Abstract
Purpose.
We tested variants in genes related to lutein and zeaxanthin status for association with age-related macular degeneration (AMD) in the Carotenoids in Age-Related Eye Disease Study (CAREDS).
Methods.
Of 2005 CAREDS participants, 1663 were graded for AMD from fundus photography and genotyped for 424 single nucleotide polymorphisms (SNPs) from 24 candidate genes for carotenoid status. Of 337 AMD cases 91% had early or intermediate AMD. The SNPs were tested individually for association with AMD using logistic regression. A carotenoid-related genetic risk model was built using backward selection and compared to existing AMD risk factors using the area under the receiver operating characteristic curve (AUC).
Results.
A total of 24 variants from five genes (BCMO1, BCO2, NPCL1L1, ABCG8, and FADS2) not previously related to AMD and four genes related to AMD in previous studies (SCARB1, ABCA1, APOE, and ALDH3A2) were associated independently with AMD, after adjusting for age and ancestry. Variants in all genes (not always the identical SNPs) were associated with lutein and zeaxanthin in serum and/or macula, in this or other samples, except for BCO2 and FADS2. A genetic risk score including nine variants significantly (P = 0.002) discriminated between AMD cases and controls beyond age, smoking, CFH Y402H, and ARMS2 A69S. The odds ratio (95% confidence interval) for AMD among women in the highest versus lowest quintile for the risk score was 3.1 (2.0–4.9).
Conclusions.
Variants in genes related to lutein and zeaxanthin status were associated with AMD in CAREDS, adding to the body of evidence supporting a protective role of lutein and zeaxanthin in risk of AMD.
Keywords: macular degeneration, carotenoids, genes
In this study of over 1600 postmenopausal women of the CAREDS, we describe the first evidence that variation in multiple genes related to carotenoid status in the blood and macula are associated with age-related macular degeneration (AMD).
Introduction
Age-related macular degeneration (AMD) is a degenerative disease of the macula and the leading cause of blindness among the elderly in developed countries. Lutein and zeaxanthin, and the lutein metabolite meso-zeaxanthin, uniquely concentrate in the macula and comprise macular pigment (MP).1–4 Increasing evidence suggests the dietary carotenoids lutein and zeaxanthin protect against pathogenic processes of AMD5–7 by absorbing an estimated 40% to 90% of incident blue light8 otherwise damaging the macula,9 and lowering oxidative stress10–12 and inflammation.12–14 Systemic antioxidant and antiinflammatory effects of lutein also have been suggested,15,16 which may influence the retina indirectly through general inflammatory processes, and the availability of antioxidants and antiinflammatory molecules.
Despite strong biological plausibility for a protective effect of macular carotenoids against AMD, the body of evidence from epidemiologic studies and clinical trials is inconsistent. A protective influence of lutein and zeaxanthin in the diet or blood on lower risk for advanced AMD is supported by the results of several epidemiologic studies17–22 and by secondary, but not primary, analyses in the Age-Related Eye Disease Study 2 (AREDS2), a multicenter, randomized controlled clinical trial of lutein and zeaxanthin supplements, and progression of AMD individuals with intermediate or advanced disease.23 A protective influence of lutein and zeaxanthin intake on early AMD sometimes,22,24–26 but not always,22,24,27–29 is observed in epidemiologic studies. Thus, a role of dietary lutein and zeaxanthin in preventing and lowering progression of AMD is unclear.
One reason for inconsistency across previous studies may be that there is a variable macular pigment response to dietary intake of macular carotenoids.30–45 While lutein and zeaxanthin are acquired only through diet or supplements, the subsequent accumulation of these carotenoids in the retina is related to many factors, including several genetic factors.28,44,46,47 Results of a recent twin study suggest 27% of macular response to dietary carotenoids is heritable.44 Studies in animal models and humans support a role for genetic variation in determining carotenoid status in the retina or serum (see prior reviews48,49). Therefore, genetic variation associated with carotenoid status in the serum and retina can provide another line of evidence for the putative role of lutein and zeaxanthin in protecting against AMD, independent from dietary estimates of exposure to macular carotenoids. Relationships of diet or serum carotenoids and AMD also might reflect other unknown, and unadjusted for, aspects of diet and lifestyle related to AMD, while genetic measures of carotenoid status would not.
To evaluate genetic evidence for relationships of lutein and zeaxanthin to AMD, we examined relationships of common single nucleotide polymorphisms (SNPs) from genes in pathways related to binding, metabolism, or transport of macular carotenoids for association with AMD. These include variants in genes related to cholesterol and carotenoid membrane transport proteins in the intestine and retina, high density lipoprotein levels in blood, carotenoid cleavage, omega-3 fatty acid status previously related to macular pigment,50 and retinopathies associated with impaired macular pigment. These SNPs were studied previously for their relation to MP optical density.47 Relationships to serum concentration of lutein and zeaxanthin are reported within.
Methods
Study Sample
The sample included participants of the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study within the Women's Health Initiative Observational Study (WHI-OS), described previously.26,28 The CAREDS study visits were conducted between 2001 and 2004 in 2005 women from WHI-OS study centers in Madison, Wisconsin (n = 694), Iowa City, Iowa (n = 631), and Portland, Oregon (n = 680). Visits included ocular photography, measurement of the optical density of MP, and questionnaires to assess risk factors for age-related eye diseases, including queries of diet, supplement use, sunlight exposure history, and eye health history. The WHI-OS study visits in 1995–1998 provided additional relevant information, including collection and storage of serum samples that were used later for genotyping and biomarker measurement, smoking history, food frequency questionnaires, physical activity, blood pressure, and anthropometrics. The CAREDS and WHI-OS procedures conformed to the Declaration of Helsinki, informed consent was obtained from all participants, and approval was granted by the Institutional Review Board at each university.
AMD Classification
Stereoscopic fundus photographs were graded by the University of Wisconsin Fundus Photograph Reading Center using the Age-Related Eye Disease Study (AREDS) protocol for grading maculopathy.51 For the present analysis, women were classified as having AMD if they had photographic evidence of either early or late stages of AMD. Early AMD was classified, in part, using criteria for AREDS category 3. This included the presence of one or more large drusen (≥125 μ) or extensive intermediate drusen (total area ≥ 360 μ when soft indistinct drusen were present or ≥650 μ when soft indistinct drusen were absent).51 Additional criteria for early AMD included having pigmentary abnormalities; an increase or decrease in pigmentation, if accompanied by at least one druse ≥ 63 μ. Advanced AMD included geographic atrophy, neovascularization, or exudation in the center subfield. The reference group included women who had neither early nor advanced AMD; generally corresponding to AREDS categories 1 and 2.51
Serum Analyses of Lutein and Zeaxanthin
Serum samples, obtained from participants in WHI baseline examinations (1994–1998) and stored at −80°C, were analyzed for levels of trans lutein and zeaxanthin at Tufts University by a reverse phase high performance liquid chromatography (HPLC) analysis52 as described previously.28
Genotyping
Genotyping was attempted for 438 SNPs from 24 carotenoid pathway genes selected based on previous evidence that suggested their capacity to encode factors influencing carotenoid status.47,49,53–58 Specific SNPs within candidate genes were chosen based on previous literature or as tag SNPs for their respective gene. Tagging was conducted using the HapMap Genome Browser Release #27 (available in the public domain at http://hapmap.ncbi.nlm.nih.gov/) CEU reference population and filtering for a minor allele frequency (MAF) ≥ 0.05 and r2 ≥ 0.80. Tagging included a 20 kilobase (kb) pair window up- and downstream of each gene. Genotyping included an additional 190 ancestry informative markers (AIMs) for northwest-southeast European ancestry and southeastern-Ashkenazi Jewish ancestry clines.59
The SNPs were genotyped at Case Western Reserve University (Cleveland, OH) using an Illumina Custom GoldenGate Assay (Illumina, Inc., San Diego, CA). DNA was extracted from the buffy coats of blood obtained at WHI-OS baseline examinations (1994–1998) that have been stored frozen at −80°C. Genotype calls were made using Illumina Genome Studio (Illumina, Inc.). The SNPs that could not be assayed successfully because of the unique chemistry on the custom Illumina assay (not designable) were genotyped using KASP Assay at LCG Genomics (Teddington, UK) and called via the KASP SNP Genotyping System. Standard quality control (QC) filters were applied,60 resulting in exclusions of SNPs with Hardy-Weinberg equilibrium (HWE) χ2 P < 1.0 × 10−6, MAF < 0.01, or genotype call rates < 95%. A total of 424 candidate SNPs and 176 AIMs passed these QC filters. For a list of 424 SNPs tested in association analyses, see the previously published Supplementary Table S1.47
Of the 2005 enrolled, DNA was requested for 1787 participants who also had data on AMD status. Of these women 1697 approved use of and had sufficient DNA for genotyping. Participants were removed from the analysis if their individual genotyping call rate was < 90% (n = 21), overall heterozygosity > 44.5% (n = 12), or genotype concordance between individuals > 95% (n = 6). These were not mutually exclusive filters and resulted in a total of 1663 CAREDS participants (98%) passing QC tests.
