Abstract
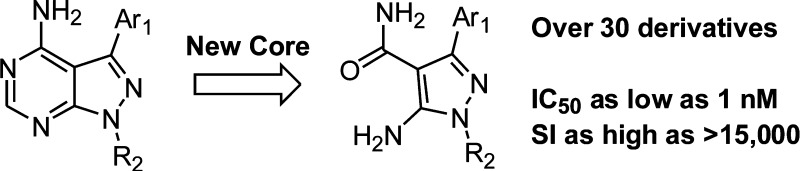
5-Aminopyrazole-4-carboxamide was used as an alternative scaffold to substitute for the pyrazolopyrimidine of a known “bumped kinase inhibitor” to create selective inhibitors of calcium-dependent protein kinase-1 from both Toxoplasma gondii and Cryptosporidium parvum. Compounds with low nanomolar inhibitory potencies against the target enzymes were obtained. The most selective inhibitors also exhibited submicromolar activities in T. gondii cell proliferation assays and were shown to be nontoxic to mammalian cells.
Keywords: Toxoplasma gondii, Cryptosporidium parvum, calcium-dependent protein kinase-1, enzyme inhibitor, selectivity
Toxoplasma gondii and Cryptosporidium parvum are apicomplexan parasites that cause serious diseases in humans (toxoplasmosis1,2 and cryptosporidiosis3) with inadequate treatment options. C. parvum infection has been implicated in 15–20% of childhood diarrhea cases in developing countries4,5 and can lead to life-threatening illness in immunocompromised persons. The only approved medicine for C. parvum infection, nitazoxanide, is expensive and not very effective for treating immunocompromised patients.3 Toxoplasmosis also leads to life-threatening situations in immunocompromised patients. T. gondii infection of pregnant women can result in severe birth defects or miscarriage. Current options are limited to sulfadiazine and pyrimethamine, which can have toxic side effects and require lifelong treatment for immunocompromised persons.1,2 Clearly, new and effective therapy for treating T. gondii or C. parvum infection is needed.
The calcium-dependent protein kinase-1 orthologues of both T. gondii (TgCDPK1) and C. parvum (CpCDPK1) have attracted interest as potential drug targets for these parasites.6−12 CDPK1 belongs to a family of serine/threonine protein kinases found in plants and Apicomplexa but not in humans or other animals. Recent genetic and chemical evidence suggests that TgCDPK1 plays a critical role in the lifecycle of T. gondii parasites by controlling the exocytosis of micronemes, which are specialized organelles that contain a number of proteins involved in parasite invasion and egress.6CpCDPK1 is likely of importance to the lifecycle of C. parvum for similar reasons.
From a drug discovery perspective, CDPK1 contains unique structural features that can be targeted for parasite-specific inhibition over human kinases. Crystal structures of TgCDPK1 and CpCDPK1 revealed an enlarged ATP-binding pocket due to the presence of the smallest amino acid, glycine, at the “gatekeeper” position adjacent to the adenine recognition site.7,8 In most kinases, the amino acid at the gatekeeper position is large although threonine or valine can be found at this position. It is extremely rare for alanine or glycine to be present at this position.13 Consequently, we and others have explored the use of so-called “bumped kinase inhibitors”14 (BKIs), which allow selective inhibition of Tg/CpCDPK1s and parasite proliferation.7,10,12 Our previous efforts have focused on generating BKIs based on a pyrazolopyrimidine (PP) scaffold (Figure 1); several of which are very potent and selective inhibitors of Tg/CpCDPK1 that show good efficacies against parasites and minimal toxicities to mammalian cells.9,11,15 We also attempted to generate selective inhibitors using an alternative acylbenzimidazole scaffold (Figure 1). Guided by compound-bound TgCDPK1 structures, we obtained potent inhibitors based on this scaffold that unfortunately lacked cellular activity.16 In our continued efforts to explore alternative scaffolds in the hope of finding compounds with different physicochemical and pharmacological profiles for drug discovery, we report here the design of Tg/CpCDPK1 inhibitors based on a 5-aminopyrazole-4-carboxamide (AP) scaffold (Figure 1).
Figure 1.
