Abstract
Plasmids carrying the CYR1 gene of yeast Saccharomyces cerevisiae, which encodes adenylate cyclase, were introduced into the cya mutant strain of Escherichia coli. The transformants had a GTP-independent adenylate cyclase activity but did not produce cAMP. The E. coli transformant carrying the yeast RAS2 or RAS2val19 gene had no adenylate cyclase activity. Transformant cells carrying both CYR1 and RAS2 produced GTP-dependent adenylate cyclase and cAMP, and those carrying CYR1 and RAS2val19 produced GTP-independent adenylate cyclase and a large amount of cAMP. Production of cAMP in the transformant carrying CYR1 and either RAS2 or RAS2val19 was confirmed by staining colonies on maltose-MacConkey plates and by measuring induction of beta-galactosidase by isopropyl beta-D-thiogalactopyranoside. Mixing a crude extract from the E. coli transformant carrying CYR1 with a crude extract from cells carrying RAS2 reconstituted the GTP-dependent adenylate cyclase. Reconstitution of the GTP-dependent adenylate cyclase was observed by mixing the plasma membrane fraction of yeast CYR1 ras1 ras2 bcy1 mutant and a crude extract from the E. coli transformant carrying RAS2 or by mixing a crude extract from the E. coli transformant carrying CYR1 and the membrane fraction of yeast cyr1 RAS1 RAS2 BCY1 mutant. The data suggest that the yeast GTP-dependent adenylate cyclase consists of catalytic and regulatory subunits encoded by the CYR1 and RAS2 genes, respectively.
Full text
PDF
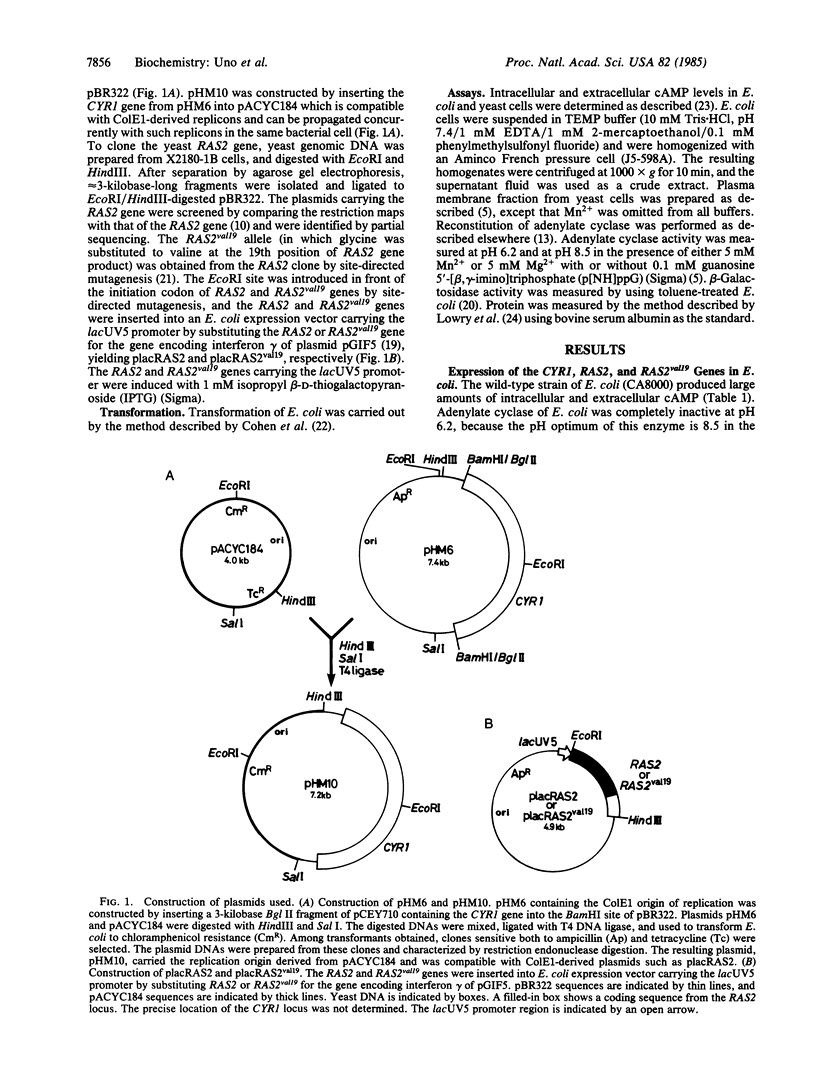

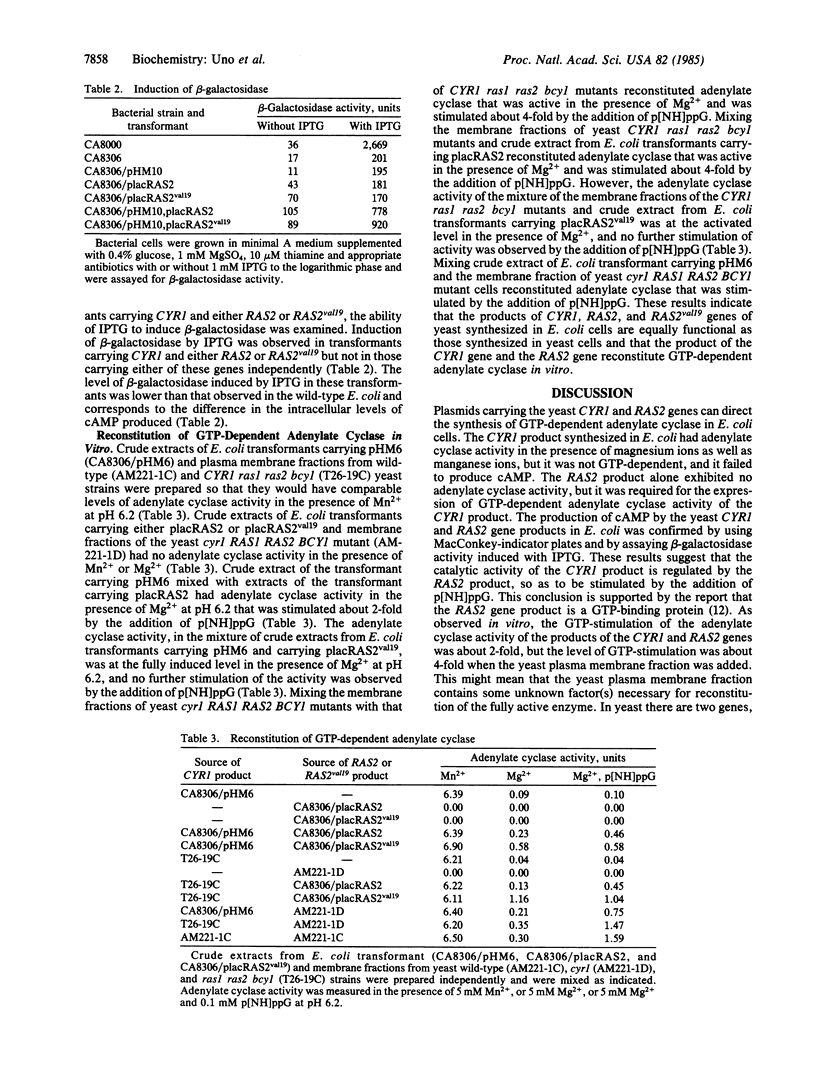

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casperson G. F., Walker N., Brasier A. R., Bourne H. R. A guanine nucleotide-sensitive adenylate cyclase in the yeast Saccharomyces cerevisiae. J Biol Chem. 1983 Jul 10;258(13):7911–7914. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- Dhar R., Nieto A., Koller R., DeFeo-Jones D., Scolnick E. M. Nucleotide sequence of two rasH related-genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Apr 25;12(8):3611–3618. doi: 10.1093/nar/12.