Statistical Analysis
Data management and statistical analyses were performed using a combination of SAS software version 9.2 (SAS Institute, Inc., Cary NC) and PLINK version 1.07.61 Of CAREDS participants, 98% are self-reported white. However, to minimize the risk of residual confounding due to population stratification within a sample of European ancestry, principal components analysis was conducted using 176 AIMs and the SmartPCA program in EIGENSOFT.62,63 The first two components accounted for 3.1% and 1.3% of the genotype variability, respectively, and were used to adjust for ancestry.
Single SNP associations with serum lutein and zeaxanthin were performed using linear regression, assuming an additive genetic model and adjusting for global (genome-wide) ancestry via the first two principal components, and lutein and zeaxanthin intake from diet and supplements.
Single SNP associations with AMD were tested using logistic regression, assuming an additive genetic model, and adjusting for age and ancestry. In the case where <15 participants were homozygous for the minor allele, a dominant model was assumed. The SNPs associated with AMD (P ≤ 0.05) in the present model were retained for consideration in risk score.
As proof of concept for the ability of variation in lutein- and zeaxanthin-related genes to impact AMD risk beyond well-established predictors, we investigated the joint influence of the variants significantly related to AMD on the ability to classify persons correctly with AMD in this sample. The joint effect of individually significant SNPs in relation to AMD was assessed by constructing a lutein- and zeaxanthin-related genetic risk score, a linear combination of the number of risk alleles for each SNP, weighted by the respective logarithm of the odds ratio (OR).64,65 Model selection for the risk score was conducted beginning with all SNPs associated with AMD (P ≤ 0.05), and then implementing backward selection (exclusion P ≤ 0.10). Receiver operating characteristic (ROC) curves were plotted to assess whether the carotenoid-related genetic risk score significantly improved classification of AMD cases and noncases, beyond well-established AMD risk factors, including age, smoking (never, <7 pack-years, ≥7 pack-years), and complement factor H (CFH) Y402H (rs1061170) and age-related maculopathy susceptibility locus 2 (ARMS2) A69S (rs10490924) genotypes.
Results
Sample Characteristics
The CAREDS sample,26 and determinants of lutein and zeaxanthin in the serum and macula28 have been described previously. In brief, the 1663 participants included in this analysis were on average 69 years of age at time of fundus photography (range, 53–86). The average body mass index was 28 kg/m2 (range, 16–62), 6% reported having diabetes and only 2.4% were self-reported current smokers. There were 337 AMD cases, 91% (n = 308) of which were early stages of AMD. Distribution of AMD risk factors were consistent when comparing participants included in the present analysis (n = 1663), relative to the 342 excluded due to lack of genetic and/or phenotypic data. The exception was that women included consumed slightly less dietary lutein and zeaxanthin compared to those not included in the study (2.3 vs. 2.5 mg/d, P = 0.02).
SNP Associations With Carotenoid Status
Associations of SNPs from lutein and zeaxanthin pathway candidate genes related to MPOD in this sample were described previously.47 The SNPS associated with serum lutein and zeaxanthin are described in Table 1. Genes associated with MPOD and levels of these carotenoids in the serum included: β-carotene 15, 15′-monooxygenase 1 (BCMO1); ATP-binding cassette, subfamily A, member 1 (ABCA1) and subfamily G member 5 (ABCG5); scavenger receptor class B member1 (SCARB1); and retinal pigment epithelium-specific protein 65kDa (RPE65). The SNPs from candidate genes associated with MPOD, but not related to serum lutein and zeaxanthin, in this sample include genes related to xanthophyll binding in human macula (glutathione S-transferase pi 1; GSTP1), high density lipoprotein status (hepatic lipase; LIPC), long chain fatty acid status (fatty acid desaturase 1 [FADS1] and elongation of very long chain fatty acids protein 2 [ELOVL2], and maculopathies associated with MPOD aldehyde dehydrogenase 3 family, member A2 [ALDH3A2]). Fatty acid desaturase 2 (FADS2) was related to MPOD, but not statistically significant after adjusting for other predictors. Genes associated uniquely with serum lutein and zeaxanthin (Table 1) and not MPOD were from stAR-related lipid transfer protein 3 (STARD3), ATP-binding cassette subfamily G member 8 (ABCG8), Niemann-Pick C1-like protein 1 (NPC1L1), and cholesteryl ester transfer protein (CETP). SNPs from some genes were not significantly and independently associated with MPOD or serum lutein and zeaxanthin in the present sample, but previously associated with carotenoid status in another sample (apolipoprotein E; ApoE57) or with genes previously associated with the accumulation of lutein and zeaxanthin in tissues of other mammals (β-carotene oxygenase 2; BCO2).55,56
Table 1.
SNPs Independently Associated With Serum Lutein and Zeaxanthin Within Each Gene, in CAREDS (N = 1643)
|
Gene |
SNP |
Genotype |
N |
Mean Serum LZ |
% Change From Reference |
P
Value |
| Xanthophyll binding in retina | ||||||
| STARD3 | rs9892427 | AA | 1394 | 0.28 | Ref. | 0.01 |
| AG or GG | 249 | 0.26 | −8% | |||
| Carotenoid cleavage | ||||||
| BCMO1 | rs11645428 | GG | 726 | 0.25 | Ref. | <0.0001 |
| AG | 732 | 0.30 | 20% | |||
| AA | 185 | 0.34 | 35% | |||
| rs6564851 | CC | 436 | 0.24 | Ref. | <0.0001 | |
| AC | 814 | 0.28 | 18% | |||
| AA | 391 | 0.32 | 33% | |||
| rs7500996 | AA | 1100 | 0.27 | Ref. | 0.0002 | |
| AG | 478 | 0.30 | 8% | |||
| GG | 63 | 0.31 | 12% | |||
| HDL transport or status | ||||||
| ABCA1 | rs2274873 | GG | 1310 | 0.29 | Ref. | 0.01 |
| AG or AA | 318 | 0.27 | −6% | |||
| rs1331924 | CC | 1209 | 0.28 | Ref. | 0.03 | |
| CG | 386 | 0.29 | 6% | |||
| GG | 42 | 0.29 | 4% | |||
| ABCG5 | rs10205816 | AA | 838 | 0.29 | Ref. | 0.03 |
| AG | 667 | 0.27 | −5% | |||
| GG | 138 | 0.28 | −5% | |||
| ABCG8 | rs13405698 | AA | 835 | 0.28 | Ref. | 0.01 |
| AG | 664 | 0.28 | 2% | |||
| GG | 142 | 0.31 | 13% | |||
| rs4953028 | GG | 491 | 0.29 | Ref. | 0.03 | |
| AG | 816 | 0.28 | −2% | |||
| AA | 336 | 0.27 | −7% | |||
| NPC1L1 | rs217430 | AA | 954 | 0.28 | Ref. | 0.03 |
| AG | 583 | 0.29 | 3% | |||
| GG | 102 | 0.30 | 9% | |||
| CETP | rs708272 | GG | 525 | 0.29 | Ref. | 0.05 |
| AG | 804 | 0.28 | −5% | |||
| AA | 313 | 0.28 | −5% | |||
| Lipid and/or carotenoid absorption | ||||||
| SCARB1 | rs10846744 | GG | 1192 | 0.27 | Ref. | 0.0001 |
| CG | 415 | 0.30 | 9% | |||
| CC | 33 | 0.31 | 15% | |||
| CD36 | rs1524598 | AA | 705 | 0.27 | Ref. | 0.04 |
| AG | 724 | 0.29 | 4% | |||
| GG | 213 | 0.29 | 6% | |||
| Genes previously related to maculopathies | ||||||
| RPE65 | rs12744671 | AA | 1307 | 0.28 | Ref. | 0.05 |
| AC | 310 | 0.27 | −3% | |||
| CC | 22 | 0.23 | −18% | |||
Adjusted for WHI baseline dietary lutein and zeaxanthin and first two principal components from principal component analysis using 176 ancestry informative markers.