Different chemical scaffolds for CDPK1 inhibitors.
Inhibitor design was based on the crystal structures of TgCDPK1 in complex with several PP and acylbenzimidazole derivatives.7,15 The 4-amino group of the PP scaffold and the adjacent N5 nitrogen atom make hydrogen bonds to the hinge region of TgCDPK1, projecting the Ar1 group at the C3 position into the hydrophobic pocket adjacent to the gatekeeper residue and the R2 group at the N1 position into the ribose-binding pocket. The acylbenzimidazole scaffold allows Ar1 and R2 substituents to be oriented in the same directions and pockets as PP-based inhibitors. It is the combined effect of Ar1 and R2 groups that enhances selectivity for CDPK1 even over human kinases that contain a relatively small gatekeeper residue, such as the threonine gatekeeper of the tyrosine kinase Src.15 Using pyrazole-4-carboxamide as an alternative core scaffold, we expected the 4-carboxamide group to replace the pyrimidine to form specific hydrogen bonds to the hinge region of CDPK1 and maintain the relative orientations of Ar1 and R2. Incorporation of the 5-amino group allows the formation of an intramolecular hydrogen bond to the amide oxygen atom in order to preorganize the compound for binding to CDPK1 and reduce loss of binding entropy. The pyrazole moiety has been utilized before as a scaffold for the discovery of kinase inhibitors such as those targeting p38α MAP kinase at an allosteric binding site outside the ATP binding pocket.17,18 However, our design explores a different functional group display and targets an alternative site within the kinase domain.
The synthesis of AP derivatives with N1 and C3 substitutions has been reported in the literature,19,20 and some of the derivatives were used as intermediates for other classes of kinase inhibitors.19 We used similar approaches to synthesize a few derivatives containing selective combinations of Ar1 and R2 groups to compare directly with matching analogues based on the PP scaffold, as well as one compound with the pyrazole scaffold without the 5-amino group. The results are shown in Table 1. It was gratifying to observe that even the first compound (1) with 2-naphthyl at C3 and t-butyl at N1 is a very potent inhibitor of both parasite CDPK1s. Compound 1 is slightly more potent than the corresponding PP scaffold analogue (compound 2). The same trend exists for the pair