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masson P., Jacquemin J. M., Culot M. Molecular cloning of the tsm0185 gene responsible for adenylate cyclase activity in Saccharomyces cerevisiae. Ann Microbiol (Paris) 1984 May-Jun;135A(3):343–351. doi: 10.1016/s0769-2609(84)80076-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Control of cell division in Saccharomyces cerevisiae mutants defective in adenylate cyclase and cAMP-dependent protein kinase. Exp Cell Res. 1983 Jun;146(1):151–161. doi: 10.1016/0014-4827(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Identification of the structural gene and nonsense alleles for adenylate cyclase in Saccharomyces cerevisiae. J Bacteriol. 1984 Jan;157(1):277–282. doi: 10.1128/jb.157.1.277-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Roy A., Danchin A. The cya locus of Escherichia coli K12: organization and gene products. Mol Gen Genet. 1982;188(3):465–471. doi: 10.1007/BF00330050. [DOI] [PubMed] [Google Scholar]
- Smigel M., Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. Mechanisms of guanine nucleotide-mediated regulation of adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:1–18. [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Walsh M., Kataoka T., Wigler M. A product of yeast RAS2 gene is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6924–6928. doi: 10.1073/pnas.81.22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Oshima T., Ohsuye K., Ono T., Mizono A., Ueno A., Nakazato H., Tsujimoto M., Higashi N., Noguchi T. Expression in Escherichia coli of chemically synthesized gene for the human immune interferon. Nucleic Acids Res. 1983 Mar 25;11(6):1707–1723. doi: 10.1093/nar/11.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- Temeles G. L., Gibbs J. B., D'Alonzo J. S., Sigal I. S., Scolnick E. M. Yeast and mammalian ras proteins have conserved biochemical properties. Nature. 1985 Feb 21;313(6004):700–703. doi: 10.1038/313700a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Adachi K., Ishikawa T. Characterization of cyclic AMP-requiring yeast mutants altered in the catalytic subunit of protein kinase. J Biol Chem. 1984 Oct 25;259(20):12508–12513. [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Ishikawa T. Characterization of cyclic AMP-requiring yeast mutants altered in the regulatory subunit of protein kinase. J Biol Chem. 1982 Dec 10;257(23):14110–14115. [PubMed] [Google Scholar]
- Yang J. K., Epstein W. Purification and characterization of adenylate cyclase from Escherichia coli K12. J Biol Chem. 1983 Mar 25;258(6):3750–3758. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]