SNP Associations With AMD
There were 24 SNPs from nine carotenoid-candidate genes associated with AMD (P ≤ 0.05) after adjusting for age and ancestry (Table 2). The SNPs with the greatest statistical significance for associations with AMD (reflecting effect size and higher minor allele frequencies) included rs2487714 downstream of ABCA1 (OR = 1.31; 95% confidence interval [CI], 1.10–1.56; P = 0.002), rs8069576 in ALDH3A2 (OR = 0.77; 95% CI, 0.64–0.92; P = 0.004), and rs2250417 in BCO2 (OR = 1.24; 95% CI, 1.04–1.48; P = 0.01). Other carotenoid-candidate genes with SNPs exhibiting significant associations with AMD included ABCG8, APOE, BCMO1, FADS2, NPC1L1, and SCARB1. Further adjusting for dietary lutein or for Y402H (CFH) and A69S (ARMS2), two well-known, strong genetic risk factors for AMD, did not significantly alter ORs and P values for each of these 24 SNPs or conclusions (data not shown).
Table 2.
SNPs Associated With AMD (P ≤ 0.05) in CAREDS After Adjusting for Age and Ancestry
|
Gene |
SNP |
OR* |
95% CI |
P
Value |
Minor Allele |
Major Allele |
Minor Allele Frequency |
| ABCA1 | rs2254884 | 1.24 | (1.03, 1.50) | 0.02 | C | A | 0.30 |
| rs2297406 | 1.21 | (1.01, 1.46) | 0.04 | A | G | 0.30 | |
| rs2472476 | 1.22 | (1.02, 1.45) | 0.03 | G | A | 0.37 | |
| rs2482432 | 0.79 | (0.66, 0.94) | 0.01 | G | A | 0.43 | |
| rs2487714 | 1.31 | (1.10, 1.56) | 0.002 | G | A | 0.47 | |
| rs2515614 | 0.81 | (0.67, 0.98) | 0.03 | C | A | 0.33 | |
| rs2740484 | 0.82 | (0.68, 0.98) | 0.03 | A | G | 0.36 | |
| rs4149263 | 1.35 | (1.09, 1.67) | 0.01 | G | A | 0.20 | |
| rs4149338 | 1.21 | (1.00, 1.45) | 0.05 | A | G | 0.28 | |
| ABCG8 | rs4148222 | 1.27 | (1.02, 1.60) | 0.04 | A | G | 0.16 |
| ALDH3A2 | rs1800869 | 1.25 | (1.02, 1.52) | 0.03 | G | C | 0.23 |
| rs2072331 | 1.25 | (1.03, 1.53) | 0.02 | C | T | 0.23 | |
| rs7215 | 0.79 | (0.66, 0.94) | 0.01 | A | G | 0.46 | |
| rs8069576 | 0.77 | (0.64, 0.92) | 0.004 | A | G | 0.43 | |
| APOE | ε-4 | 0.73 | (0.56, 0.95) | 0.02 | |||
| BCMO1 | rs11645428 | 0.80 | (0.66, 0.97) | 0.02 | A | G | 0.33 |
| rs16955008 | 1.31 | (1.02, 1.68) | 0.04 | A | C | 0.12 | |
| BCO2 | rs12796114 | 0.80 | (0.65, 0.99) | 0.04 | C | A | 0.26 |
| rs2250417 | 1.24 | (1.04, 1.48) | 0.01 | A | G | 0.46 | |
| FADS2 | rs174627 | 1.28 | (1.01, 1.62) | 0.04 | A | G | 0.15 |
| rs526126 | 1.29 | (1.05, 1.60) | 0.02 | C | G | 0.19 | |
| NPC1L1 | rs10234070 | 0.73 | (0.55, 0.99) | 0.04 | A | G | 0.11 |
| rs217428 | 0.81 | (0.67, 1.00) | 0.05 | C | A | 0.26 | |
| SCARB1 | rs9919713† | 0.60 | (0.36, 0.98) | 0.04 | A | T | 0.05 |
OR is the per-minor allele effect (additive genetic model).
Dominant model is assumed.
Genetic Risk Model
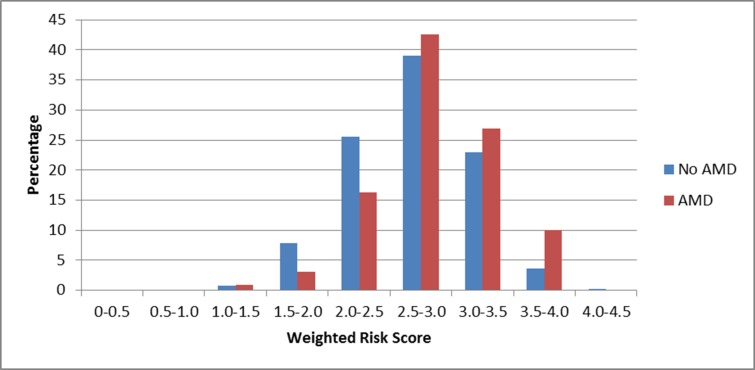
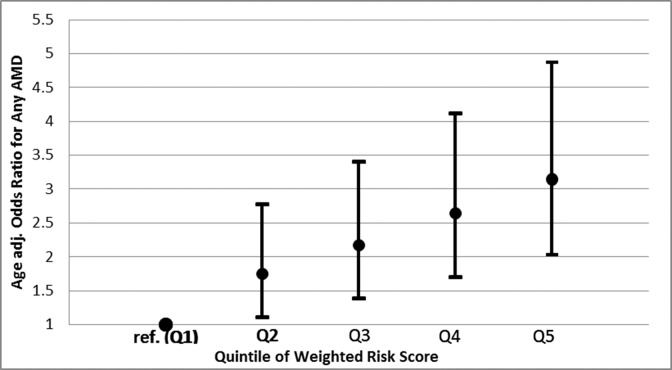
To evaluate the potential impact of carotenoid-related genes on AMD risk, beyond known AMD genetic risk factors, we created a genetic risk score for AMD and tested the extent to which adding the risk score altered the percentage of AMD cases that could be detected by a model containing age, smoking, Y402H, and A69S. Model selection resulted in the inclusion of the following 9 SNPs: rs2254884, rs2487714, and rs4149263 from ABCA1; rs8069576 from ALDH3A2; APOE ε4; rs2250417 from BCO2; rs526126 from FADS2; rs10234070 from NPC1L1; and rs9919713 from SCARB1 (Table 3). The SNPs included in this model, the gene they are tagging, their respective weights (logarithm of the ORs) for each copy of the risk allele, and the individual statistical significance within the risk score are outlined in Table 3. The mean weighted genetic risk score was higher in AMD cases compared to noncases, 2.84 vs. 2.66, respectively (P = 1.7 × 10−9; Fig. 1). The odds of AMD for those in the highest quintile of genetic risk relative to the lowest quintile of risk was 3.15 (95% CI, 2.03–4.87; Fig. 2). Genes with the largest contribution to the score, based on likelihood ratio test statistic with degrees of freedom equal to number of SNPs respective to the gene were ABCA1 (P = 6.7 × 10−5) and SCARB1 (P = 0.006).
Table 3.