of compounds 3 and 4, which contain 6-ethoxy-2-naphthyl groups at their C3 positions. Compound 5, which lacks the 5-amino group of compound 3, is an important control in this series. Compound 5 possesses 30-fold lower potency against the target enzymes relative to 3, indicating that keeping the 5-amino group on the pyrazole scaffold to restrict the conformation and orientation of the 4-carboxamide group through an anticipated intramolecular hydrogen bond is very desirable. These examples provided confidence that our design approach for CDPK1 inhibitors is working as expected.
Table 1. Initial Assessment of CDPK1 Inhibitors Based on a 5-Aminopyrazole-4-carboxamide Scaffold.
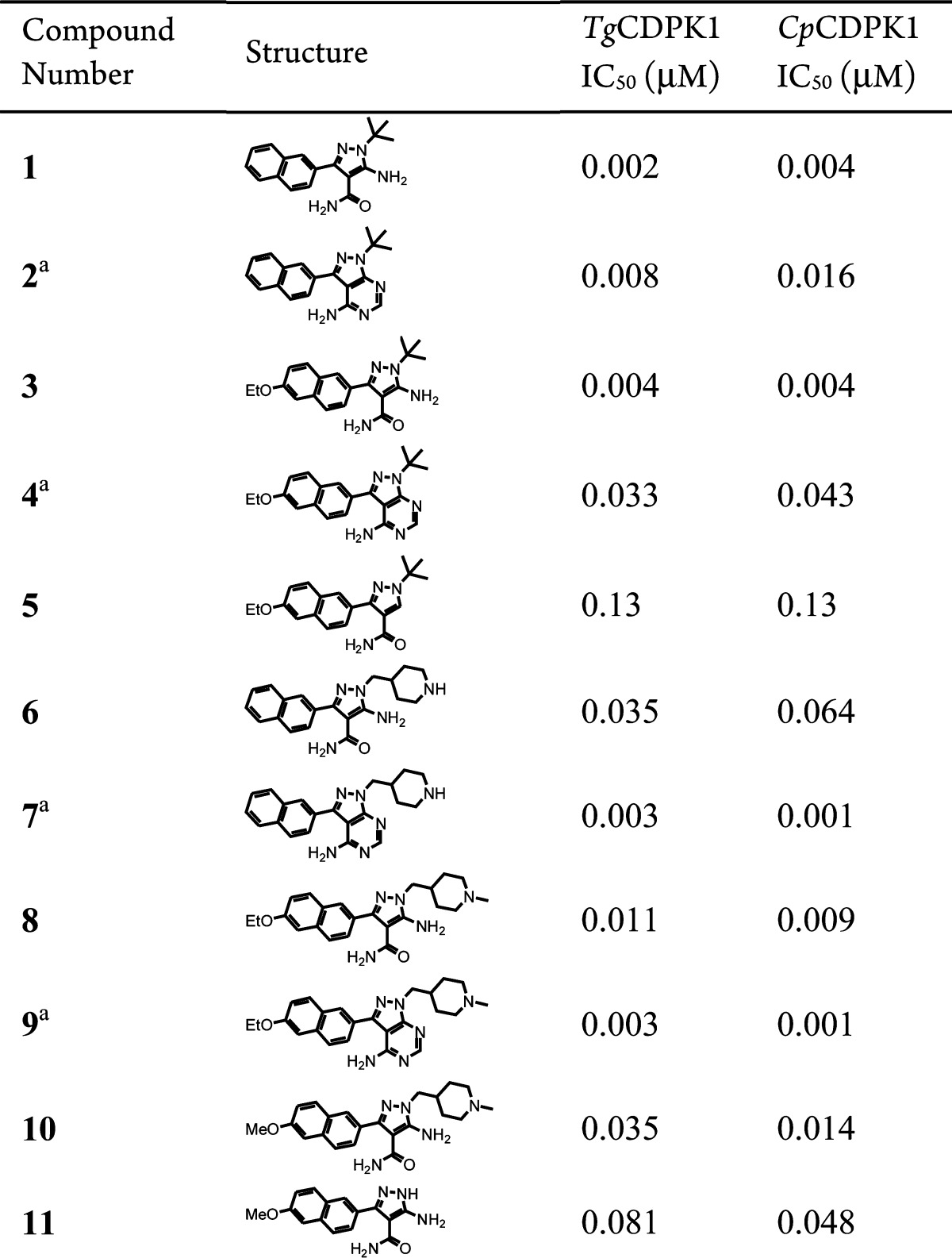
Reported in ref (11). Assay was performed in a pH 7.5 buffer containing 1 mM EGTA, 2 mM CaCl2, 10 μM ATP, and 40 μM syntide-2 peptide substrate (PLARTLSVAGLPGKK–OH). Enzyme concentration: 2.0 nM of TgCDPK1 or 0.80 nM of CpCDPK1.
Additional interesting structure–activity relationship (SAR) information was revealed through compounds 6–11 in Table 1 that explore the effect of substitutions at the N1 position containing a piperidine moiety. In our previous investigations, incorporation of a piperidine moiety at N1 on the PP scaffold had several beneficial effects. It improved compound solubility, inhibitory potency against the parasite enzyme, and selectivity over mammalian enzymes (compounds 7 and 9 versus compounds 2 and 4).11,15 However, a similar improvement in potency is not preserved in the new AP series, as both compounds 6 and 8 lose potency compared to the matched PP analogues 7 and 9, or when compared to compounds 1 and 3 with the same core scaffold but containing a t-butyl group at the N1 position. From compounds 10 and 11, it seems that the contribution of a methyl piperidine moiety at the N1 position to inhibitory potency is very limited. Possible reasons for the different SAR of the two series of compounds may include differences in electronic and conformational features of the two cores. The PP core is a flat fused ring system, while the exocyclic amide group of 5-amino-pyrazole-4-caboxamide may adopt conformations that deviate from perfect planarity. Nevertheless, it is clear from data presented in Table 1 that the SAR we obtained with PP scaffold is not directly transferrable to the AP series, and new SAR needs to be derived.
