 2000;24(3):223–32. [PubMed] [Google Scholar]
- 22.Gackowska L, Michalkiewicz J, Krotkiewski M, et al. Combined effect of different lactic acid bacteria strains on the mode of cytokines pattern expression in human peripheral blood mononuclear cells. J Physiol Pharmacol. 2006;57 (Suppl 9):13–21. [PubMed] [Google Scholar]
- 23.Elmadfa I, Klein P, Meyer AL. Immune-stimulating effects of lactic acid bacteria in vivo and in vitro. Proc Nutr Soc. 2010;69(3):416–20. doi: 10.1017/S0029665110001710. [DOI] [PubMed] [Google Scholar]
- 24.Li CY, Lin HC, Lai CH, et al. Immunomodulatory effects of lactobacillus and Bifidobacterium on both murine and human mitogen-activated T cells. Int Arch Allergy Immunol. 2011;156(2):128–36. doi: 10.1159/000322350. [DOI] [PubMed] [Google Scholar]
- 25.Mane J, Pedrosa E, Loren V, et al. A mixture of Lactobacillus plantarum CECT 7315 and CECT 7316 enhances systemic immunity in elderly subjects. A dose-response, double-blind, placebo-controlled, randomized pilot trial. Nutr Hosp. 2011;26(1):228–35. [PubMed] [Google Scholar]
- 26.Yuan L, Ward LA, Rosen BI, et al. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70(5):3075–83. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Li G, Wen K, et al. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010;23(2):135–49. doi: 10.1089/vim.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer RC, Bohl EH, Kohler EM. Procurement and Maintenance of Germ-Free Seine for Microbiological Investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen K, Li G, Bui T, et al. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine. 2011;30(6):1198–207. doi: 10.1016/j.vaccine.2011.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward LA, Rosen BI, Yuan L, et al. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77 ( Pt 7):1431–41. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 31.To TL, Ward LA, Yuan L, et al. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. 1998;79 ( Pt 11):2661–72. doi: 10.1099/0022-1317-79-11-2661. [DOI] [PubMed] [Google Scholar]
- 32.Parreno V, Hodgins DC, de Arriba L, et al. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J Gen Virol. 1999;80 ( Pt 6):1417–28. doi: 10.1099/0022-1317-80-6-1417. [DOI] [PubMed] [Google Scholar]
- 33.Yuan L, Geyer A, Saif LJ. Short-term immunoglobulin A B-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotobiotic pigs inoculated with Wa human rotavirus. Immunology. 2001;103(2):188–98. doi: 10.1046/j.1365-2567.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciorba MA, Riehl TE, Rao MS, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61(6):829–38. doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth A, Yan F, Polk DB, et al. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G1060–9. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87(3–4):147–60. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan L, Wen K, Azevedo MS, et al. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;26(26):3322–31. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe JH, Ertelt JM, Way SS. Foxp3(+) regulatory T cells, immune stimulation and host defence against infection. Immunology. 2012;136(1):1–10. doi: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F, Li G, Wen K, et al. Lactobacillus rhamnosus GG on rotavirus induced injury of ileal epithelium in gnotobiotic pigs. Journal of Pediatric Gastroenterology & Nutrition. 2013 doi: 10.1097/MPG.0b013e3182a356e1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen HR, Larsen CN, Kaestel P, et al. Immunomodulating potential of supplementation with probiotics: a dose-response study in healthy young adults. FEMS Immunol Med Microbiol. 2006;47(3):380–90. doi: 10.1111/j.1574-695X.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 41.Rochet V, Rigottier-Gois L, Sutren M, et al. Effects of orally administered Lactobacillus casei DN-114 001 on the composition or activities of the dominant faecal microbiota in healthy humans. Br J Nutr. 2006;95(2):421–9. doi: 10.1079/bjn20051625. [DOI] [PubMed] [Google Scholar]
- 42.West NP, Pyne DB, Peake JM, et al. Probiotics, immunity and exercise: a review. Exerc Immunol Rev. 2009;15:107–26. [PubMed] [Google Scholar]
- 43.Li XQ, Zhu YH, Zhang HF, et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: intestinal microbiota and immune imbalances. PLoS One. 2012;7(7):e40666. doi: 10.1371/journal.pone.0040666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paineau D, Carcano D, Leyer G, et al. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol Med Microbiol. 2008;53(1):107–13. doi: 10.1111/j.1574-695X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 45.Fang H, Elina T, Heikki A, et al. Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol. 2000;29(1):47–52. doi: 10.1111/j.1574-695X.2000.tb01504.x. [DOI] [PubMed] [Google Scholar]