SNPs Included in Carotenoid Genetic Risk Score for AMD
|
Gene |
SNP |
Beta |
Risk Allele |
OR (95% CI) |
P
Value |
| ABCA1 | rs2254884 | 0.27 | C | 1.31 (1.08, 1.59) | 0.01 |
| rs2487714 | 0.27 | G | 1.31 (1.10, 1.56) | 0.003 | |
| rs4149263 | 0.27 | G | 1.30 (1.05, 1.62) | 0.02 | |
| ALDH3A2 | rs8069576 | 0.21 | G | 1.23 (1.03, 1.47) | 0.02 |
| APOE | ε-4 | 0.29 | 1.34 (1.02, 1.76) | 0.04 | |
| BCO2 | rs2250417 | 0.21 | A | 1.24 (1.04, 1.47) | 0.02 |
| FADS2 | rs526126 | 0.29 | C | 1.33 (1.07, 1.65) | 0.01 |
| NPC1L1 | rs10234070 | 0.27 | G | 1.31 (0.97, 1.77) | 0.08 |
| SCARB1* | rs9919713 | 0.72 | T | 2.06 (1.24, 3.43) | 0.01 |
Dominant genetic model assumed.
Figure 1.
Distribution of the weighted genetic risk score in cases (red) and noncases (blue). P value for difference in mean weighted risk score between cases and noncases = 1.7 × 10−9.
Figure 2.
OR (95% CI) for age-related macular degeneration in the CAREDS by quintile of the weighted genetic risk score (P value for trend across increasing quintile = 1.6 × 10−7).
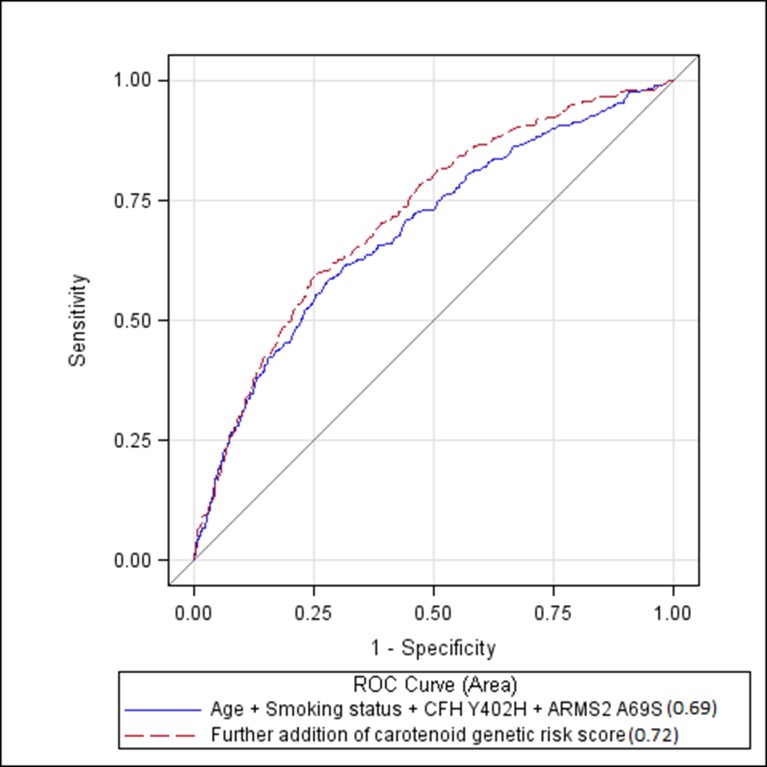
The area under the curve (AUC) for a model containing age, smoking, CFH Y402H, and ARMS2 A69S was 0.69 (95% CI, 0.65–0.72). Addition of the genetic risk score, derived from variants in carotenoid candidate genes, significantly increased the AUC to 0.72 (95% CI, 0.69–0.75; P = 0.002; Fig. 3).
Figure 3.
ROC curve showing improvement of discrimination between AMD cases and noncases when adding the carotenoid genetic risk score to a model containing age, smoking status, CFH Y402H, and ARMS2 A69S (AUC = 0.72 vs. 0.69, P = 0.002).
Discussion
In this sample of over 1600 women over age 55, we observed that variation in multiple genes related to lutein and zeaxanthin physiology or status was associated with AMD. These results add to the increasing body of genetic and epidemiologic evidence48 suggesting a protective role of lutein and zeaxanthin against AMD, beyond evidence from previous observational studies of protective relationships between lutein and zeaxanthin in the diet or serum17–22,24–26 or macula.66–68 Results of the present study primarily reflect relations to early AMD, which predominated among AMD cases in the present sample. Not accounting for single and multiple gene loci related to lutein and zeaxanthin in the serum and macula may have limited the ability to detect protective associations of lutein and zeaxanthin intake to early AMD in some previous studies.22,24–29 Protection of lutein and zeaxanthin against AMD progression is suggested by secondary, but not primary, analyses in AREDS2. In secondary analyses, participants with low dietary intake of these carotenoids at baseline and participants who consumed supplements that replaced β-carotene with lutein in the original AREDS formulation had lower progression from intermediate to advanced AMD.23,69
These data strengthen the body of evidence in support of a protective role of lutein and zeaxanthin against AMD development. This is because associations of AMD with carotenoid status-related genes would not be influenced by measurement error of dietary carotenoids, nor confounded by other unknown, and unadjusted for determinants of carotenoid status and dietary and lifestyle correlates that may influence associations in observational studies. Also, polymorphisms in genes related to carotenoid status in tissues could reflect conditions that influence macular carotenoid exposure over decades and protect against early AMD development on a subclinical level, whereas levels of carotenoids in the diet and blood change over time, which can mask causal relationships to AMD.
Several common variants in carotenoid candidate genes were related to the optical density of MP or serum carotenoids in the present sample,47 or independent samples.57,58,70 In the present sample, these SNPs only explained 5% of variation in MPOD. However, the percent variability explained could be underestimated due to our tagSNP approach, which does not include causal SNPs, exclusion of numerous unknown variants (rare and common) underlying this polygenic trait, and failure to account for interactions with other genes or environmental factors. Further exploration of joint and interacting genetic variables in relation to carotenoid status are the subject of continued investigation in this research group.
Relationships of individual tagSNPs to AMD were modest in the present study. However, when considered jointly in a risk score, there was a strong relationship with odds for AMD (OR = 3.15). Many studies have demonstrated that for complex traits, combining multiple loci, each with individually low to moderate effects, into a weighted genetic risk score improves case prediction.64,65 Our results in CAREDS are consistent with this polygenic model. Modest effects of some individual SNPs with AMD also may be explained by the indirect association they have with AMD via carotenoid status.
Improvement in Classification of AMD Risk
As further proof of concept that lutein and zeaxanthin status is related to AMD, we observed that a risk score combining nine SNPs from seven genes, which are related to levels of lutein and zeaxanthin in blood and macula, significantly increases the ability to classify AMD cases and noncases beyond four well-established AMD risk factors. While several carotenoid-related SNPs have been associated with AMD in previous studies (discussed below), variants from five genes (BCMO1, BCO2, NPC1L1, ABCG8, and FADS2) have not been related previously to AMD. We acknowledge that the criteria set for model selection may have influenced specific SNPs included in this model. For this reason, further evaluation of the SNPs in Table 2, in relation to early and advanced AMD risk in prospective studies, and in separate populations, is needed to continue evaluating specific SNPs, which might have predictive value in the general population.
Mechanisms Relating Genotypes to Lutein and Zeaxanthin Status and AMD
Genetic variants related to AMD in the present study were within genes related to: (1) cholesterol and carotenoid membrane transport proteins in the intestine and retina (SCARB1, NPCL1L1, and ABCA1) and/or high density lipoprotein levels in blood (SCARB1, APOE, and ABCA1), (2) carotenoid cleavage enzymes (BCMO1 and BCO2), (3) omega-3 fatty acid status (FADS2), and (4) an inherited retinopathy associated with the complete absence of macular pigment (ALDH3A2). Different variants within many of these same genes were related previously to levels of lutein and zeaxanthin in serum (ABCA1, ABCG8, SCARB1, NPC1L1, and BCMO1, Table 1) or macula (ABCA1, SCARB1, BCMO1, and ALDH3A2).47 Because the tagSNP strategy used in CAREDS does not necessarily measure causal loci, the exact SNPs reported for associations with serum and macular levels of the carotenoids are not the same as those reported in association with AMD. The causal loci within these carotenoid-related genes, and mechanisms of their action in relation to carotenoid status and AMD risk, need to be determined.