We divide the SAR work into two major parts, as listed in Tables 2 and 3. For derivatives shown in Table 2, the Ar1 is fixed as 2-naphthyl, and the N1 substitution R2 is varied with small aliphatic chains and rings, some of which contain nitrogen or oxygen atoms to decrease lipophilicity. Overall, these small substitutions do not improve potency over the t-butyl analogue 1, but variants such as 18, with a trifluoroethyl substitution, have potency very close to compound 1. Similar substituents may serve as an alternative to the t-butyl group in the future to optimize pharmacokinetic properties. The relatively small range of potency changes for the R2 variations may be partly due to the fact that the R2 group is expected to occupy the ribose-binding pocket, a region that is exposed to solvent and can accommodate various functional groups.
Table 2. SAR Study of N1 Substitution.


Table 3. SAR Study of C3 Substitution.
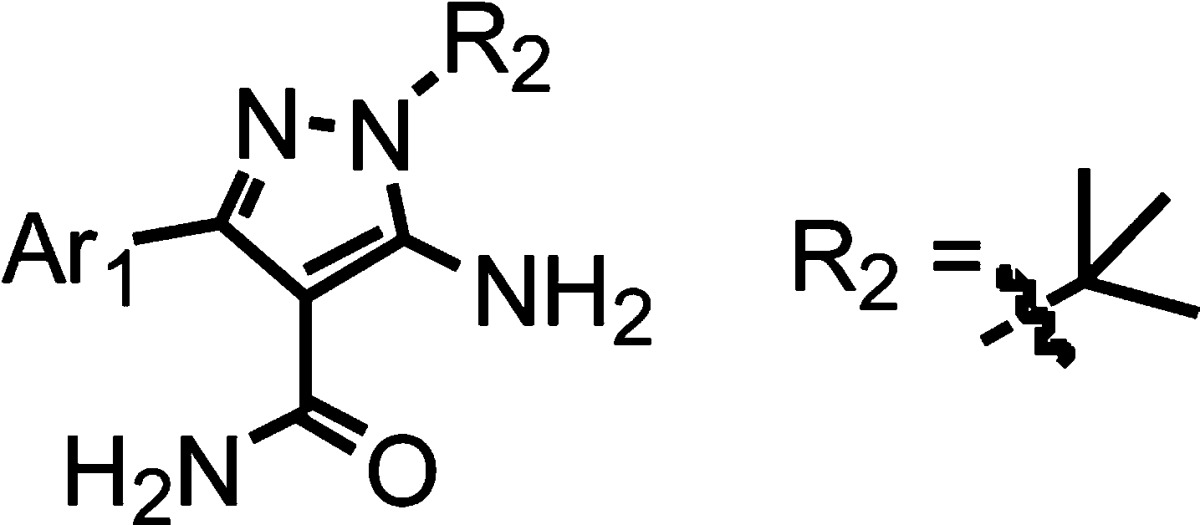

Because the t-butyl group seems to be optimal for the N1 position, it was fixed for the analogues in Table 3, which contain a range of substituted and fused double ring Ar1 systems at the C3 position. Compounds 24–27 are naphthyl-based substituents, with various alkoxy groups at the 6-position. These compounds possess good inhibitory potency, similar to the PP series.11 Compounds 27–33 explore fused ring systems that contain 5-membered rings and hydrophilic substitutions. Unfortunately, all the modifications led to a loss of potency, indicating that these fused rings do not fit the binding pocket well. Coming back to two 6-membered ring fusions, while chromenone and quinolin-2-yl rings led to poor inhibitors (34–36), quinolin-3-yl or quinolin-6-yl were tolerated (37–39). Overall, 6-alkoxy-naphth-2-yl and 7-alkoxy-quinolin-3-yl groups are the best aromatic C3 substituents investigated (compounds 25, 26, 38, and 39).