Variants in Genes Related to Cholesterol and Carotenoid Membrane Transport
Carotenoids, like other fat soluble vitamins, are transported into the body and tissues in conjunction with membrane receptors, which influence the uptake of cholesterol and other lipids.71 Common variants in many genes encoding proteins related to cholesterol and carotenoid transport were related to AMD in the CAREDS sample (Table 2). Variants within SCARB172 and ABCA173–77 have been related to AMD in previous studies. To our knowledge, this is the first report of an association of variants in ABCG8 and NPC1L1 to AMD.
Absorption of carotenoids into the body from the intestine occurs partly by a facilitated process involving plasma membrane receptor proteins scavenger receptor class B type 1 (SR-B1), Niemann-Pick type C1 Like 1 (NPC1L1), and ATP-binding cassette transporter, subfamily A (ABCA1), which also transport cholesterol and other lipids.78,79 The SR-B1, a plasma membrane receptor for HDL encoded for by SCARB1, mediates cholesterol efflux and carotenoid uptake in the retinal pigment epithelium facilitating transport of macular xanthophylls over β-carotene.80 Consistently, variants in SCARB1 have been associated with macular density of lutein and zeaxanthin in the CAREDS47 and serum levels of lutein in CAREDS (Table 1) and two independent cohorts.70
The gene ABCA1 encodes the adenosine triphosphate-binding cassette transporter A1 with a known role in cholesterol efflux from cells. Mutations in ABCA1 in chickens and humans with Tangier's disease lead to low levels of HDL.81,82 Half of xanthophyll carotenoids are carried on HDLs, different from carotenoids which do not accumulate abundantly in the macula, such as β-carotene, which is carried only on LDLs.83 This may explain why mutations in this gene impact lutein transport, and result in carotenoid deficiencies in chickens.82,84 A common variant in ABCA1 (rs1929841) was associated with macular density of lutein and zeaxanthin, and different variants to serum lutein and zeaxanthin (Table 1) in this sample.47
Other HDL-related loci associated with AMD in previous studies include LPL,77,85 LIPC,74,75,77,85 and CETP.73,74,77 In the present study, one SNP each in LPL (rs12678919) and CETP (rs708272) were weakly associated with AMD (P = 0.2). Furthermore, a SNP within CETP was associated with serum lutein and zeaxanthin (Table 1), and one in LIPC was associated with MP,47 providing evidence for potential mediation of AMD risk through lutein and zeaxanthin status.
The ABCG8 protein is part of an ABCG5/8 heterodimer complex, which is critical to sterol homeostasis. Two variants in the ABCG8 gene were independently related to levels of lutein and zeaxanthin in serum (Table 1), but none was related to MP in CAREDS. However, different variants in ABCG5 were related independently to lutein and zeaxanthin in the serum (Table 1) and macula (rs10179921).47 Allelic variation within ABCG5 has been related to plasma response to cholesterol and lutein after eating eggs.86
Different variants in NPC1L1 and ABCG8, along with variants from two other carotenoid-related genes BCMO1 and CD36, explained 25% of the variation in plasma lutein and 38% of the variation of MP optical density in a separate sample of 29 males.58 The NPC1L1 protein was demonstrated recently to be related to lutein transport in Caco-2 cells.87
Like several past studies,75,88,89 we observed that women who had one or two APOE ε4 alleles had lower risk for AMD. This seems counterintuitive, as having ε4 alleles is associated with higher risk of mortality and risk for other chronic diseases of aging. In one previous study, having ε4 alleles was associated higher MPOD.57 We observed ε4 alleles to be associated with lower, rather than higher, MPOD, although this was not statistically significant. An explanation for the protective effect of ε4 on AMD that involves opposing of cellular cholesterol export from the retinal pigment epithelium and into Bruch's membrane has been suggested.90
The protective associations with allelic variants in SCARB1 and ABCA1 to AMD in this study also might reflect processes other than carotenoid accumulation and transport. Variants in these genes have been related to serum HDL levels and the process of reverse cholesterol export, in which cholesterol from peripheral tissues is transported to the liver for elimination in bile.91 Therefore, it may be that the variants related to AMD in this study reflect this general process and the presence of conditions that prevent the accumulation of lipids in Bruch's membrane, thought to encourage the pathogenic process of AMD.92 Knock-out mice for SR-B193 fed high fat diets or ApoE deficient mice94 display retinal phenotypes characterized by subretinal lipid accumulations and damage, similar to dry AMD. In addition, new roles for HDL in inflammation have been proposed, as HDL also contains proteins associated with the acute phase response and complement regulation.91
Variants in Carotenoid Cleavage Enzyme Genes
Two variants each from BCMO1 and BCO2, both carotenoid cleavage enzymes, were associated with AMD for the first time in the present study. The β,β-carotene 15,15′-monooxygenase 1 is a cytosolic enzyme that cleaves symmetrically and β,β-carotene 9′,10′-dioxygenase 2 is a mitochondrial enzyme that cleaves asymmetrically, resulting in different cleavage products with numerous, incompletely understood biological effects; one well-known product is retinal from cleavage of pro-vitamin A carotenoids, like β-carotene.95,96 In the present sample, having one or two A alleles in rs11645428 (BCMO1) was associated with higher levels of lutein and zeaxanthin in serum (Table 1) and the macula,47 and a 20% lower odds for AMD. Women with another variant (rs6564851) related to higher serum (Table 1) and macular levels47 also were less likely to have AMD, but this was not statistically significant (P = 0.16). Having these two BCMO1 variants were associated previously with higher circulating levels of lutein and zeaxanthin in other samples,97 and higher catalytic activity of the BCMO1 enzyme in women98; that is, higher conversion of β-carotene to retinal leading to lower circulating levels of β-carotene. These SNPs are located upstream from the coding region of BCMO1, a region that may alter transcriptional activity.
Although lutein and zeaxanthin are not thought to be substrates for BCMO1, even low levels of specificity for this substrate might lead to cleavage products that are responsible for a protective association with AMD. However, the body of evidence currently suggests an alternative explanation: a protective effect on AMD might be indirect and related to biological competition between pro-vitamin A carotenoids, like β-carotene, and xanthophyll carotenoids for absorption in the intestine and into tissues. Several BCMO1 variants associated with high circulating levels of β-carotene are associated with low circulating levels of lutein and zeaxanthin.97
The opposing relationships between serum levels of β-carotene and macular carotenoids are consistent with observations in some studies that β-carotene supplementation leads to lower circulating levels of lutein and zeaxanthin.23,99 Further evidence of interactions between xanthophyll carotenoids and β-carotene in relation to AMD risk were observed in secondary analyses conducted in AREDS2; lutein supplementation without β-carotene, compared to with β-carotene, was associated with lower risk for progression of advanced AMD.23
Associations of BCMO1 to AMD also might partly explain some inconsistency in observed relationships between diet lutein and AMD in past studies. Data in CAREDS suggests that variants in this gene may influence the macular response to dietary macular carotenoids: Women with lutein intakes in the lowest tertile (having a median lutein and zeaxanthin intake of 1.0 mg/d) and two A alleles for rs11645428 had higher mean MP optical density than those with one or no A alleles (0.40 ± 0.03, 0.32 ± 0.02, and 0.31 ± 0.02, respectively; P < 0.01).47 Moreover, MP in women with two A alleles, despite low intake of lutein and zeaxanthin, was not significantly different than MP in women with much higher intakes (median 2.1 and 4.1 mg/d in the in the second and third tertiles for intake of lutein and zeaxanthin).47 This suggests the hypothesis that having either two A alleles for BCMO1 rs11645428 or intake of lutein and zeaxanthin > 2 mg/d is associated with higher carotenoids in the serum, macula, and with lower risk for AMD. Further, supplementation with macular carotenoids might lower AMD risk to a greater extent in persons without A alleles for rs11645428. This remains to be tested in trials.
Having two minor alleles for rs2250417 in BCO2 was associated with almost a 50% increased risk for AMD. Having one or two minor alleles for rs12796114 was associated with an approximately 25% lower risk for AMD. Lutein and zeaxanthin, and other carotenoids with a hydroxylated β-ionone ring (such as cryptoxanthin) are substrates for the BCO2 enzyme. Moreover, in mammals, BCO2 mutations result in accumulation of lutein and zeaxanthin in skin of livestock.55,56 However, in the present study, BCO2 variants appear to be unrelated to lutein and zeaxanthin levels in the blood and macular pigment, suggesting tissue specific influences. Studies in BCO2-deficient mice and human cells in culture indicate that excess carotenoids can impair respiration and induce oxidative stress in the mitochondria, and BCO2 may protect against this.10 The fact that oxidative stress is known to promote AMD and the demonstrated role for BCO2 in limiting oxidative stress in mitochondria100 suggests that these associations might reflect lower oxidative stress and damage to retinal pigment epithelium mitochondria. New evidence in mice suggests that BCO2 might be important for vitamin A synthesis from asymmetric carotenoids, like β-cryptoxanthin.101
Common BCMO1 and BCO2 variants also may be related to AMD via mechanisms involving an influence of activity of the enzymes they encode on lipid homeostasis and inflammation, although these lines of evidence are early in development. A BCMO1 variant previously identified as related to serum carotenoid levels, recently was associated with HDL in two populations.102 BCMO1103 and BCO2100 knock-out mice have been observed to have hepatic lipid accumulation. The BCMO1-deficient mice have elevated levels of insulin and leptin, suggesting an obese phenotype.104 Obesity is known to be a state of chronic low-grade inflammation.105 Common variants in BCO2 and IL8, which is near BCO2, also were related to serum concentrations of a proinflammatory cytokine in genome-wide association studies.106 One of these, rs2115763 in BCO2, is in weak linkage disequilibrium with rs2250417 (r2 = 0.51 in HapMap CEU population), which we report here to be associated with AMD.
Variants Related to the Synthesis of Long-Chain Omega-3 Fatty Acids
A variant in one gene that encodes an essential enzyme for the synthesis of docosahexaenoic acid (DHA)107 was associated with AMD in the present study. Associations between variants in this gene, FADS2, and AMD have not been reported previously, to our knowledge. Previous evidence suggests that this association might be related to better accumulation of macular pigments. Dietary omega-3 fatty acid intake and variants in other genes associated with long-chain fatty acid synthesis (FADS1 and ELOVL2) were associated with higher MP in this study sample47 and higher plasma levels of long chain omega-3 fatty acids are related to higher MP in a separate sample.50 In one human trial, a trend for MP accretion in the foveal center was observed when DHA108 was added to lutein and zeaxanthin supplements. Results of previous studies suggest that DHA increases HDL and HDL subfractions,109–111 so a mechanism for better accretion of macular pigments might be secondary to increased transport of lutein into the macula in HDLs. Alternatively, lower status for omega-3 fatty acids may have influenced the foveal architecture, which subsequently influenced the ability to accumulate macular pigments. In rhesus monkeys, dietary levels of omega-3 fatty acids affected the retinal pigment epithelium cell density and the response to xanthophyll supplementation.112
An alternative explanation for relationships of FADS2 variants and AMD could be direct protection by DHA against the development or progression of AMD as has been observed in previous observational studies.113,114 This may be secondary to known antiinflammatory effects of omega-3 fatty acids.115 Although omega-3 supplements, with or without lutein did not prevent the progression to advanced AMD in the AREDS2 study in people supplementing with high dose antioxidants,69 this study may have been too short (5 years), to observe a protective effect of long chain omega-3 fatty acids on disease progression. The FADS2 variants (and other unknown genetic influences on omega-3 fatty acids status) could influence DHA status over a lifetime. Whether this is related to or independent of macular carotenoids requires further study.
Variants Associated With Age-Related Maculopathies
Results of the present analysis of four common variants in ALDH3A2 relating to AMD are consistent with a concurrent analysis in the AREDS cohort > 65 years of age in which different sequence variants in the same gene were observed to be related to advanced AMD over 12-years of follow-up.116 Full search of results from the recent AMD Consortium analysis revealed two SNPs reported in Table 1 (rs1800869 and rs2072331) replicated with marginally significant associations with advanced AMD (P = 0.05), and consistent direction of effect.117 Rare mutations in the ALDH3A2 gene result in Sjögren-Larsson Syndrome.118 This condition results in neural and cutaneous defects as well as in macular dystrophy in the retinal ganglion cells and inner plexiform layer, and complete lack of macular pigment despite normal levels of carotenoids in serum.119 We observed common variants within this gene related to MP, but not to levels of serum carotenoids in the present sample, suggesting influence is limited to the macula.47 This gene encodes a lipid metabolic enzyme (aldehyde dehydrogenase 3 family member 2 protein), which catalyzes the oxidation of a variety of short and medium chain fatty aldehydes to fatty acids. In SLS patients, fatty aldehydes and alcohols accumulate in body tissues, and are thought to lead to Mueller cell degeneration with possible photooxidative stress as a result of a lack of macular pigment.119 It is unclear whether a lack of MP could be a cause or consequence of mutation or genotypic variation in the ALDH3A2 gene.
Limitations
In addition to limitations addressed above, the following limit conclusions that can be drawn from evidence presented here. This study included only women and mainly persons of self-reported European ancestry. In addition, the availability of prevalent (existing) AMD as an outcome, rather than incident (newly developed AMD after exposure assessment) could result in associations that reflect confounding related to survivor bias or change in carotenoid exposure subsequent to AMD development. Further work is warranted in large, prospective studies of older men and women of varied ancestry to understand more fully the impact genes related to carotenoid status have on AMD status. In addition, there is a need to understand further the modifying influence, if any, of other suspected AMD risk factors.
Summary
The results demonstrated associations between genetic determinants of serum or macular carotenoids and AMD, which are independent of dietary lutein and zeaxanthin. Thus, they provided independent evidence to strengthen the existing body of evidence suggesting a role of carotenoids in the prevention of AMD, and provide direction for future work to better understand the direct and indirect effects these carotenoid-related genes have on AMD. The degree to which these observations have value in clinical prediction of AMD remains to be determined.
Acknowledgments
The authors thank all CAREDS and WHI Investigators who have contributed over the years. A short list of investigators who have contributed to WHI science can be found in the Supplementary Material. The authors also thank Gregory Hageman, PhD, of the Moran Eye Center, University of Utah Health Care, Salt Lake City, Utah, for contributions regarding selection of genetic SNPS to evaluate.
Supported by the National Institutes of Health, National Eye Institute (Grants EY013018, EY016886), the Retina Research Foundation and Research to Prevent Blindness (CAREDS Study), and by the National Heart, Lung, and Blood Institute (Contracts N01 WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221; The Women's Health Initiative, to which CAREDS is ancillary).