We were able to obtain a crystal structure of the TgCDPK1-35 complex at 2.0 Å. Superposition of the structures of TgCDPK1 in complex with 35 and with a PP analogue 2(7) shows clearly that the new AP core can preserve the projections of N1 and C3 substitutions as well as hydrogen bond interactions with TgCDPK1 seen for the PP core (Figure 2). This structure confirms our original design strategy. The amide group of the AP core is essentially on top of the aminopyrimidine moiety of the PP core. Further away, on the naphthyl/quinolinyl and t-butyl substitutions, the matching carbons are 0.5–0.6 Å apart. It is not clear if the deviations are caused by the difference in the aromatic rings or by the overall electronic configurations of the two inhibitors. It is certainly not sufficient to explain why the two series of inhibitors have different tolerance of methyl piperidine substituents on the N1 position (Table 1) since these substitutions project distally into the ribose binding pocket and the solvent.
Figure 2.

Close-up view of inhibitors in an overlay of TgCDPK1-bound 35 (2.0 Å, PDB 4M84, green structure; nitrogen in cyan, oxygen in red, and sulfur in yellow) and 2 (PDB 3I7C, ref (7); carbon in orange and water in pink). The key hydrogen bonds to the backbone oxygen of Glu129 and NH of Tyr131 are the same in both scaffolds.
A select group of compounds with low nanomolar IC50s for CDPK1s were tested for inhibition of a mammalian kinase with a small gatekeeper residue (Src), for inhibition of T. gondii cell proliferation and for cytotoxicity against a mammalian cell line (CRL8155) using reported procedures.11 The results are summarized in Table 4. Some of the compounds were several thousand-fold selective for TgCDPK1 over Src. Most inhibited parasite proliferation at submicromolar concentrations, and all of them demonstrated low toxicity to mammalian cells. These compounds are good candidates for further investigation of pharmacological properties and efficacies in animal models by oral administration. For example, 39 was given orally to mice at 10 mg/kg and showed an average Cmax of 10 μM at 60 min after dosing and an area under the curve (AUC) of 2500 μM·min. It was further profiled for kinome-wide selectivity using a panel of 80 human kinases representing different subfamilies of the kinome tree.21 Compound 39 did not show significant inhibition at 10 μM for 78 of the 80 kinases using a fluorescence based competition assay.22 The remaining two kinases, Kdr and Prkcn, were inhibited >1000-fold weaker by 39 than CpCDPK1 (Supporting Information). Follow-up optimization to improve compound solubility and extend half-life by oral administration is currently underway.
Table 4. Further Characterization of Select Inhibitors.
| compd | Src IC50 (μM) | selectivity index (Src/TgCDPK1) | T. gondii EC50 (μM) | cytotoxicity (CRL8155) EC50 (μM) |
|---|---|---|---|---|
| 1 | 1.3 | 650 | 0.26 | >40 |
| 3 | 7.3 | 1825 | 0.39 | >30 |
| 18 | 0.98 | 245 | 0.26 | >30 |
| 19 | 2.7 | 225 | 0.32 | >40 |
| 25 | 6.9 | >3500 | 0.072 | >40 |
| 38 | >10 | >1600 | 1.1 | >40 |
| 39 | >30 | >15000 | 0.22 | >30 |
In summary, we have developed potent and selective inhibitors of Tg/CpCDPK1 based on a 5-aminopyrazole-4-carboxamide scaffold. Preliminary SAR studies led to compounds with excellent selectivity over Src, good efficacy in T. gondii proliferation assays, and low toxicity to mammalian cells. This clearly demonstrates that the 5-aminopyrazole-4-carboxamide scaffold can effectively replace the pyrazolopyrimidine core of the BKI series, enabling design of novel CDPK1 inhibitors that offer additional opportunities for tuning their pharmacokinetic properties in our drug discovery efforts.
Glossary
ABBREVIATIONS
- AP
5-aminopyrazole-4-carboxamide
- BKI
bumped kinase inhibitor
- CDPK1
calcium-dependent protein kinase-1
- PP
pyrazolopyrimidine
- SAR
structure–activity relationship
Supporting Information Available
Synthetic procedures, compound characterization data, crystallography table, and kinase selectivity table. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession Codes
The PDB accession code for the X-ray crystal structure of TgCDPK1-35 complex is 4M84.