Disclosure: K.J. Meyers, None; J.A. Mares, None; R.P. Igo Jr, None; B. Truitt, None; Z. Liu, None; A.E. Millen, None; M. Klein, None; E.J. Johnson, Bausch & Lomb (S); C.D. Engelman, None; C.K. Karki, None; B. Blodi, None; K. Gehrs, None; L. Tinker, None; R. Wallace, None; J. Robinson, None; E.S. LeBlanc, None; G. Sarto, None; P.S. Bernstein, None; J.P. SanGiovanni, None; S.K. Iyengar, None
References
- 1. Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985; 25: 1531–1535 [DOI] [PubMed] [Google Scholar]
- 2. Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988; 29: 850–855 [PubMed] [Google Scholar]
- 3. Bone RA, Landrum JT, Friedes LM, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997; 64: 211–218 [DOI] [PubMed] [Google Scholar]
- 4. Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001; 72: 215–223 [DOI] [PubMed] [Google Scholar]
- 5. Shaban H, Richter C. A2E and blue light in the retina: the paradigm of age-related macular degeneration. Biol Chem. 2002; 383: 537–545 [DOI] [PubMed] [Google Scholar]
- 6. Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Chem. 2000; 45: 115–134 [DOI] [PubMed] [Google Scholar]
- 7. Hageman G, Luthert P, Victor-Chong N, Johnson L, Anderson D, Mullins R. An integrated hypothesis that considers drusen as biomarkers of immune-medicated processes at the RPE-Brunch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20: 705–732 [DOI] [PubMed] [Google Scholar]
- 8. Landrum JT, Bone RA. Mechanistic Evidence for Eye Diseases and Carotenoids. In: Krinski NI, ST Mayne, Sics H. eds Carotenoids in Health and Disease. New York, NY: Marcel Dekker, 2004: 445–472 [Google Scholar]
- 9. Barker FM II, Snodderly DM, Johnson EJ, et al. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci. 2011; 52: 3934–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005; 26: 459–516 [DOI] [PubMed] [Google Scholar]
- 11. Liu A, Chang J, Lin Y, Shen Z, Bernstein PS. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J Lipid Res. 2010; 51: 3217–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sasaki M, Ozawa Y, Kurihara T, et al. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009; 50: 1433–1439 [DOI] [PubMed] [Google Scholar]
- 13. Izumi-Nagai K, Nagai N, Ohgami K, et al. Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2007; 27: 2555–2562 [DOI] [PubMed] [Google Scholar]
- 14. Bian Q, Gao S, Zhou J, et al. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic Biol Med. 2012; 53: 1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang MX, Jiao JH, Li ZY, Liu RR, Shi Q, Ma L. Lutein supplementation reduces plasma lipid peroxidation and C-reactive protein in healthy nonsmokers. Atherosclerosis. 2013; 227: 380–385 [DOI] [PubMed] [Google Scholar]
- 16. Tian Y, Kijlstra A, van der Veen RL, Makridaki M, Murray IJ, Berendschot TT. The effect of lutein supplementation on blood plasma levels of complement factor d, c5a and c3d. PLoS One. 2013; 8: e73387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand. 2002; 80: 368–371 [DOI] [PubMed] [Google Scholar]
- 18. Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age related macular degeneration. JAMA. 1994; 272: 1413–1420 [PubMed] [Google Scholar]
- 19. Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003; 44: 2461–2465 [DOI] [PubMed] [Google Scholar]
- 20. SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. 2007; 125: 1225–1232 [DOI] [PubMed] [Google Scholar]
- 21. Fletcher AE, Bentham GC, Agnew M, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008; 126: 1396–1403 [DOI] [PubMed] [Google Scholar]
- 22. Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr. 2008; 87: 1837–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309: 2005–2015 [DOI] [PubMed] [Google Scholar]
- 24. Mares-Perlman JA, Fisher AI, Klein R, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001; 153: 424–432 [DOI] [PubMed] [Google Scholar]
- 25. Ho L, van Leeuwen R, Witteman JC, et al. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam study. Arch Ophthalmol. 2011; 129: 758–766 [DOI] [PubMed] [Google Scholar]
- 26. Moeller SM, Mehta NR, Tinker LF, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2006; 124: 1–24 [DOI] [PubMed] [Google Scholar]
- 27. Robman L, Vu H, Hodge A, et al. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol. 2007; 42: 720–726 [DOI] [PubMed] [Google Scholar]
- 28. Mares JA, LaRowe TL, Snodderly DM, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr. 2006; 84: 1107–1122 [DOI] [PubMed] [Google Scholar]
- 29. VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 1998; 148: 204–214 [DOI] [PubMed] [Google Scholar]
- 30. Hammond BR Jr, Johnson EJ, Russell RM, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997; 38: 1795–1801 [PubMed] [Google Scholar]
- 31. Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003; 133: 992–998 [DOI] [PubMed] [Google Scholar]
- 32. Johnson EJ, Hammond BR, Yeum KJ, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000; 71: 1555–1562 [DOI] [PubMed] [Google Scholar]
- 33. Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci. 2000; 41: 3322–3326 [PubMed] [Google Scholar]
- 34. Koh HH, Murray IJ, Nolan D, Carden D, Feather J, Beatty S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Exp Eye Res. 2004; 79: 21–27 [DOI] [PubMed] [Google Scholar]
- 35. Kvansakul J, Rodriguez-Carmona M, Edgar D, et al. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol Opt. 2006; 26: 362–371 [DOI] [PubMed] [Google Scholar]
- 36. Aleman TS, Duncan JL, Bieber ML, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci. 2001; 42: 1873–1881 [PubMed] [Google Scholar]
- 37. Aleman TS, Cideciyan AV, Windsor EA, et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Invest Ophthalmol Vis Sci. 2007; 48: 1319–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bone RA, Landrum JT, Cao Y, Howard AN, Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr Metab (Lond). 2007; 4: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schalch W, Cohn W, Barker FM, et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007; 458: 128–135 [DOI] [PubMed] [Google Scholar]
- 40. Trieschmann M, Beatty S, Nolan JM, et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res. 2007; 84: 718–728 [DOI] [PubMed] [Google Scholar]
- 41. Richer SP, Stiles W, Graham-Hoffman K, et al. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration: the Zeaxanthin and Visual Function Study (ZVF) FDA IND #78, 973. Optometry. 2011; 82: 667–680 [DOI] [PubMed] [Google Scholar]
- 42. Connolly EE, Beatty S, Thurnham DI, et al. Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study. Curr Eye Res. 2010; 35: 335–351 [DOI] [PubMed] [Google Scholar]
- 43. Nolan JM, Akkali MC, Loughman J, Howard AN, Beatty S. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp Eye Res. 2012; 101: 9–15 [DOI] [PubMed] [Google Scholar]
- 44. Hammond CJ, Liew SM, Van Kuijk FJ, et al. The heritability of macular response to supplemental lutein and zeaxanthin: a classical twin study. Invest Ophthalmol Vis Sci. 2012; 53: 4963–4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ma L, Yan SF, Huang YM, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology. 2012; 119: 2290–2297 [DOI] [PubMed] [Google Scholar]
- 46. Liew SHM, Gilbert CE, Spector TD, et al. Heritability of macular pigment: a twin study. Invest Ophthalmol Vis Sci. 2005; 46: 4430–4436 [DOI] [PubMed] [Google Scholar]
- 47. Meyers KJ, Johnson EJ, Bernstein PS, et al. Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Invest Ophthalmol Vis Sci. 2013; 54: 2333–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. SanGiovanni JP, Neuringer M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field. Am J Clin Nutr. 2012; 96: 1223S–1233S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res. 2011; 56: 228–240 [DOI] [PubMed] [Google Scholar]
- 50. Delyfer MN, Buaud B, Korobelnik JF, et al. Association of macular pigment density with plasma omega-3 fatty acids: the PIMAVOSA Study. Invest Ophthalmol Vis Sci. 2012; 53: 1204–1210 [DOI] [PubMed] [Google Scholar]
- 51. Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001; 132: 668–681 [DOI] [PubMed] [Google Scholar]
- 52. Yeum KJ, Booth SL, Sadowski JA, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996; 64: 594–602 [DOI] [PubMed] [Google Scholar]
- 53. Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004; 279: 49447–49454 [DOI] [PubMed] [Google Scholar]
- 54. Bhosale P, Li B, Sharifzadeh M, et al. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009; 48: 4798–4807 [DOI] [PubMed] [Google Scholar]
- 55. Tian R, Pitchford WS, Morris CA, Cullen NG, Bottema CD. Genetic variation in the beta, beta-carotene-9′, 10′-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim Genet. 2010; 41: 253–259 [DOI] [PubMed] [Google Scholar]
- 56. Vage DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 2010; 11: 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Loane E, McKay GJ, Nolan JM, Beatty S, Apolipoprotein E. Genotype is associated with macular pigment optical density. Invest Ophthalmol Vis Sci. 2010; 51: 2636–2643 [DOI] [PubMed] [Google Scholar]
- 58. Borel P, de Edelenyi FS, Vincent-Baudry S, et al. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med. 2011; 43: 47–59 [DOI] [PubMed] [Google Scholar]
- 59. Price AL, Butler J, Patterson N, et al. Discerning the ancestry of European Americans in genetic association studies. PLoS Genet. 2008; 4: e236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laurie CC, Doheny KF, Mirel DB, et al. Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol. 2010; 34: 591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81: 559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006; 38: 904–909 [DOI] [PubMed] [Google Scholar]