K.R.K. was supported by a training scholarship from the University of Washington Plein Endowment for Geriatric Pharmacy Research. J.A.G. was supported by a training grant from the National Institute of Allergy and Infectious Diseases (Grant T32AI007509). Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases and the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R01AI089441, R01GM086858, and T32AI007509. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Schwartzman J. D.; Maguire J. H.. Toxoplasmosis. In Tropical Infectious Diseases: Principles, Pathogens and Practice, 3rd ed.; Guerrant R. L., Walker D. H., Weller P. F., Eds.; Saunders: Edinburgh, U.K., 2011; Chapter 103, pp 722–728. [Google Scholar]
- Montoya J. G.; Boothroyd J. C.; Kovacs J. A.. Toxoplasma gondii. In Mandell, Douglas, & Bennett’s Principles and Practice of Infectious Diseases, 7th ed.; Mandell G. L., Bennett J. E., Dolin R., Eds.; Churchill: Livingston, U.K., 2010; Chapter 279, pp 3495–3526. [Google Scholar]
- White A. C.Cryptosporidium Species. In Mandell, Douglas, & Bennett’s Principles and Practice of Infectious Diseases, 7th ed.; Mandell G. L., Bennett J. E., Dolin R., Eds.; Churchill: Livingston, U.K., 2010; Chapter 283, pp 3547–3560. [Google Scholar]
- Samie A.; Bessong P. O.; Obi C. L.; Sevilleja J. E.; Stroup S.; Houpt E.; Guerrant R. L. Cryptosporidium species: preliminary descriptions of the prevalence and genotype distribution among school children and hospital patients in the Venda region, Limpopo Province, South Africa. Exp. Parasitol. 2006, 114, 314–322. [DOI] [PubMed] [Google Scholar]
- Ajjampur S. S.; Rajendran P.; Ramani S.; Banerjee I.; Monica B.; Sankaran P.; Rosario V.; Arumugam R.; Sarkar R.; Ward H.; Kang G. Closing the diarrhoea diagnostic gap in Indian children by the application of molecular techniques. J. Med. Microbiol. 2008, 57, 1364–1368. [DOI] [PubMed] [Google Scholar]
- Lourido S.; Shuman J.; Zhang C.; Shokat K. M.; Hui R.; Sibley L. D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 2010, 465, 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K. K.; Larson E. T.; Keyloun K. R.; Castaneda L. J.; DeRocher A. E.; Inampudi K. K.; Kim J. E.; Arakaki T. L.; Murphy R. C.; Zhang L.; Napuli A. J.; Maly D. J.; Verlinde C. L. M. J.; Buckner F. S.; Parsons M.; Hol W. G. J.; Merritt E. A.; Van Voorhis W. C. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010, 17, 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernimont A. K.; Artz J. D.; Finerty P.; Lin Y.-H.; Amani M.; Allali-Hassani A.; Senisterra G.; Vedadi M.; Tempel W.; Mackenzie F.; Chau I.; Lourido S.; Sibley L. D.; Hui R. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat. Struct. Mol. Biol. 2010, 17, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. C.; Ojo K. K.; Larson E. T.; Castellanos-Gonzalez A.; Perera B. G. K.; Keyloun K. R.; Kim J. E.; Bhandari J. G.; Muller N. R.; Verlinde C. L. M. J.; White A. C.; Merritt E. A.; Van Voorhis W. C.; Maly D. J. Discovery of Potent and Selective Inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med. Chem. Lett. 2010, 1, 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T.; Kato K.; Kobayashi K.; Watanabe S.; Kurokawa H.; Gong H.; Pandey K.; Takemae H.; Akashi H. Use of the kinase inhibitor analog 1NM-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryotic Cell 2010, 9, 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M.; Murphy R. C.; Geiger J. A.; DeRocher A. E.; Zhang Z.; Ojo K. K.; Larson E. T.; Perera B. G.; Dale E. J.; He P.; Reid M. C.; Fox A. M.; Mueller N. R.; Merritt E. A.; Fan E.; Parsons M.; Van Voorhis W. C.; Maly D. J. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J. Med. Chem. 2012, 55, 2416–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido S.; Zhang C.; Lopez M.; Tang K.; Barks J.; Wang Q.; Wildman S. A.; Shokat K.; Sibley L. D. Optimizing small molecule inhibitors of calcium-dependent protein kinase 1 to prevent infection by Toxoplasma gondii. J. Med. Chem. 2013, 56, 3068–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Kenski D. M.; Paulson J. L.; Bonshtien A.; Sessa G.; Cross J. V.; Templeton D. J.; Shokat K. M. A second-site suppressor strategy for chemical genetic analysis of diverse protein kinases. Nat. Methods 2005, 2, 435–441. [DOI] [PubMed] [Google Scholar]
- Bishop A. C.; Kung C.-Y.; Shah K.; Witucki L.; Shokat K. M.; Liu Y. Generation of monospecific nanomolar tyrosine kinase inhibitors via a chemical genetic approach. J. Am. Chem. Soc. 1999, 121, 627–631. [Google Scholar]
- Larson E. T.; Ojo K. K.; Murphy R. C.; Johnson S. M.; Zhang Z.; Kim J. E.; Leibly D. J.; Fox A. M.; Reid M. C.; Dale E. J.; Perera B. G.; Kim J.; Hewitt S. N.; Hol W. G.; Verlinde C. L.; Fan E.; Van Voorhis W. C.; Maly D. J.; Merritt E. A. Multiple determinants for selective inhibition of apicomplexan calcium-dependent protein kinase CDPK1. J. Med. Chem. 2012, 55, 2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Ojo K. K.; Johnson S. M.; Larson E. T.; He P.; Geiger J. A.; Castellanos-Gonzalez A.; White A. C. Jr.; Parsons M.; Merritt E. A.; Maly D. J.; Verlinde C. L.; Van Voorhis W. C.; Fan E. Benzoylbenzimidazole-based selective inhibitors targeting Cryptosporidium parvum and Toxoplasma gondii calcium-dependent protein kinase-1. Bioorg. Med. Chem. Lett. 2012, 22, 5264–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pargellis C.; Tong L.; Churchill L.; Cirillo P. F.; Gilmore T.; Graham A. G.; Grob P. M.; Hickey E. R.; Moss N.; Pav S.; Regan J. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat. Struct. Mol. Biol. 2002, 9, 268–272. [DOI] [PubMed] [Google Scholar]
- Kroe R. R.; Regan J.; Proto A.; Peet G. W.; Roy T.; Landro L. D.; Fuschetto N. G.; Pargellis C. A.; Ingraham R. H. Thermal denaturation: A method to rank slow binding, high-affinity P38α MAP kinase inhibitors. J. Med. Chem. 2003, 46, 4669–4675. [DOI] [PubMed] [Google Scholar]
- Markwalder J. A.; Arnone M. R.; Benfield P. A.; Boisclair M.; Burton C. R.; Chang C.-H.; Cox S. S.; Czerniak P. M.; Dean C. L.; Doleniak D.; Grafstrom R.; Harrison B. A.; Kaltenbach R. F.; Nugiel D. A.; Rossi K. A.; Sherk S. R.; Sisk L. M.; Stouten P.; Trainor G. L.; Worland P.; Seitz S. P. Synthesis and biological evaluation of 1-aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-one inhibitors of cyclin-dependent kinases. J. Med. Chem. 2004, 47, 5894–5911. [DOI] [PubMed] [Google Scholar]
- Bobko M. A.; Kaura A. C.; Evans K. A.; Su D.-S. Novel synthesis of 5-amino-3-bromo-1-(tert-butyl)-1H-pyrazole-4-carbonitrile: A versatile intermediate for the preparation of 5-amino-3-aryl-1-(tert-butyl)-1H-pyrazole-4-carboxamides. Org. Lett. 2012, 14, 3906–3908. [DOI] [PubMed] [Google Scholar]
- Manning G.; Whyte D. B.; Martinez R.; Hunter T.; Sudarsanam S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1918. [DOI] [PubMed] [Google Scholar]
- Lebakken C. S.; Riddle S. M.; Singh U.; Frazee W. J.; Eliason H. C.; Gao Y.; Reichling L. J.; Marks B. D.; Vogel K. W. Development and applications of a broad-coverage, TR-FRET-based kinase binding assay platform. J. Biomol. Screening 2009, 14, 924–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