- 63. Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006; 2: e190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk to disease from genome-wide association studies. Genome Res. 2007; 17: 1520–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen H, Poon A, Yeung C, et al. A genetic risk score combining ten psoriasis risk loci improves disease prediction. PLoS One. 2011; 6: e19454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001; 42: 235–240 [PubMed] [Google Scholar]
- 67. Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001; 42: 439–446 [PubMed] [Google Scholar]
- 68. Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002; 109: 1780–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chew EY, Clemons T, SanGiovanni JP, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012; 119: 2282–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McKay GJ, Loane E, Nolan JM, et al. Investigation of genetic variation in scavenger receptor class B, member 1 (SCARB1) and association with serum carotenoids. Ophthalmology. 2013; 120: 1632–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reboul E, Borel P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res. 2011; 50: 388–402 [DOI] [PubMed] [Google Scholar]
- 72. Zerbib J, Seddon JM, Richard F, et al. rs5888 variant of SCARB1 gene is a possible susceptibility factor for age-related macular degeneration. PLoS One. 2009; 4: e7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and HDL-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010; 107: 7401–7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Neale BM, Fagerness J, Reynolds R, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A. 2010; 107: 7395–7400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peter I, Huggins GS, Ordovas JM, Haan M, Seddon JM. Evaluation of new and established age-related macular degeneration susceptibility genes in the Women's Health Initiative Sight Exam (WHI-SE) Study. Am J Ophthalmol. 2011; 152: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fauser S, Smailhodzic D, Caramoy A, et al. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 5525–5528 [DOI] [PubMed] [Google Scholar]
- 77. Buitendijk GH, Rochtchina E, Myers C, et al. Prediction of age-related macular degeneration in the general population: the three continent AMD consortium. Ophthalmology. 2013; 120: 2644–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005; 135: 2305–2312 [DOI] [PubMed] [Google Scholar]
- 79. Reboul E, Abou L, Mikail C, et al. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem J. 2005; 387: 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008; 49: 1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schaefer EJ, Santos RD, Asztalos BF. Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol. 2010; 21: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Attie AD, Hamon Y, Brooks-Wilson AR, et al. Identification and functional analysis of a naturally occurring E89K mutation in the ABCA1 gene of the WHAM chicken. J Lipid Res. 2002; 43: 1610–1617 [DOI] [PubMed] [Google Scholar]
- 83. Romanchik JE, Morel DW, Harrison EH. Distributions of carotenoids and alpha-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J Nutr. 1995; 125: 2610–2617 [DOI] [PubMed] [Google Scholar]
- 84. Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-Deficient WHAM chicks having a mutant ABCA1 transporter. Invest. Ophthalmol. Vis. Sci. 2007; 48: 4226–4231 [DOI] [PubMed] [Google Scholar]
- 85. Merle BM, Maubaret C, Korobelnik JF, et al. Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: the Alienor study. PLoS One. 2013; 8: e79848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Herron KL, McGrane MM, Waters D, et al. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J Nutr. 2006; 136: 1161–1165 [DOI] [PubMed] [Google Scholar]
- 87. Sato Y, Suzuki R, Kobayashi M, et al. Involvement of cholesterol membrane transporter Niemann-Pick C1-like 1 in the intestinal absorption of lutein. J Pharm Pharm Sci. 2012; 15: 256–264 [DOI] [PubMed] [Google Scholar]
- 88. McKay GJ, Patterson CC, Chakravarthy U, et al. Evidence of association of APOE with age-related macular degeneration: a pooled analysis of 15 studies. Hum Mutat. 2011; 32: 1407–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Adams MK, Simpson JA, Richardson AJ, et al. Apolipoprotein E gene associations in age-related macular degeneration: the Melbourne Collaborative Cohort Study. Am J Epidemiol. 2012; 175: 511–518 [DOI] [PubMed] [Google Scholar]
- 90. Curcio CA, Johnson M, Huang JD, Aging Rudolf M. age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009; 28: 393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhu X, Parks JS. New roles of HDL in inflammation and hematopoiesis. Annu Rev Nutr. 2012; 32: 161–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res. 2010; 51: 451–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Provost AC, Vede L, Bigot K, et al. Morphologic and electroretinographic phenotype of SR-BI knockout mice after a long-term atherogenic diet. Invest Ophthalmol Vis Sci. 2009; 50: 3931–3942 [DOI] [PubMed] [Google Scholar]
- 94. Dithmar S, Curcio CA, Le NA, Brown S, Grossniklaus HE. Ultrastructural changes in Bruch's membrane of apolipoprotein E-deficient mice. Invest Ophthalmol Vis Sci. 2000; 41: 2035–2042 [PubMed] [Google Scholar]
- 95. von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr. 2010; 30: 35–56 [DOI] [PubMed] [Google Scholar]
- 96. Lietz G, Oxley A, Boesch-Saadatmandi C, Kobayashi D. Importance of beta, beta-carotene 15,15′-monooxygenase 1 (BCMO1) and beta, beta-carotene 9′,10′-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res. 2012; 56: 241–250 [DOI] [PubMed] [Google Scholar]
- 97. Ferrucci L, Perry JR, Matteini A, et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009; 84: 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the beta-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012; 142: 161S–165S [DOI] [PubMed] [Google Scholar]
- 99. Albanes D, Virtamo J, Taylor PR, Rautalahti M, Pietinen P, Heinonen OP. Effects of supplemental beta-carotene, cigarette smoking, and alcohol consumption on serum carotenoids in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1997; 66: 366–372 [DOI] [PubMed] [Google Scholar]
- 100. Amengual J, Lobo GP, Golczak M, et al. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011; 25: 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, et al. Two carotenoid-oxygenases contribute to mammalian pro-vitamin A metabolism. J Biol Chem. 2013; 288: 34081–34096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Clifford AJ, Rincon G, Owens JE, et al. Single nucleotide polymorphisms in CETP, SLC46A1, SLC19A1, CD36, BCMO1, APOA5, and ABCA1 are significant predictors of plasma HDL in healthy adults. Lipids Health Dis. 2013; 12: 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hessel S, Eichinger A, Isken A, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. Nov 16. 2007; 282: 33553–33561 [DOI] [PubMed] [Google Scholar]
- 104. Ford NA, Elsen AC, Erdman JW Jr. Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr Res. 2013; 33: 733–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012; 249: 218–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. He M, Cornelis MC, Kraft P, et al. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol. 2010; 30: 885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stoffel W, Holz B, Jenke B, et al. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 2008; 27: 2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008; 87: 1521–1529 [DOI] [PubMed] [Google Scholar]
- 109. Nelson GJ, Schmidt PC, Bartolini GL, Kelley DS, Kyle D. The effect of dietary docosahexaenoic acid on plasma lipoproteins and tissue fatty acid composition in humans. Lipids. 1997; 32: 1137–1146 [DOI] [PubMed] [Google Scholar]
- 110. Foulon T, Richard MJ, Payen N, et al. Effects of fish oil fatty acids on plasma lipids and lipoproteins and oxidant-antioxidant imbalance in healthy subjects. Scand J Clin Lab Invest. 1999; 59: 239–248 [DOI] [PubMed] [Google Scholar]
- 111. Thomas TR, Smith BK, Donahue OM, Altena TS, James-Kracke M, Sun GY. Effects of omega-3 fatty acid supplementation and exercise on low-density lipoprotein and high-density lipoprotein subfractions. Metabolism. 2004; 53: 749–754 [DOI] [PubMed] [Google Scholar]
- 112. Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004; 45: 3244–3256 [DOI] [PubMed] [Google Scholar]
- 113. SanGiovanni JP, Chew EY, Agron E, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008; 126: 1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Reynolds R, Rosner B, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. 2013; 120: 1020–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83 (6 suppl): 1505S–1519S [DOI] [PubMed] [Google Scholar]
- 116. SanGiovanni JP, Neuringer MN. The promise of molecular genetics for investigating the influence of macular xanthophylls on advanced age-related macular degeneration. In: Landrum JT, Nolan JM. eds Carotenoids and Retinal Disease. Chapter 6. Boca Raton, FL: CRC Press; 2013: 93–128 [Google Scholar]
- 117. Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013; 45: 433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Online Mendelian Inheritance in Man, OMIM. Baltimore, MD: Johns Hopkins University, MIM Number: #270200; Last edited: 09/19/2012; Available at: http://omim.org/. Accessed 07/19/2013 [Google Scholar]
- 119. van der Veen RL, Fuijkschot J, Willemsen MA, Cruysberg JR, Berendschot TT, Theelen T. Patients with Sjögren-Larsson syndrome lack macular pigment. Ophthalmology. 2010; 117: 966–971 [DOI] [PubMed] [Google Scholar]